Abstract
Purpose
The optimal treatment strategy for ductal carcinoma in situ (DCIS) continues to evolve and should consider the consequences of initial treatment on the likelihood, type, and treatment of recurrences.
Methods
We conducted a retrospective cohort study using two data sources of patients who experienced a recurrence (DCIS or invasive cancer) following breast-conserving surgery (BCS) for index DCIS: patients with an index DCIS diagnosed from 1997 to 2008 at the academic institutions of the National Comprehensive Cancer Network (NCCN; N = 88) and patients with an index DCIS diagnosed from 1990 to 2001 at community-based integrated healthcare delivery sites of the Health Maintenance Organization Cancer Research Network (CRN) (N = 182).
Results
Just under half of local recurrences in both cohorts were invasive cancer. While 40 % of patients in both cohorts underwent mastectomy alone at recurrence, treatment of the remaining patients varied. In the earlier CRN cohort, most other patients underwent repeat BCS (39 %) with only 18 % receiving mastectomy with reconstruction, whereas only 16 % had repeat BCS and 44 % had mastectomy with reconstruction in the NCCN cohort. Compared with patients not treated with radiation, those who received radiation for index DCIS were less likely to undergo repeat BCS (NCCN: 6.6 vs. 37 %, p = 0.001; CRN: 20 vs. 48 %, p = 0.0004) and more likely to experience surgical complications after treatment of recurrence (NCCN: 15 vs. 4 %, p = 0.17; CRN: 40 vs. 25 %, p = 0.09).
Conclusion
We found that treatment of recurrences after BCS and subsequent complications may be affected by the use of radiotherapy for the index DCIS. Initial treatment of DCIS may have long-term implications that should be considered.
With increasing adoption of screening mammography, the incidence of ductal carcinoma in situ (DCIS) has risen dramatically over the past 25 years.1 Currently, DCIS represents half of the malignancies diagnosed by mammographically-directed biopsies2–5 and over one-quarter of new breast cancer diagnoses in the US.1,6 Despite the large number of women affected by this disease, the optimal treatment approach remains controversial and continues to evolve.
Given that DCIS does not metastasize, management focuses on local control to prevent progression to invasive carcinoma. Four randomized clinical trials have assessed the benefit of radiation therapy (XRT) after breast-conserving surgery (BCS) in patients with DCIS.7–10 All have demonstrated that XRT reduces the risk of a second ipsilateral event by 50–60 %. While these local recurrences are equally likely to be DCIS or invasive cancer with its inherent risk of regional and/or distant metastases, no trial demonstrated a difference in survival.
Despite these trials, uncertainty about the best approach persists in practice. Population-based analyses show that approximately half of the patients with DCIS receive XRT following breast conservation while half do not, although use of radiotherapy has increased substantially over time.11–13 In addition, there is significant geographic variation in the utilization of XRT following BCS for DCIS.11 Taken together, these observations imply a lack of consensus on the role of radiation in this setting, perhaps due to differences in interpretation of how the only two outcomes currently reported in the clinical trials data, namely recurrence and survival, should impact practice.
While it is not often discussed as such, the treatment of DCIS is preventive.29 The goal of treatment is to avoid progression to an invasive cancer with its associated risk of metastases. A decrease in local recurrence is a clear advantage of adding radiation to the treatment regimen for index DCIS, but there are also disadvantages to this approach. The desire to avoid radiation is the motivation behind ongoing work to risk-stratify patients in order to make more informed decisions regarding the use of radiation in this setting.14 Standard external beam radiation usually requires 6 weeks of daily treatments that can be logistically difficult for many patients. While newer approaches such as hypofractionation, partial breast irradiation, and brachytherapy attempt to overcome this limitation, they are not yet standard of care in the treatment of DCIS.15–20 Furthermore, XRT may impede wound healing and increase complication rates, as well as complicate decision making should a patient experience a local recurrence.21–23
Although reports utilizing adjuvant brachytherapy for recurrence are increasing, most practitioners will not offer repeat BCS in the setting of prior radiation (especially for an invasive recurrence) as it is not usually given to the same breast more than once due to the limits of normal tissue tolerance.24–26 Should a mastectomy be required, reconstruction is more challenging, particularly implant or tissue expander-based reconstruction, given scarring and the decreased pliability of the chest wall after radiation.21,27
Despite the likely long-term impact on patient-reported outcomes, there are currently few data to help patients and providers account for these consequences of treatment decisions for index DCIS. We sought to help address this evidence gap by characterizing the type and treatment of local recurrence after DCIS as a function of initial treatment decisions.
