Abstract
Randomized trials have demonstrated that male circumcision (MC) reduces heterosexual acquisition of HIV, herpes simplex virus type-2, human papillomavirus (HPV), and genital ulcer disease (GUD) among men, and reduces HPV, GUD, bacterial vaginosis and trichomoniasis among female partners. The pathophysiology behind these effects is multifactorial, relying on anatomic and cellular changes. MC is cost-effective and potentially cost saving in both the US and Africa. The WHO and Joint United Nations Program on HIV/AIDS proposed reaching 80% MC coverage in HIV endemic countries, but current rates fall far behind targets. Barriers to scale-up include supply-side and demand-side challenges. In the US, neonatal MC rates are decreasing, but the American Academy of Pediatrics now recognizes the medical benefits of MC and supports insurance coverage. While MC is a globally valuable tool to prevent HIV and other sexually transmitted infections, it is under-utilized. Further research is needed to address barriers to MC uptake.
Keywords: male circumcision, HIV, AIDS, herpes simplex virus type-2 (HSV-2), human papillomavirus (HPV), penile cancer, cervical cancer, bacterial vaginosis and Tricomonas vaginalis, trichomoniasis, sub-Saharan Africa, America, policy, cost-effectiveness
Three randomized trials from Africa conclusively demonstrated that male circumcision (MC) reduces heterosexual HIV acquisition by 53–60%, triggering widespread efforts to promote MC as part of a comprehensive HIV prevention strategy. In 2007, the World Health Organization (WHO), in conjunction with the Joint United Nations Program on HIV/AIDS (UNAIDS), issued a formal policy statement in support of MC: “Male circumcision should now be recognized as an efficacious intervention for HIV prevention…Promoting male circumcision should be recognized as an additional, important strategy for the prevention of heterosexually acquired HIV infection in men”(1).
Five years later, in 2012, the American Academy of Pediatrics (AAP) took a similar step in support of the procedure among newborns, revising their policy statement to note, “preventive health benefits of elective circumcision of male newborns outweigh the risks of the procedure.” While the AAP did not find that the health benefits of circumcision were substantial enough to warrant recommending routine circumcision for male newborns, the policy noted, “the benefits of circumcision are sufficient to justify access to this procedure for families choosing it and to warrant third-party payment for circumcision of male newborns (2).” The American College of Obstetricians and Gynecologists has endorsed this statement.
These policy statements have arisen in the context of a growing body of evidence supporting the medical benefits of MC. However, despite this evidence, MC rates in developed countries have declined in recent years, and uptake of MC services in the developing world has been slow. In this review, we will discuss the global rates of MC, medical evidence on effects of the practice, projections of health and financial impact from large-scale modeling studies, implementation strategies for MC programs, and barriers to scale-up.
MC Prevalence
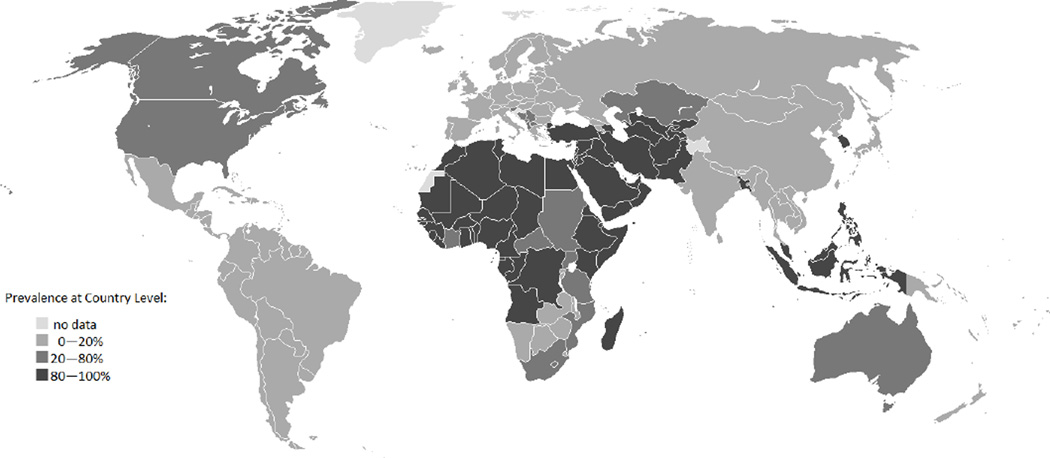
Rates of MC vary across countries, and differ between social, cultural, and religious groups. MC has not been traditional for many men historically; only 30% of men worldwide are circumcised for religious, cultural, medical, or other reasons (3) (Figure 1).
Figure 1.
Male circumcision prevalence by country according to the World Health Organization (http://www.who.int/hiv/pub/malecircumcision/globaltrends/en/index.html).
Judaism and Islam are two religions where MC has traditionally been an important ritual. Jewish male infants are typically circumcised on their 8th day of life, and rates of MC among Jewish men in the United States, Israel, and the United Kingdom exceed 98%(3). MC among Muslim men accounts for more than two-thirds of all circumcision globally. While Islam does not dictate a specific day for the procedure to be performed, it is typically conducted between birth and puberty. Most other religions adopt a neutral stance on MC.
Rates of MC for non-religious reasons vary substantially across countries. While in the United States, 75% of men 15 or older are circumcised for non-religious reasons, only 6% of males in the United Kingdom are circumcised (3). Rates in Europe have decreased since 1949, when a British Medical Journal article concluded that MC was not medically justified (3; 4). MC is uncommon in Central and South America, and in most of Asia, with the exceptions of Korea and the Philippines (5–8). Within Africa, rates vary substantially. While men in parts of West and North Africa and men from specific ethnic or tribal groups such as the Xhosa men in South Africa, are traditionally circumcised, the practice is not mainstream for other groups (3). The age at which MC is performed also varies across communities; neonatal MC is common in Ghana, but MC among boys or young adults is more common in Burkina Faso, Kenya, South Africa, Zambia, and Zimbabwe(3).