METHODS
Study Design
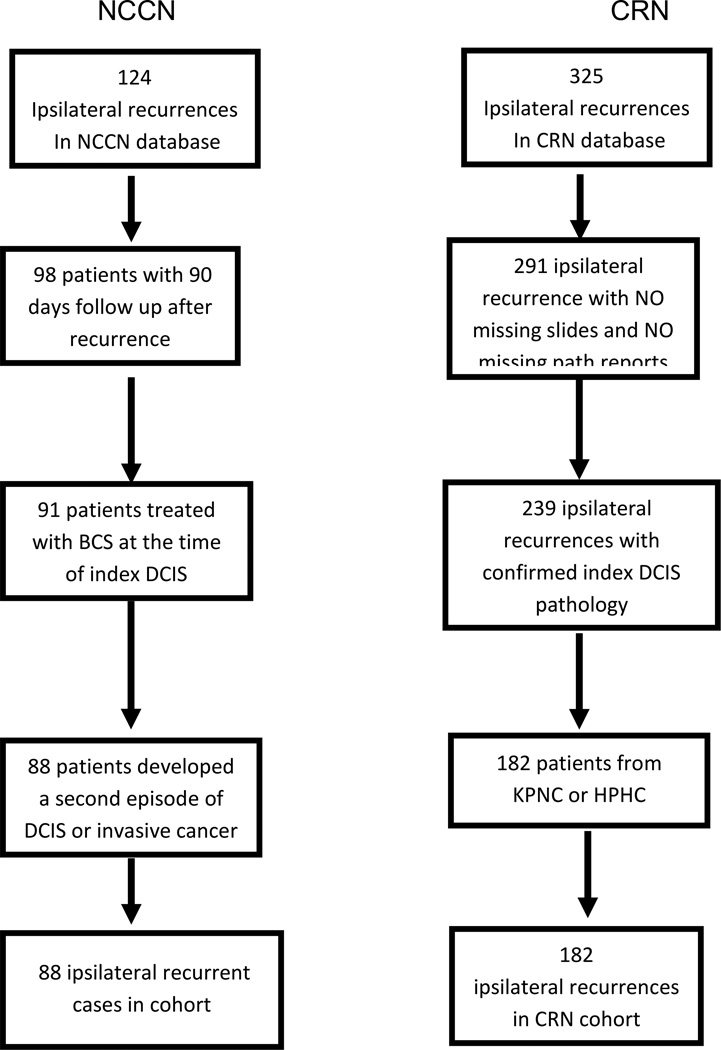
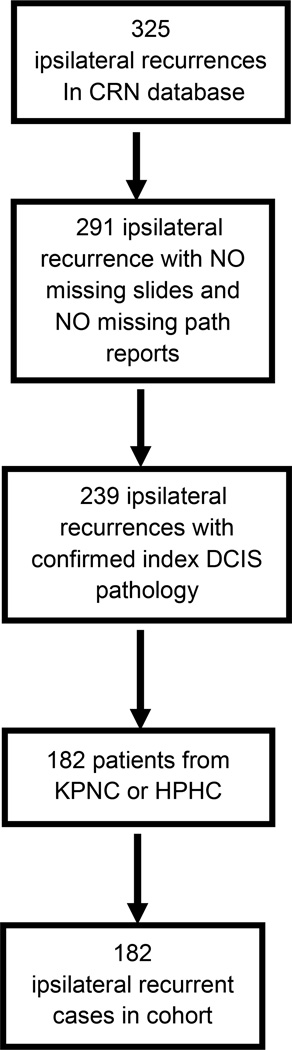
This is a retrospective cohort study of patients using two existing data sources: the National Comprehensive Cancer Network (NCCN) Oncology Outcomes Database and the Cancer Research Network (CRN) DCIS study.13 Figures 1 and 2 provide an overview of the identification of the two analytic cohorts.
FIG. 1.
Development of the analytic cohort from the NCCN data sources. NCCN National Comprehensive Cancer Network, BCS breast-conserving surgery, DCIS ductal carcinoma in situ
FIG. 2.
Development of the analytic cohorts from the CRN DCIS Study. CRN Cancer Research Network, DCIS ductal carcinoma in situ, KPNC Kaiser Permanente Northern California, HPHC Harvard Pilgrim Health Care
National Comprehensive Cancer Network (NCCN)
Since 1997, the NCCN Oncology Outcomes Database has prospectively collected patient and tumor characteristics, treatments, and outcomes for women with newly diagnosed breast cancer (including DCIS) receiving surgery or systemic therapy for the incident cancer at participating NCCN member institutions. Data from the medical records are collected prospectively at baseline, 4, 9, and 18 months following the first visit date at the NCCN and annually thereafter by trained abstractors at each institution. Rigorous data quality-assurance processes were in place for the study, including initial and follow-up data management training; online edit checking during web-based data entry; programmed logic checks against the pooled data repository; routine quality assurance reports to the centers for rectification by the data managers; and onsite audits of a random sample of source documents against the submitted data within the first few months of data collection, and annually thereafter. The patient is considered lost to follow-up and only reviewed for survival if they have not had a visit at the NCCN institution for more than 2 consecutive years.
Nine academic comprehensive cancer centers contributed data to this analysis: City of Hope National Medical Center, Duarte, CA; Dana-Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; H. Lee Moffitt Cancer Center and Research Institute at the University of South Florida, Tampa, FL; Massachusetts General Hospital, Boston, MA; The Ohio State University Comprehensive Cancer Center—James Cancer Hospital and Solove Research Institute, Columbus, OH; Roswell Park Cancer Institute, Buffalo, NY; The University of Texas MD Anderson Cancer Center, Houston, TX; and University of Michigan Comprehensive Cancer Center, Ann Arbor, MI. The Institutional Review Board (IRB) at each center has approved the parent study, data collection process, data transmission methods, and data storage protocols. This particular analysis was granted an exemption by the Dana Farber-Partners Cancer Center Office for Human Research Studies.
During the study period, 4,577 index DCIS patients were identified. Among these, 2,939 patients underwent BCS, 1,638 were treated with mastectomy. Of those treated with BCS, 81 % had adjuvant XRT. Patients included in this analysis had an initial index diagnosis of DCIS treated with BCS at one of the NCCN institutions between 1 July 1997 and 31 December 2008 and were diagnosed with an ipsilateral recurrence as their first recurrent site at least 90 days after presentation (N = 88). Demographic, clinical, and treatment data were collected at the time of the index diagnosis. At the time of recurrence, we obtained disease characteristics as well as information on definitive surgical treatment (BCS, mastectomy, and mastectomy with reconstruction). If patients had multiple documented operations in the NCCN Outcomes Database (e.g. BCS and subsequent mastectomy), the patient’s medical record was reviewed to determine the first attempt at definitive treatment. For patients who received mastectomy as their definitive surgery, the receipt of breast reconstruction was determined by identifying a procedure date for a breast reconstruction surgery code that occurred within 365 days of the procedure code for mastectomy and prior to the date of an additional recurrence. Major complications that required a repeat procedure, such as incision and drainage, wound evacuation, and flap debridement, were collected if noted in the medical record. Minor complications such as seromas or wound infections treated with antibiotics were not recorded.