MC for HIV Prevention Among Heterosexual Men
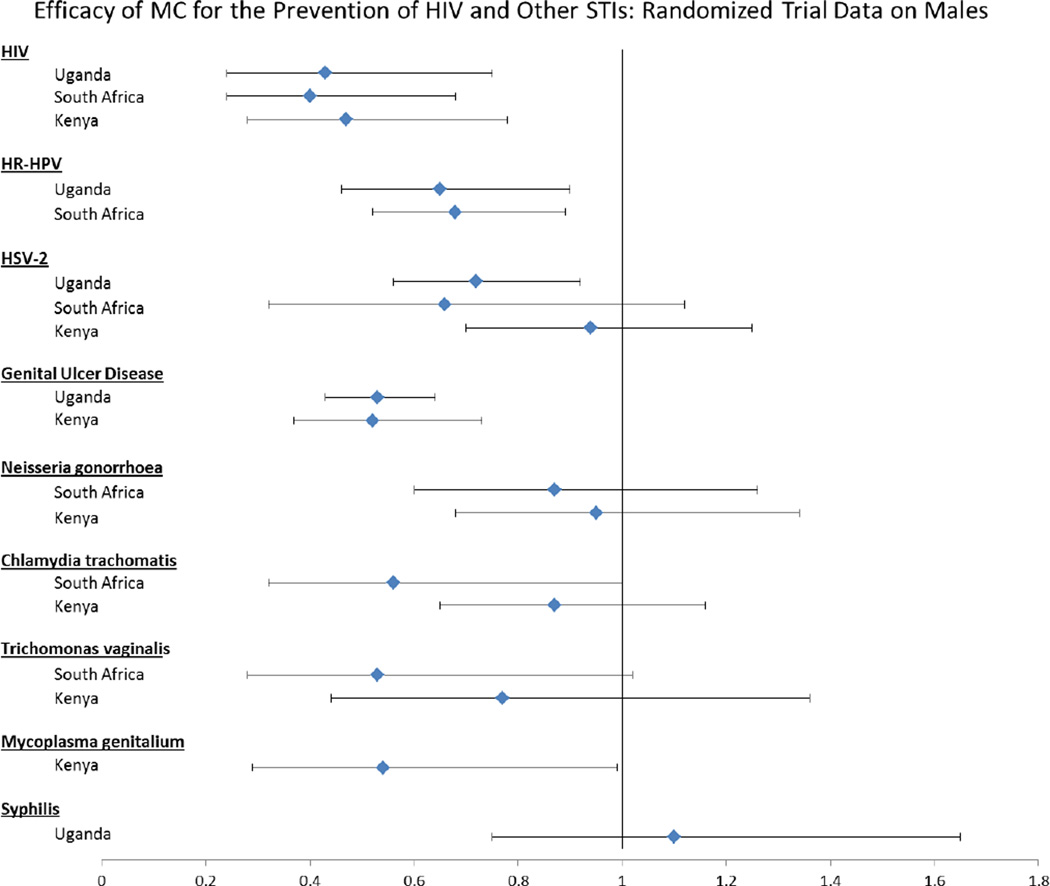
Prior to 2002, more than 30 published ecological and observational studies had suggested that HIV acquisition is lower among circumcised men than uncircumcised men (9). However, these studies were unable to exclude the possibility that the association found between HIV infection and circumcision status was due to confounding by sexual behaviors or other factors. Thus, three large randomized controlled trials enrolling more than 10,000 adult men were conducted in South Africa, Kenya, and Uganda. The trials showed that MC decreased heterosexual HIV acquisition by 50–60% (10–12), and that this effect was significant across trial settings. While the trials varied by surgical procedure used, urban/rural status of the community, and eligibility criteria including age, all trials enrolled HIV-negative men and randomized them to circumcision upon enrollment or control. Men were followed for 21–24 months. The intent-to-treat efficacy estimates found in the South African, Kenyan, and Ugandan trials were strikingly consistent, at 60% (95%CI 32% – 76%) (12), 53% (95%CI 22% – 72%) (11) and 57% (95%CI 25% – 76%) (10), respectively (Figure 2).
Figure 2.
The data shown in this figure are from publications of the three randomized controlled trials that presented the efficacy of male circumcision using different statistical methods. Specifically, for HIV, HSV-2 (South Africa), C. trachomatis (Kenya), T. vaginalis (Kenya) and N. gonorrhoeae (Kenya) the ratio expressed above is an incidence rate ratio. For HR-HPV, N gonorrhoeae (South Africa), and GUD the ratio expressed above is a prevalence rate ratio. For HSV-2 (Uganda) and syphilis, the ratio expressed above is a hazard ratio. For T. vaginalis (South Africa) and C. trachomatis (South Africa) the ratio expressed above is an odds ratio. For HSV-2 (Kenya) the data are expressed as a risk ratio. All ratios are adjusted (except South African HSV-2, Kenyan HSV-2, Kenyan bacterial STIs) and represent an intention-to-treat analysis (except Kenyan HSV-2).
Post-trial follow-up demonstrated that the efficacy of MC to reduce HIV acquisition actually increased over time. In an analysis three years post-MC trial closure in Uganda, MC was associated with a 73% reduction in HIV acquisition risk (13). The Kenyan trial also demonstrated that the protective efficacy of MC had increased when evaluated at 42 months post-circumcision (14).
MC and Viral Sexually Transmitted Infections (STIs)
Circumcision in the Ugandan and Kenyan randomized trials was associated with decreased frequency of genital ulcers (10; 15), suggesting that MC may reduce sexually transmitted infections (STIs) associated with genital lesions (Figure 2). Among men in the Uganda trial who were herpes simplex virus type 2 (HSV-2) and HIV negative, the probability of HSV-2 seroconversion over two years was 7.8% in the circumcised group, but 10.3% among control group men (adjusted hazard ratio 0.72, 95%CI 0.56–0.92) (16; 17). In the South African trial, HSV-2 incidence was 3.54/100 person-years among uncircumcised men and 2.33/100 person-years among circumcised men, with an unadjusted incidence rate ratio of 0.66 (95% CI 0.32 – 1.12). (18). The Kenyan trial, however, did not find a difference in HSV-2 acquisition between circumcised men and uncircumcised men (19).
Prior to these trials, observational studies had suggested that MC may decrease risk of infection with high-risk human papillomavirus (HR-HPV), which can lead to penile cancer (20–23). The South African trial evaluated penile HR-HPV prevalence at the urethra at 21 months post-enrollment (24). The prevalence of HR-HPV was significantly lower among intervention arm men (14.8%) than control arm men (22.3%), with an adjusted PRR of 0.68 (95%CI 0.52–0.89) (Figure 2). The Ugandan MC trial evaluated HR-HPV prevalence at the glans/coronal sulcus (16). At enrollment, HR-HPV prevalence was comparable in both study arms, but the point prevalence of HR-HPV at the two year visit was lower in the intervention arm (18.0%) than in the control arm (27.9%), with an adjusted PRR of 0.65 (95%CI 0.46 – 0.90). MC decreased the overall HR-HPV viral load (25), and the decrease in HR-HPV prevalence was due to both reduced acquisition of new HR-HPV infections and increased clearance of pre-existing HR-HPV infection in HIV-negative men (26; 27). The Ugandan MC trial also showed that MC reduces HR-HPV on the penile shaft (28). In addition to HIV-negative men, MC of HIV-positive men decreased penile high-risk HPV prevalence (RR=0.77, 95%CI 0.62–0.97) (29). These trial findings indicate that circumcision should be accepted as an efficacious intervention for reducing HSV-2 and HR-HPV.