The Health Maintenance Organization (HMO) Cancer Research Network
The CRN is a consortium of 14 community-based integrated healthcare delivery systems with more than 12 million enrollees. The CRN DCIS study included 2,995 patients diagnosed between 1990 and 2001 with a first primary unilateral DCIS treated with BCS at Kaiser Permanente Northern California (KPNC), Kaiser Permanente Southern California (KPSC), and Harvard Pilgrim Health Care (HPHC).13 Patients were eligible if they were <85 years of age at diagnosis. Exclusion criteria included mastectomy within 6 months of diagnosis or care for the index DCIS from medical providers outside the three health plans (<3 %).
A standardized medical record review by trained abstractors was conducted to identify recurrences and to confirm diagnosis and treatment information obtained from the cancer registry (KPNC and KPSC) or the electronic medical record (HPHC). Information was also abstracted on age at diagnosis, race, family history of breast cancer, body mass index, method of DCIS detection, and presence of re-excision or other breast surgery.
Follow-up for recurrences began 6 months after diagnosis of DCIS and ended at the time of recurrence, prophylactic mastectomy of the ipsilateral breast (i.e. not for recurrence), diagnosis of contralateral breast cancer, diagnosis of other non-breast invasive cancer, death, or last chart note.
For the current study, a secondary chart review by trained abstractors was performed at KPNC and HPHC on patients who had recurrences (n = 182). Information was abstracted on the pathology and treatment of the recurrence, as well as both major and minor postoperative complications. Additionally, for quality assurance a blinded re-review was conducted on 10 % of the 182 patient records which resulted in close to 100 % agreement for all additional variables. In particular, for type and timing of reconstruction, there was 100 % agreement.
Data Analysis
We examined the patient, tumor, and treatment characteristics at index DCIS for each of the cohorts. We then determined the proportion of patients who received mastectomy alone, BCS, or mastectomy with reconstructions at the time of recurrence. We also identified the timing and type of reconstructive procedure in the subset of patients undergoing reconstruction within each cohort. Specifically, we report the proportion undergoing immediate versus delayed reconstruction and the proportion receiving tissue expander or implant reconstruction versus autologous tissue flap reconstruction. For this analysis, reconstructions that included both an implant and an autologous tissue flap (e.g. latissimus dorsi rotational flap) were considered as autologous tissue. We also evaluated major surgical complications, including incision and drainage, wound evacuation, flap debridement, or other complications related to surgery.
We then compared choice of surgical procedure, type and timing of reconstruction, and rate of surgical complications for patients who received adjuvant radiation treatment at the time of their index DCIS with those who underwent BCS alone. The NCCN and CRN data are each evaluated separately given the distinct characteristics of their populations and the different time periods represented. Statistical testing was performed using Fisher’s exact test, Chi square, or t test, as appropriate. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Characteristics and Treatment of Ipsilateral Recurrence Following Ductal Carcinoma In Situ (DCIS)
Table 1 describes the two cohorts included in this analysis, including variables related to sociodemographics, treatment of index DCIS, and tumor characteristics of the ipsilateral recurrence. In each cohort, just under half of the recurrences were invasive cancer, with 8.5 % experiencing a node-positive recurrence. Approximately 40 % of patients in each cohort underwent a mastectomy alone at the time of their recurrence; however, there were significant differences in the treatment of the remaining patients (Table 1). Of the remaining patients in the CRN cohort, 71 underwent repeat BCS (39 %), while 32 (18 %) received mastectomy with reconstruction. These numbers were reversed in the NCCN cohort, with 14 (16 %) of patients receiving BCS and 39 (44 %) having a mastectomy with reconstruction performed. In both cohorts, patients who received repeat BCS were significantly older than patients who received mastectomy alone, who were in turn older than patients who received mastectomy with reconstruction (mean age: NCCN 60.6 vs. 56.2 vs. 48.8 years, p = 0.0004; CRN 59.3 vs. 56.2 vs. 47.8 years, p < 0.0001).
TABLE 1.
Patient characteristics and treatment of recurrences after BCS in two cohorts of women with DCIS
| NCCN (N = 88) |
CRN (N = 182) |
|
|---|---|---|
| Demographics | ||
| Patient age [years; mean (SD)] | 53.6 (11.3) | 55.9 (11.6) |
| BMI [mean (SD)] | 27.2 (6.4) | 26.3 (4.8) |
| Race | ||
| Caucasian | 71 (80.7 %) | 131 (72.0) |
| Black | 7 (8.0 %) | 17 (9.3 %) |
| Other | 10 (11.4 %) | 34 (18.7 %) |
| Index DCIS | ||
| Received radiation | 61 (69.3 %) | 60 (33.0 %) |
| Time to recurrence [mean no. of months (SD)] | 39.7 (26.2) | 45.4 (32.0) |
| In-breast recurrence | ||
| DCIS only | 52 (59.1 %) | 97 (53.3 %) |
| Invasive component | 36 (40.9 %) | 85 (46.7 %) |
| Surgical treatment | ||
| BCS | 14 (15.9 %) | 71 (39.0 %) |
| Mastectomy alone | 35 (39.8 %) | 79 (43.4 %) |
| Mastectomy + reconstruction | 39 (44.3 %) | 32 (17.6 %) |
| Timing of reconstruction | ||
| Immediate | 36 (92.3 %) | 23 (71.9 %) |
| Delayed | 3 (7.7 %) | 9 (28.1 %) |
| Type of reconstruction | ||
| Autologous tissue | 26 (66.7 %) | 5 (15.6 %) |
| Implant/expander | 13 (33.3 %) | 27 (84.4 %) |
| Received radiation | ||
| BCS | 7 (70.0 %) | 19 (73.1 %) |
| Mastectomy only | 3 (30.0 %) | 6 (23.1 %) |
| Mastectomy + reconstruction | 0 | 1 (3.8 %) |
| Received chemotherapy | 11 (12.5 %) | 20 (11.0 %) |
| Surgical complicationsb | 10 (11.3 %) | 54 (29.7 %) |
Recurrence including both DCIS and invasive cancer
The first cohort includes those patients who were diagnosed with an ipsilateral recurrence of DCIS or invasive cancer following an index case of DCIS in NCCN between 1997 and 2008. The other includes patients with an ipsilateral recurrence following treatment for index DCIS at a CRN institution between 1990 and 2001
BCS breast-conserving surgery, DCIS ductal carcinoma in situ, NCCN National Comprehensive Cancer Network, CRN Cancer Research Network, BMI body mass index
NCCN data collection was limited to major complications that were documented in medical records, while the CRN data includes both major and minor complications
The Impact of Radiation on Local Treatment of Ipsilateral Recurrence Following DCIS
Among patients who recurred in the NCCN cohort, 69 % of patients had received adjuvant radiation at the time of their index DCIS compared with 33 % in the CRN cohort. There was no statistically significant difference in age, body mass index, or race for patients who received radiation following BCS for their index DCIS compared with those who did not (Table 2). There was also no statistically significant difference in time to recurrence. Patients who received radiation were significantly less likely to undergo repeat BCS than those who did not receive radiation (NCCN: 6.6 vs. 37 %, p = 0.001; CRN: 20 vs. 48 %, p = 0.0004). Among patients who did not receive radiation for the index DCIS and received reconstruction for the recurrence, approximately 85 % in both cohorts received reconstruction with implants or tissue expanders. As for patients who received radiation for the index DCIS and received reconstruction for the recurrence, only 7 of 32 (22 %) patients in the NCCN cohort received implants or tissue expanders, while the likelihood remained 8 of 10 (80 %) in the CRN cohort. Major complications following an operation for recurrence occurred in 9 of 61 (15 %) patients who had received radiation for their index DCIS compared with 1 of 27 (4 %) of those who had not at NCCN institutions, but this observed difference did not reach statistical significance (p = 0.17). Within the CRN cohort, surgical complications were also observed more commonly in patients who had received radiation at the time of their index treatment (24 of 60 (40 %) vs. 30 of 122 (25 %), p = 0.09).