MC and Bacterial STIs
The effect of MC on the risk of bacterial STI acquisition among men was evaluated by all three randomized trials. In the South African trial, MC decreased both Trichomonas vaginalis (adjusted OR 0.53, 95%CI 0.28 – 1.02) and Chlamydia trachomatis (adjusted OR 0.56, 95%CI 0.32 – 1.00), with borderline statistical significance (Figure 1) (30). However, MC had no impact on Neisseria gonorrhoeae (adjusted PRR 0.87, 95%CI 0.60 – 1.26) (24). The randomized trial in Kenya did not find a reduction in bacterial infections of Neisseria gonorrhoeae (OR 0.95, 95%CI 0.68–1.38), Chlamydia trachomatis (OR 0.87, 95%CI 0.65–1.16, p=0.325), or Trichomonas vaginalis infection (OR 0.77, 95%CI 0.44–1.36) (31), but did find an protective effect for Mycosplasma gentailium (adjusted OR 0.54; 95% CI: 0.29–0.99) (32). In the Ugandan trial, MC did not reduce syphilis acquisition (adjusted hazard ratio 1.10, 95%CI 0.75–1.65) (16). These data suggest that MC has no significant effect on bacterial STIs, particularly those of urethral pathogenicity.
MC and Indirect Effects on Female Partners
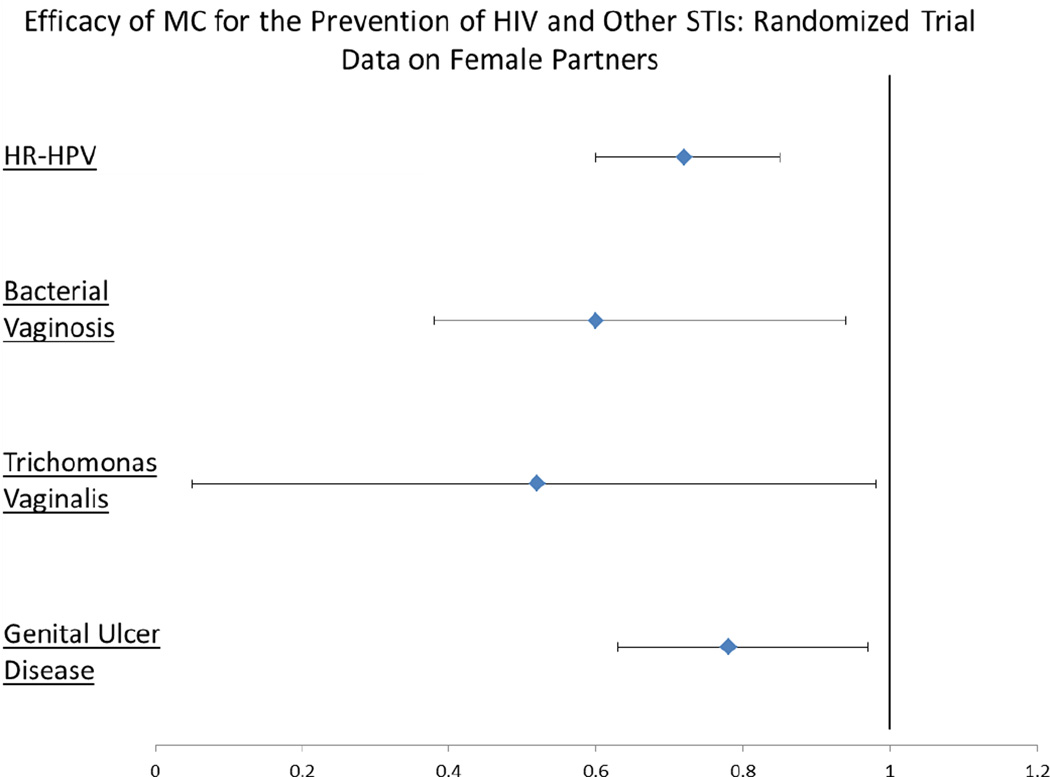
Observational studies found conflicting results regarding the effect of MC on a female partner’s risk of cervical cancer, caused by HR-HPV (21; 33; 34). The randomized controlled trial in Uganda simultaneously enrolled female partners of the male trial participants to clarify this effect. While HPV prevalence of female partners of HIV-negative men were similar between arms at enrollment, female HR-HPV prevalence at year two was 27.8% in the intervention and 38.7% in the control arm (PRR=0.72, 95%CI 0.60–0.85) (Figure 3); this reduced prevalence was due to decreased acquisition and increased clearance among the female partners (35). The decrease in cervical cancer found in observational studies may be due to the reduction in overall HPV viral load of female partners of circumcised men (36). Contrary to findings in HIV-negative men, MC of HIV-positive men did not affect HR-HPV transmission to female partners (PRR = 1.07, 95%CI 0.86–1.32)(37).
Figure 3.
The data shown above are from publications of the Ugandan randomized controlled trial that presented the efficacy of male circumcision on STIs among female partners. For HR-HPV, bacterial vaginosis, T. vaginalis, and GUD, the ratio expressed above is a prevalence rate ratio. All ratios are adjusted (except HR-HPV) and represent an intention-to-treat analysis.
In addition to reduced HPV prevalence, the female partners of HIV-negative circumcised men in the Ugandan randomized trial had decreased genital ulcer disease (adjPRR 0.78, 95%CI 0.63 – 0.97), trichomonas infection (adjusted PRR 0.52, 95%CI 0.05 – 0.98), and bacterial vaginosis (BV) (adjPRR 0.60, 95%CI 0.38 – 0.94) as compared to the partners of uncircumcised men (Figure 3) (38). The decreased risk of BV is likely due to a reduction in overall penile bacterial load and reduced pro-inflammatory anaerobic bacteria among circumcised men (39; 40). MC does not have an impact on HSV-2 acquisition among female partners (41). Overall, however, MC’s benefits to female partners are substantial.
The role of MC to prevent HIV transmission to female partners is not clear. Among 163 HIV-positive men and their HIV-negative female partners enrolled in a randomized controlled trial in Uganda, MC had no effect on male-to-female HIV transmission over two years of observation (adjusted HR 1.49, 95%CI 0.62–3.57, p=0.37) (42). This trial, however, was terminated prematurely due to futility. In a stratified analysis, HIV transmission risk was significantly increased among couples who resumed sex prior to complete healing of the circumcision wound (42). However, two observational studies found that MC decreased HIV transmission to female partners (43; 44). The difference in findings between the trials and observational studies may be due to the age at which circumcision was performed: the observational studies assessed men who had been circumcised as infants, while in the randomized trials, circumcision was performed among adults. It is possible that incomplete wound healing among HIV-positive men enrolled in the trial may have offset potential longer-term effects of circumcision on male-to-female HIV transmission. While the direct potential benefits of MC for female HIV transmission are not clear, modeling studies have suggested that MC will likely lead to an indirect benefit to females by decreasing HIV prevalence among male partners (45).
Generalizability of MC Trial Results
While the dynamics and burden of HIV in the developed world may be different than that in Africa, supporting evidence from the United States suggests that trial findings may be externally valid. Observational studies in the United States involving heterosexual men who had primarily been circumcised in childhood have found protective medical effects of MC consistent with those found in the trials. In a study among men with known heterosexual HIV exposure visiting a Baltimore, Maryland STI clinic, HIV prevalence among uncircumcised men was 22%, but only 10% among circumcised men (adjusted PRR 0.49, 95%CI 0.26 – 0.93) (46). Additional studies among men in Florida and Arizona found MC to be protective against HPV infection of the urethra, glans/corona and penile shaft, with an adjusted odds ratio of 0.53 (95% CI, 0.28 – 0.99) (47), and circumcised men to be more likely to clear oncogenic HPV infection (48). Thus, the results of the African trials also appear to be relevant to heterosexuals at high risk for STIs in the developed world.
While MC clearly reduces STIs among heterosexual men, its effects among men who have sex with men (MSM) are unclear. While some observational studies of MSM have found that MC is associated with decreased HIV infection (49; 50), others have found no protective effect (15; 51–53). A meta-analysis incorporating more than 50,000 MSM did not find an association between HIV status and being circumcised (OR = 0.95, 95%CI 0.81–1.11) (54). MSM engage in both insertive and receptive sexual practices, but MC may exhibit preventative effects only for insertive intercourse. Several studies of men who participate exclusively in insertive anal intercourse have found that uncircumcised men have a higher risk of HIV infection than circumcised men (53; 55). Thus, any protective effect of circumcision among MSM may not be observed if studies do not differentiate between these practices.
Pathophysiology of MC to Reduce STIs
The pathophysiology underlying the ability of MC to reduce HIV and other sexually transmitted infections is likely multifactorial, with multiple anatomic factors that may favor protection. Circumcision is associated with a decreased frequency of genital ulcers (10). The Ugandan trial found reduced genital ulcers among both HSV-2 negative and HSV-2 positive men (56). Viral infections may enter through genital ulcers or microtears in the preputial mucosa. It has been estimated the 11% of reduced HIV acquisition in circumcised men is attributable to reductions in symptomatic genital ulcers and an additional 9% of reduced HIV is mediated by reduced HSV-2 incidence (56). It has also been shown that HIV is higher among men with larger foreskin surface area (57). The male foreskin creates a warm, moist subpreputial cavity that may assist in viral and anaerobic bacterial survival. The foreskin of uncircumcised men is retracted over the shaft during intercourse when the penis is erect. This exposes the preputial mucosa to vaginal and cervical fluids that may more easily penetrate the inner foreskin mucosa and lead to infection (58; 59). Circumcision removes this rich vascular tissue with a thin keratin layer and it becomes replaced with scar tissue. Thus, there is biological plausibility to support the findings that circumcision reduces viral STIs.
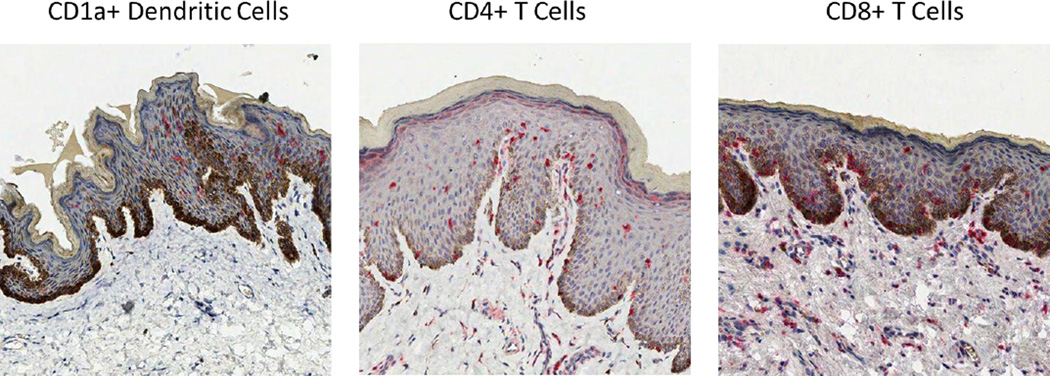
In addition to anatomic factors, there are likely cellular factors that play a role in protection among circumcised men. It has been hypothesized that the inner mucosa of the foreskin is lightly keratinized compared to the epithelium of the shaft, coronal sulcus and glans, which may facilitate mucosal access of HIV, HSV-2 or HPV to the epithelium (59; 60), suggesting that it is easier for HIV to establish infection via the inner mucosal surface than cervical tissue (61). However, more recent evidence suggests that keratin thickness is similar between the internal and external foreskin surfaces (62; 63). While keratin thickness may not explain the protective mechanism of MC, the foreskin mucosa contains a high density of dendritic (Langerhans) cells, CD4+ T cells and CD8+ T cells (Figure 4), which are all targets for HIV infection (59; 64; 65), and HIV is able to penetrate the foreskin infecting Langerhans cells (66; 67). In HSV-2 infected men, there is an increased CD4+ T-cell density in the foreskin (64), which may help to explain why HSV-2–infected men are at increased risk of HIV acquisition.
Figure 4.
CD1a+ dendritic cells, CD4+ T cells, and CD8+ T cells are present in the foreskin. The cells are highlighted by red precipitate using immunohistochemistry. While the CD4+ and CD8+ T cells are found both the epidermis and dermis, the dendritic cells are primarily located in the epidermis.
Scale-up and Potential for Large-scale Impact
With a strong biological basis for protection against HIV and other STIs, the WHO and Joint United Nations Program on HIV/AIDS established recommendations to reach 80% MC coverage among men aged 15–49 in 13 countries in eastern and southern Africa with high HIV prevalence and low MC rates (Botswana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Uganda, the United Republic of Tanzania, Zambia, and Zimbabwe) by 2015(68; 69). The US President’s Emergency Plan for AIDS Relief (PEPFAR) is supporting MC scale-up efforts in these priority countries, in addition to supporting a scale-up program in Ethiopia (69).