TABLE 2.
Relationship of radiation treatment of index DCIS and treatment of ipsilateral recurrence
| NCCN | CRN | |||||
|---|---|---|---|---|---|---|
| BCS + XRT (N = 61) |
BCS alone (N = 27) |
p value | BCS + XRT (N = 60) |
BCS alone (N = 122) |
p value | |
| Demographics | ||||||
| Age [years; mean (SD)] | 53.5 (10.5) | 54.1 (13.1) | 0.81 | 55.1 (8.9) | 56.3 (12.7) | 0.54 |
| BMI [mean (SD)] | 27.8 (6.2) | 26.1 (6.9) | 0.27 | 26.4 (4.7) | 26.2 (4.9) | 0.82 |
| Race | ||||||
| Caucasian | 48 (78.7 %) | 23 (85.2 %) | 0.27 | 43 (71.7 %) | 88 (72.1 %) | 0.91 |
| Black | 5 (8.2 %) | 2 (7.4 %) | 5 (8.3 %) | 12 (9.8 %) | ||
| Other | 8 (13.1 %) | 2 (7.4 %) | 12 (20.0 %) | 22 (18.0 %) | ||
| In-breast recurrence | ||||||
| Time from initial diagnosis to recurrence | 41.1 (27.4) | 36.9 (23.5) | 0.49 | 48.4 (31.7) | 43.9 (32.1) | 0.37 |
| DCIS only | 39 (63.9 %) | 13 (48.2 %) | 0.24 | 34 (56.7 %) | 63 (51.6 %) | 0.52 |
| Invasive component | 22 (36.1 %) | 14 (51.9 %) | 26 (43.3 %) | 59 (48.4 %) | ||
| Surgical treatment | ||||||
| Repeat BCS | 4 (6.6 %) | 10 (37.0 %) | 0.0009 | 12 (20.0 %) | 59 (48.4 %) | 0.0003 |
| Mastectomy alone | 25 (41.0 %) | 10 (37.0 %) | 38 (63.3 %) | 41 (33.6 %) | ||
| Mastectomy + reconstruction | 32 (52.5 %) | 7 (25.9 %) | 10 (16.7 %) | 22 (18.0 %) | ||
| Timing of reconstruct | ||||||
| Immediate | 30 (93.8 %) | 6 (85.7 %) | 0.46 | 9 (90.0 %) | 14 (63.6 %) | 0.21 |
| Delayed | 2 (6.3 %) | 1 (14.3 %) | 1 (10 %) | 8 (36.4 %) | ||
| Type of reconstruction | ||||||
| Autologous tissue | 25 (78.1 %) | 1 (14.3 %) | 0.003 | 2 (20.0 %) | 3 (13.6 %) | 0.21 |
| Implant/expander | 7 (21.9 %) | 6 (85.7 %) | 8 (80.0 %) | 19 (86.4 %) | ||
| XRT for recurrence | ||||||
| BCS | 0 (0.0 %) | 7 (70.0 %) | a | 1 (8.3 %) | 18 (30.5 %) | a |
| Mastectomy only | 0 (0.0 %) | 3 (30.0 %) | 0 (0 %) | 6 (14.6 %) | ||
| Mastectomy + reconstruction | 0 (0.0 %) | 0 (0.0 %) | 0 (0 %) | 1 (4.5 %) | ||
| Received chemotherapy | 5 (8.2 %) | 6 (22.2 %) | 0.085 | 6 (10.0 %) | 14 (11.5 %) | 0.76 |
| Surgical complicationb | 9 (14.8 %) | 1 (3.7 %) | 0.17 | 24 (40.0 %) | 30 (24.6 %) | 0.09 |
Recurrence including both DCIS and invasive cancer
DCIS ductal carcinoma in situ, NCCN National Comprehensive Cancer Network, CRN Cancer Research Network, BCS breast-conserving surgery, XRT radiation therapy, BMI body mass index
p-Value not calculated due to cells containing a value of zero
Includes all complications in the CRN cohort, and only major complications that were evident in medical records in the NCCN cohort
DISCUSSION
The study of the longer-term impact of initial treatment options for DCIS has been limited due to the lack of an appropriate cohort with sufficient numbers of patients with local recurrence following treatment of an index DCIS. To address these inherent limitations, we studied the impact of initial treatment choice on the operative treatment decisions and postoperative complications in two multiinstitutional cohorts of patients with local recurrence following BCS for index DCIS, namely the NCCN Oncology Outcomes Database and the CRN DCIS Study.