Multiple analyses have attempted to model the potential health and financial impact of large-scale MC programs. Reaching the 80% coverage target in these 14 countries would require 20.33 million MC procedures by 2015, and sustaining this 80% coverage level would require an additional 8.4 million MC procedures from 2016–2025 (69). MC scale-up programs which reach coverage targets in sub-Saharan Africa by 2015 are projected to prevent 22% of new HIV infections through 2025 (69). In a high HIV prevalence setting, each averted HIV infection would require 5 to 15 circumcision procedures (70). Furthermore, models suggest that as an HIV prevention method, MC is highly cost-effective and potentially cost saving after a period of scale-up (71; 72). While MC programs require initial expenditure for the procedure, potential adverse events, and other components of scale-up, savings are expected to accrue over time as circumcised men, their female partners, and others throughout the community avoid infection with HIV and other STIs. MC scale-up to 80% coverage across priority countries in eastern and southern Africa by 2015 is associated with a net savings of $16.51 billion through averted HIV alone (71). Although estimates have varied based on the specifics of the model used and the population studied, a systematic review from 2009 found that reported cost per HIV infection averted through MC ranged from $174 to $2808 (72). These values are comparable to those for other interventions to prevent HIV, such as voluntary testing and counseling, antiretroviral therapy for prevention, and interventions to reduce mother-to-child transmission. Incorporating averted cases of non-HIV STIs in cost-effectiveness models increases cost savings associated with MC scale-up (73).
Additional studies have examined the health and financial impact of MC in developed countries such as the United States (74; 75). Even in countries with low HIV prevalence, MC may be a highly cost-effective method to reduce incidence of heterosexually-transmitted HIV, other STIs, and infant urinary tract infections (UTIs)(76). An analysis using efficacy estimates from the African trials suggested that in the US, each MC procedure performed is associated with a decrease of $313 in net direct medical costs(75). These cost savings are expected to arise from reduced HIV, HPV, HSV-2, and infant UTIs among circumcised males, in addition to averted HPV, BV, and Trichomoniasis among female partners. Thus, MC is highly cost-effective in both Africa and the developed world.
Implementation
Proposals to scale up MC programs in eastern and southern Africa incorporate an initial “catch-up” phase, to focus on reaching uncircumcised adult males likely to be sexually active and at risk of HIV infection, as well as a “sustainability” phase, to focus on routinely offering MC to infants or adolescents (69). Reaching coverage targets will require massive and rapid roll-out of services, frequently across regions where MC has not been a routine practice. Thus far, implementation in target areas has been slow, with rates of MC lagging far behind the goal of 80% coverage. As of 2010, only 2.7% of the total number of MC procedures needed to be done to reach this coverage level had been performed. Of all priority countries, only Kenya appears to be on track to achieving the 80% coverage target (77).
Barriers to Scale-up
Rapid scale-up of MC in eastern and southern Africa is likely to require extensive human and capital resources (78; 79) to ensure an adequate supply of safe, high-quality MC services. Furthermore, effective programs may also need to incorporate demand-generation activities to ensure acceptability and uptake among men, particularly within communities where MC is not routine.
In many African countries, the supply of available human resources for health (physicians, surgical specialists, nurses, counselors, other support staff) falls far below the amount needed to achieve MC coverage targets (79). In addition, priority countries for MC also face other health concerns, and shifting all resources to MC scale-up may lead to neglect of these other health issues. However, various supply-side innovations may be able to improve surgical, non-surgical, and human resource efficiencies and help to minimize disruptions to other aspects of the healthcare system. Conventional MC programs have employed a one doctor-one assistant team responsible for surgically performing each MC, using a forceps-guided, dorsal slit, or sleeve resection method. This model allows each team to perform 8–10 MC procedures daily (79). However, increasing the number of surgical bays in an operating room and task shifting or task sharing specific components of the procedure to nurses or other staff may allow for higher volumes of procedures. In addition, disposable or pre-bundled MC kits may reduce preparation time. Temporarily increasing healthcare professionals available for MC through targeted recruitment or redeployment may also improve the supply of services. The development of new non-surgical MC methods, such as the Shang Ring or PrePex, may allow for further task shifting to non-surgically trained health care workers (81; 82).
Acceptance of MC among men who have not traditionally engaged in the practice of MC varies across settings. A review of studies on the acceptability in sub-Saharan Africa suggests that 65% of uncircumcised men are willing to become circumcised, with this portion varying from 29% in Uganda to 87% in Swaziland. In addition, the review suggested that 69% of women favored circumcised partners, and that the majority of men (71%) and women (81%) were willing to circumcise their sons (83). Various demand-generation activities have been used to encourage engagement, both from men and from the broader community. Communication efforts have attempted to raise awareness and increase acceptability using educational materials, mass media and entertainment, and peer educators (68). Some efforts have tried to address concerns of men, including issues of pain, cost, and reduced sexual pleasure. In addition, these messages have been clear that MC is not 100% protective against HIV, in an attempt to reduce any adverse risk compensation following scale-up. There was no demonstration of change in sexual behaviors among circumcised men during or after the Kenyan or Ugandan MC trials(10; 11; 84). Targeted incentives to uncircumcised males may also have some effect on demand, although a study from Malawi found that vouchers to subsidize the cost of MC were primarily effective among lower-risk men (85).
As scale-up programs begin to shift from targeting adult males at risk of HIV to a “sustainability phase” focused on a younger population, demand generation activities and the way services are supplied may need to be adjusted. Proposals to incorporate MC as a routine activity among neonatal males may face challenges if births do not often occur in health facilities. Alternatively, it may be possible to use schools or youth centers to offer services to young men before becoming sexually active(86).
Despite encouraging findings on the medical benefits of MC, Medicaid, the public insurance system for low income individuals in the United States, has recently eliminated coverage for neonatal MC in 18 states(87). Medicaid pays for more than one-third of all in-hospital MC procedures(88). Private insurers in the US are also trending towards decreasing coverage for the procedure(88). Alongside coverage limitations, rates of MC in the United States have been declining dramatically over the past 20 years; while men born in the 1970s and 1980s had an MC rate of approximately 79%, the MC rate among males born in 1999 was 62.5%, and by 2010, the rate among newborns was below 55% (75; 89; 90). These trends may have important health and financial implications (75; 91). Furthermore, lack of Medicaid coverage for the procedure may lead to exaggerated socioeconomic disparities in STI-related health (91; 92). Thus, it is clear that the practice of MC may be a policy-relevant issue in developed countries such as the US, as well as in Africa. The recent revision of a policy statement by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists in support of access to MC may begin to reverse these current trends (2).
It is clear that MC is a potentially valuable tool for the prevention of HIV and other STIs, with relevance for sub-Saharan Africa as well as other countries globally. Thus far, however, it appears to be an under-utilized method. Further research is needed to understand why uptake of MC services has been low compared to target coverage levels, and to develop innovative methods to address this gap.
Acknowledgments
Funding/Support
SK and AART are supported by the Doris Duke Charitable Foundation (grant 2011036) and AART is also supported by NIH 1K23AI093152-01A1. TCQ is supported by the Division of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Conflicts of Interest
The authors deny any conflicts of interest.
References
- 1.UNAIDS. New data on male circumcision and HIV prevention: policy and programme implications. Montreux: UNAIDS; 2007. [Google Scholar]
- 2.American Academy of Pediatrics Task Force on C. Male circumcision. Pediatrics. 2012;130:e756–e785. doi: 10.1542/peds.2012-1990. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization., Joint United Nations Programme on HIV/AIDS. Male circumcision : global trends and determinants of prevalence, safety, and acceptability. Geneva: World Health Organization : UNAIDS; 2008. London School of Hygiene and Tropical Medicine. [Google Scholar]
- 4.Gairdner D. The fate of the foreskin, a study of circumcision. British medical journal. 1949;2:1433–1437. doi: 10.1136/bmj.2.4642.1433. illust. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RB. Circumcision practice in the Philippines: community based study. Sexually transmitted infections. 2005;81:91. doi: 10.1136/sti.2004.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku JH, Kim ME, Lee NK, Park YH. Circumcision practice patterns in South Korea: community based survey. Sexually transmitted infections. 2003;79:65–67. doi: 10.1136/sti.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang MG, Kim DS. Extraordinarily high rates of male circumcision in South Korea: history and underlying causes. BJU international. 2002;89:48–54. [PubMed] [Google Scholar]
- 8.Kim DS, Lee JY, Pang MG. Male circumcision: a South Korean perspective. BJU international. 1999;83(Suppl 1):28–33. doi: 10.1046/j.1464-410x.1999.0830s1028.x. [DOI] [PubMed] [Google Scholar]
- 9.Siegfried N, Muller M, Volmink J, Deeks J, Egger M, et al. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane database of systematic reviews. 2003 doi: 10.1002/14651858.CD003362. CD003362. [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 11.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 12.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS medicine. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray PH, Edwards DM, O'Callaghan MJ, Cuskelly M. Parenting stress in mothers of preterm infants during early infancy. Early human development. 2012;88:45–49. doi: 10.1016/j.earlhumdev.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Bailey RC, Moses S, Parker C, Agot K, Maclean I, et al. The protective effect of male circumcision is sustained for at least 42 months: results from the Kisumu, Kenya trial; XVII International AIDS Conference; 2008. Abstract THAC0501. [Google Scholar]
- 15.Mehta S, Parker C, Ndinya-Achola J, Moses S, Maclean IW, et al. MMC is not protective against HSV-2 incidence but halves the risk of GUD incidence: results from the randomized trial of MMC to reduce HIV in Kisumu, Kenya; Eighteenth Conference on Retroviruses and Opportunistic Infections; 2011. Abstract 147LB. [Google Scholar]
- 16.Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. The New England journal of medicine. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobian AAR, Charvat B, Ssempijja V, Kigozi G, Serwadda D, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infections among men in Rakai, Uganda. The Journal of infectious diseases. 2009;199:945–949. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobngwi-Tambekou J, Taljaard D, Lissouba P, Zarca K, Puren A, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. The Journal of infectious diseases. 2009;199:958–964. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta SD, Moses S, Parker CB, Agot K, Maclean I, Bailey RC. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. Aids. 2012;26:1141–1149. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoen EJ, Oehrli M, Colby C, Machin G. The highly protective effect of newborn circumcision against invasive penile cancer. Pediatrics. 2000;105:E36. doi: 10.1542/peds.105.3.e36. [DOI] [PubMed] [Google Scholar]
- 21.Castellsague X, Bosch FX, Munoz N, Meijer CJ, Shah KV, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. The New England journal of medicine. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC, et al. Human papillomavirus infection in men attending a sexually transmitted disease clinic. The Journal of infectious diseases. 2003;187:1064–1070. doi: 10.1086/368220. [DOI] [PubMed] [Google Scholar]
- 23.Lajous M, Mueller N, Cruz-Valdez A, Aguilar LV, Franceschi S, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–1716. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 24.Auvert B, Sobngwi-Tambekou J, Cutler E, Nieuwoudt M, Lissouba P, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in orange farm, South Africa. The Journal of infectious diseases. 2009;199:14–19. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson LE, Gravitt P, Tobian AA, Kigozi G, Serwadda D, et al. Male circumcision reduces penile high-risk human papillomavirus viral load in a randomised clinical trial in Rakai, Uganda. Sex Transm Infect. 2013;89:262–266. doi: 10.1136/sextrans-2012-050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray RH, Serwadda D, Kong X, Makumbi F, Kigozi G, et al. Male Circumcision Decreases Acquisition and Increases Clearance of High-Risk Human Papillomavirus in HIV-Negative Men: A Randomized Trial in Rakai, Uganda. The Journal of infectious diseases. 2010;201:1455–1462. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobian AA, Kigozi G, Gravitt PE, Xiao C, Serwadda D, et al. Human papillomavirus incidence and clearance among HIV-positive and HIV-negative men in Rakai, Uganda. AIDS. 2012;26:1555–1565. doi: 10.1097/QAD.0b013e328353b83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobian AA, Kong X, Gravitt PE, Eaton KP, Kigozi G, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. International journal of cancer. Journal international du cancer. 2011;129:2970–2975. doi: 10.1002/ijc.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serwadda D, Wawer MJ, Makumbi F, Kong X, Kigozi G, et al. Circumcision of HIV-Infected Men: Effects on High-Risk Human Papillomavirus Infections in a Randomized Trial in Rakai, Uganda. The Journal of infectious diseases. 2010;201:1463–1469. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, Lissouba P, Puren A, Auvert B. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sexually transmitted infections. 2009;85:116–120. doi: 10.1136/sti.2008.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta SD, Moses S, Agot K, Parker C, Ndinya-Achola JO, et al. Adult Male Circumcision Does Not Reduce the Risk of Incident Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis Infection: Results from a Randomized, Controlled Trial in Kenya. The Journal of infectious diseases. 2009;200:370–378. doi: 10.1086/600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta SD, Gaydos C, Maclean I, Odoyo-June E, Moses S, et al. The effect of medical male circumcision on urogenital Mycoplasma genitalium among men in Kisumu, Kenya. Sex Transm Dis. 2012;39:276–280. doi: 10.1097/OLQ.0b013e318240189c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drain PK, Halperin DT, Hughes JP, Klausner JD, Bailey RC. Male circumcision, religion, and infectious diseases: an ecologic analysis of 118 developing countries. BMC infectious diseases. 2006;6:172. doi: 10.1186/1471-2334-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinton LA, Reeves WC, Brenes MM, Herrero R, Gaitan E, et al. The male factor in the etiology of cervical cancer among sexually monogamous women. International journal of cancer. Journal international du cancer. 1989;44:199–203. doi: 10.1002/ijc.2910440202. [DOI] [PubMed] [Google Scholar]