We found that a similar number of patients in both cohorts underwent mastectomy alone at recurrence, but that treatment of the remaining patients was different. In the CRN cohort, most patients underwent repeat BCS, while few received mastectomy with reconstruction. The opposite was true in the NCCN cohort where only a minority of patients had repeat BCS and the majority had mastectomy with reconstruction. This difference reflects, at least in part, variation in the utilization of post-BCS radiation for index DCIS in the two cohorts. Results of the original CRN DCIS study, with diagnosis years from 1990 to 2001, reported that 53 % of patients treated with BCS received XRT as part of their index treatment; with XRT increasing from 26 % in the early 1990s to 61 % by the early 2000s.13 In contrast, the rate of post-BCS radiation in the NCCN cohort (diagnosis years 1997–2008) was 81 % (results not published). While this in part may reflect institutional differences, the differences likely also reflect the general increase over time in the use of XRT for patients treated with BCS. Patients in both cohorts who had been previously irradiated had lower rates of repeat BCS for recurrence, but the magnitude of the difference was greater in the NCCN due to the higher rates of initial radiation. Within the CRN, the twofold decrease in the use of repeat BCS corresponded to a twofold increase in the use of mastectomy alone, suggesting that patients who would have received BCS had they not been irradiated, received mastectomy alone in the setting of prior radiation. In contrast, the likelihood of mastectomy alone was similar regardless of previous irradiation within the NCCN despite the large decrease in the use of BCS, suggesting these patients went on to receive mastectomy with reconstruction.
Previous irradiation was associated with a twofold increase in the likelihood of mastectomy with reconstruction in the NCCN cohort, while there was no difference in the likelihood of mastectomy with reconstruction based on radiation in the CRN cohort. Our results suggest that patients who have received prior radiation may be candidates for reconstruction at the tertiary cancer centers within the NCCN, but perhaps less likely in other settings. This may relate to the increased use of autologous tissue flaps at NCCN centers. In the CRN cohort, 80 % of all patients, regardless of receipt of previous radiation, had TE or implants. While a similar likelihood was seen for NCCN patients who had not received radiation, in the setting of radiation 80 % received tissue flap reconstruction. This suggests that NCCN centers may offer autologous flaps to patients who are not candidates for TE/implants due to prior irradiation, while CRN sites may choose not to offer any type of reconstruction. However, caution must be used in interpreting these results as it is unclear how much of this difference reflects the difference in timing of the study periods for the two cohorts. Yang and colleagues reported a threefold increase in the use of immediate breast reconstruction between 2000 and 2009.28
These two cohorts were chosen because they offset each other’s limitations to provide a more generalizable understanding of the impact of initial treatment for DCIS at the time of recurrence. Limited follow-up within the NCCN led to small numbers of patients in the recurrent cohort. Moreover, only major complications were abstracted, leading to a possible underestimation of the true impact of radiation on postoperative complications at the time of recurrence. This explains the difference in complication rates between the cohorts. Additionally, practice at the tertiary cancer centers which comprise the NCCN cohort may not reflect general practice across the US. The use of CRN data addresses these limitations by providing a significantly larger and more diverse cohort of patients experiencing a recurrence following BCS for an index case of DCIS. Additionally, we were able to collect more detailed complication data for this cohort.