- 35.Wawer MJ, Tobian AA, Kigozi G, Kong X, Gravitt PE, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet. 2011;277:209–218. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis MA, Gray RH, Grabowski MK, Serwadda D, Kigozi G, et al. Male circumcision decreases high-risk human papillomavirus viral load in female partners: A randomized trial in Rakai, Uganda. International journal of cancer. Journal international du cancer. 2013 doi: 10.1002/ijc.28100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobian AA, Kong X, Wawer MJ, Kigozi G, Gravitt PE, et al. Circumcision of HIV-infected men and transmission of human papillomavirus to female partners: analyses of data from a randomised trial in Rakai, Uganda. The Lancet infectious diseases. 2011;11:604–612. doi: 10.1016/S1473-3099(11)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. American journal of obstetrics and gynecology. 2009;200:42 e1–42 e7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, et al. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. mBio. 2013;4 doi: 10.1128/mBio.00076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta SD, Green SJ, Maclean I, Hu H, Bailey RC, et al. Microbial diversity of genital ulcer disease in men enrolled in a randomized trial of male circumcision in Kisumu, Kenya. PloS one. 2012;7:e38991. doi: 10.1371/journal.pone.0038991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobian AA, Kigozi G, Redd AD, Serwadda D, Kong X, et al. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. J Infect Dis. 2012;205:486–490. doi: 10.1093/infdis/jir767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wawer MJ, Makumbi F, Kigozi G, Serwadda D, Watya S, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374:229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. Aids. 2000;14:2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 44.Baeten JM, Donnell D, Kapiga SH, Ronald A, John-Stewart G, et al. Male circumcision and risk of male-to-female HIV-1 transmission: a multinational prospective study in African HIV-1-serodiscordant couples. AIDS. 2010;24:737–744. doi: 10.1097/QAD.0b013e32833616e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallett TB, Alsallaq RA, Baeten JM, Weiss H, Celum C, et al. Will circumcision provide even more protection from HIV to women and men? New estimates of the population impact of circumcision interventions. Sexually transmitted infections. 2011;87:88–93. doi: 10.1136/sti.2010.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warner L, Ghanem KG, Newman DR, Macaluso M, Sullivan PS, Erbelding EJ. Male Circumcision and Risk of HIV Infection among Heterosexual African American Men Attending Baltimore Sexually Transmitted Disease Clinics. The Journal of infectious diseases. 2009;199:59–65. doi: 10.1086/595569. [DOI] [PubMed] [Google Scholar]
- 47.Nielson CM, Schiaffino MK, Dunne EF, Salemi JL, Giuliano AR. Associations between Male Anogenital Human Papillomavirus Infection and Circumcision by Anatomic Site Sampled and Lifetime Number of Female Sex Partners. The Journal of infectious diseases. 2009;199:7–13. doi: 10.1086/595567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu B, Wu Y, Nielson CM, Flores R, Abrahamsen M, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. The Journal of infectious diseases. 2009;199:362–371. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 49.Buchbinder SP, Vittinghoff E, Heagerty PJ, Celum CL, Seage GR, 3rd, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. Journal of acquired immune deficiency syndromes. 2005;39:82–89. doi: 10.1097/01.qai.0000134740.41585.f4. [DOI] [PubMed] [Google Scholar]
- 50.Kreiss JK, Hopkins SG. The association between circumcision status and human immunodeficiency virus infection among homosexual men. The Journal of infectious diseases. 1993;168:1404–1408. doi: 10.1093/infdis/168.6.1404. [DOI] [PubMed] [Google Scholar]
- 51.El Bcheraoui C, Greenspan J, Kretsinger K, Chen R. Rates of selected neonatal male circumcision-associated severe adverse events in the United States, 2007–2009; XVIII International AIDS Conference; 2010. Abstract THAC0104. [Google Scholar]
- 52.Millett GA, Ding H, Lauby J, Flores S, Stueve A, et al. Circumcision status and HIV infection among Black and Latino men who have sex with men in 3 US cities. Journal of acquired immune deficiency syndromes. 2007;46:643–650. doi: 10.1097/QAI.0b013e31815b834d. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez J, Sal YRVG, Hughes JP, Baeten JM, Fuchs J, et al. Male circumcision and risk of HIV acquisition among men who have sex with men. AIDS. 2010 [Google Scholar]
- 54.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. JAMA : the journal of the American Medical Association. 2008;300:1674–1684. doi: 10.1001/jama.300.14.1674. [DOI] [PubMed] [Google Scholar]
- 55.Lane T, Raymond HF, Dladla S, Rasethe J, Struthers H, et al. Lower risk of HIV infection among circumcised MSM: results from the Soweto Men's Study. 5th International AIDS Society Conference; Cape Town, South Africa. 2009. [Google Scholar]
- 56.Gray RH, Serwadda D, Tobian AA, Chen MZ, Makumbi F, et al. Effects of genital ulcer disease and herpes simplex virus type 2 on the efficacy of male circumcision for HIV prevention: Analyses from the Rakai trials. PLoS Med. 2009;6:e1000187. doi: 10.1371/journal.pmed.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kigozi G, Wawer M, Ssettuba A, Kagaayi J, Nalugoda F, et al. Foreskin surface area and HIV acquisition in Rakai, Uganda (size matters) AIDS. 2009;23:2209–2213. doi: 10.1097/QAD.0b013e328330eda8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabo R, Short RV. How does male circumcision protect against HIV infection? Bmj. 2000;320:1592–1594. doi: 10.1136/bmj.320.7249.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. Aids. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 60.Gray RH, Bailey RC, Morris BJ. Keratinization of the adult male foreskin and implications for male circumcision. AIDS. 2010;24:1381. doi: 10.1097/QAD.0b013e3283392555. author reply -2. [DOI] [PubMed] [Google Scholar]
- 61.Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinh MH, McRaven MD, Kelley Z, Penugonda S, Hope TJ. Keratinization of the adult male foreskin and implications for male circumcision. AIDS. 2010;24:899–906. doi: 10.1097/QAD.0b013e3283367779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dinh MH, Hirbod T, Kigozi G, Okocha EA, Cianci GC, et al. No difference in keratin thickness between inner and outer foreskins from elective male circumcisions in Rakai, Uganda. PLoS One. 2012;7:e41271. doi: 10.1371/journal.pone.0041271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson KE, Redd AD, Quinn TC, Collinson-Streng AN, Cornish T, et al. Effects of HIV-1 and HSV-2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. The Journal of infectious diseases. 2011;203:602–609. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson KE, Sherman ME, Ssempiija V, Tobian AA, Zenilman JM, et al. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. Aids. 2009;23:1807–1815. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dinh M, Barry S, Anderson M, Polniak M, McCoombe SG, et al. HIV-1 interactions and infection in adult male foreskin explant cultures; Sixteenth Conference on Retroviruses and Opportunistic Infections; 2009. Abstract 502. [Google Scholar]
- 67.Fischetti L, Barry SM, Hope TJ, Shattock RJ. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. Aids. 2009;23:319–328. doi: 10.1097/QAD.0b013e328321b778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.(UNAIDS) Scaling-up male circumcision programmes in the Eastern and Southern Africa Region: Country update meeting to share lessons, explore opportunities and overcome challenges to scale-up. Proc. Joint United Nations Programme on HIV/AIDS., Arusha, Tanzania. 2010 [Google Scholar]
- 69.(UNAIDS) Joint Strategic Action Framework to Accelerate the Scale-Up of Voluntary Medical Male Circumcision for HIV Prevention in Eastern and Southern Africa: 2012–2016. Geneva: UNAIDS: Joint United Nations Programme on HIV/AIDS; 2011. [Google Scholar]
- 70.UNAIDS/WHO/SACEMA Expert Group on Modelling the Impact and Cost of Male Circumcision for HIV Prevention. Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS medicine. 2009;6:e1000109. doi: 10.1371/journal.pmed.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Njeuhmeli E, Forsythe S, Reed J, Opuni M, Bollinger L, et al. Voluntary medical male circumcision: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PLoS medicine. 2011;8:e1001132. doi: 10.1371/journal.pmed.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uthman OA, Popoola TA, Uthman MM, Aremu O. Economic evaluations of adult male circumcision for prevention of heterosexual acquisition of HIV in men in sub-Saharan Africa: a systematic review. PloS one. 2010;5:e9628. doi: 10.1371/journal.pone.0009628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kacker S, Frick KD, Quinn TC, Gray RH, Tobian A. Financial Implications of Male Circumcision Scale-Up for the Prevention of HIV and Other Sexually Transmitted Infections in a Sub-Saharan African Community. Sexually transmitted diseases. 2013 doi: 10.1097/OLQ.0b013e3182945e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sansom SL, Prabhu VS, Hutchinson AB, An Q, Hall HI, et al. Cost-effectiveness of newborn circumcision in reducing lifetime HIV risk among U.S. males. PloS one. 2010;5:e8723. doi: 10.1371/journal.pone.0008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kacker S, Frick KD, Gaydos CA, Tobian AA. Costs and effectiveness of neonatal male circumcision. Archives of pediatrics & adolescent medicine. 2012;166:910–918. doi: 10.1001/archpediatrics.2012.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoen EJ, Colby CJ, Ray GT. Newborn circumcision decreases incidence and costs of urinary tract infections during the first year of life. Pediatrics. 2000;105:789–793. doi: 10.1542/peds.105.4.789. [DOI] [PubMed] [Google Scholar]
- 77.Dickson KE, Tran NT, Samuelson JL, Njeuhmeli E, Cherutich P, et al. Voluntary medical male circumcision: a framework analysis of policy and program implementation in eastern and southern Africa. PLoS medicine. 2011;8:e1001133. doi: 10.1371/journal.pmed.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Auvert B, Marseille E, Korenromp EL, Lloyd-Smith J, Sitta R, et al. Estimating the resources needed and savings anticipated from roll-out of adult male circumcision in Sub-Saharan Africa. PloS one. 2008;3:e2679. doi: 10.1371/journal.pone.0002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curran K, Njeuhmeli E, Mirelman A, Dickson K, Adamu T, et al. Voluntary medical male circumcision: strategies for meeting the human resource needs of scale-up in southern and eastern Africa. PLoS medicine. 2011;8:e1001129. doi: 10.1371/journal.pmed.1001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organization. Use of Devices for Adult Male Circumcision in Public Health HIV Prevention Programmes. Conclusions of the WHO Technical Advisory Group on Innovations in Male Circumcision. 2012 Feb; http://whqlibdoc.who.int/hq/2012/WHO_HIV_2012.4_eng.pdf.
- 81.Bitega JP, Ngeruka ML, Hategekimana T, Asiimwe A, Binagwaho A. Safety and efficacy of the PrePex device for rapid scale-up of male circumcision for HIV prevention in resource-limited settings. Journal of acquired immune deficiency syndromes. 2011;58:e127–e134. doi: 10.1097/QAI.0b013e3182354e65. [DOI] [PubMed] [Google Scholar]
- 82.Barone MA, Ndede F, Li PS, Masson P, Awori Q, et al. The Shang Ring device for adult male circumcision: a proof of concept study in Kenya. Journal of acquired immune deficiency syndromes. 2011;57:e7–e12. doi: 10.1097/QAI.0b013e3182158967. [DOI] [PubMed] [Google Scholar]
- 83.Westercamp N, Bailey RC. Acceptability of male circumcision for prevention of HIV/AIDS in sub-Saharan Africa: a review. AIDS and behavior. 2007;11:341–355. doi: 10.1007/s10461-006-9169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong X, Kigozi G, Nalugoda F, Musoke R, Kagaayi J, et al. Assessment of changes in risk behaviors during 3 years of posttrial follow-up of male circumcision trial participants uncircumcised at trial closure in Rakai, Uganda. American journal of epidemiology. 2012;176:875–885. doi: 10.1093/aje/kws179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chinkhumba J, Godlonton S, Thornton R. Demand for Medical Male Circumcision. Working Paper. 2012 http://ipl.econ.duke.edu/bread/papers/working/335.pdf. [Google Scholar]
- 86.Wise J. Demand for male circumcision rises in a bid to prevent HIV. Bulletin of the World Health Organization. 2006;84:509–511. [PMC free article] [PubMed] [Google Scholar]
- 87.Tobian AA, Gray RH. The medical benefits of male circumcision. JAMA : the journal of the American Medical Association. 2011;306:1479–1480. doi: 10.1001/jama.2011.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark SJ, Kilmarx PH, Kretsinger K. Coverage of newborn and adult male circumcision varies among public and private US payers despite health benefits. Health affairs. 2011;30:2355–2361. doi: 10.1377/hlthaff.2011.0776. [DOI] [PubMed] [Google Scholar]
- 89.Xu F, Markowitz LE, Sternberg MR, Aral SO. Prevalence of circumcision and herpes simplex virus type 2 infection in men in the United States: the National Health and Nutrition Examination Survey (NHANES), 1999–2004. Sexually transmitted diseases. 2007;34:479–484. doi: 10.1097/01.olq.0000253335.41841.04. [DOI] [PubMed] [Google Scholar]
- 90.Centers for Disease C, Prevention. Trends in in-hospital newborn male circumcision--United States, 1999–2010. MMWR. Morbidity and mortality weekly report. 2011;60:1167–1168. [PubMed] [Google Scholar]
- 91.Leibowitz AA, Desmond K, Belin T. Determinants and policy implications of male circumcision in the United States. American journal of public health. 2009;99:138–145. doi: 10.2105/AJPH.2008.134403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leibowitz AA, Desmond K. Infant male circumcision and future health disparities. Archives of pediatrics & adolescent medicine. 2012;166:962–963. doi: 10.1001/archpediatrics.2012.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]