Despite this approach, our study remains limited by a small sample size prohibiting multivariable or stratified analysis and described differences in the populations and time periods preclude the use of both data sources for a composite analysis. While we were able to document significant variation in age, suggesting that operative risk and life expectancy are considered in treatment decisions following a recurrence, we do not have sufficient power to control for this in our analysis. In addition, there are a number of other factors that influence decision making for DCIS, such as extent of disease, receptor status, and histologic grade, which were not reliably available in these datasets. Future work is needed to understand the roles that these factors play in treatment decisions at the time of recurrence. In addition, the increased rate of complications observed may reflect the increased use of more extensive surgical interventions following radiation (i.e. these patients were more likely to have mastectomy or mastectomy with reconstruction with their increased risk of complications), but we were underpowered to investigate this further. Breast cancer care accounts for the largest healthcare expense in oncology, with the majority of cost associated with treatment of early-stage disease.29 Therefore, determining the optimal approach to the treatment of DCIS has important implications, not only for individual patients but also for the American healthcare system. Yet, despite its prevalence, DCIS remains a difficult disease to study. There are few data sources that reliably report recurrences, especially given the significant time delay between initial diagnosis and recurrence. Given these limitations, decision modeling had been used to project long-term outcomes in terms of breast preservation for DCIS.30,31 These models suggest a decrement in lifetime breast preservation with XRT, because although radiation decreases the likelihood of recurrence, mastectomy is the standard surgical procedure should recurrence occur.
CONCLUSIONS
Using multiple data sources, we present data regarding the impact of index treatment approaches at the time of local recurrence. Our results suggest that the use of radiation for index DCIS has a significant impact on surgical decision making and may influence the likelihood of complications at the time of a recurrence. Although the risk of local recurrence is significantly mitigated with the addition of adjuvant XRT after BCS for DCIS, the use of XRT can limit surgical options for the treatment of a local recurrence should it occur. However, our study found that 6.6 % of patients who had undergone BCS and radiation in the NCCN group and 20 %of such patients in the CRN group received repeat BCS. If repeat BCS is further studied and found to be an acceptable treatment in some patients with recurrence after radiation, the decrement in lifetime breast preservation with XRT noted by modeling analyses will be mitigated or reversed.30,31
It is not clear the extent to which patients and providers consider the long-term sequelae of these treatment options when choosing their initial treatment. However, given the lack of survival advantage with the addition of adjuvant radiation, such long-term outcomes are important considerations.
ACKNOWLEDGMENT
This work was supported by Contract No. HHSA29020050016I from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services, as part of the Developing Evidence to Inform Decisions About Effectiveness program. The views expressed in this article are those of the authors, and no official endorsement by AHRQ or the US Department of Health and Human Services is intended or should be inferred. This work was also supported by grant no. CA89393 from the National Cancer Institute to the Dana-Farber Cancer Institute, Grant No. R01 CA 81302 to Kaiser Permanente Northern California, and grant no. 2U19CA079689 to Group Health Cooperative (Laurel Habel, Project Leader).
Footnotes
CONFLICT OF INTEREST Caprice C. Greenberg, Laurel A. Habel, Melissa E. Hughes, Larissa Nekhlyudov, Ninah Achacoso, Luana Acton, Deborah Schrag, Wei Jiang, Stephen Edge, Jane C. Weeks, and Rinaa S. Punglia have no financial disclosures or conflicts of interest to disclose.
REFERENCES
- 1.Sumner WE, 3rd, Koniaris LG, Snell SE, et al. Results of 23,810 cases of ductal carcinoma-in situ. Ann Surg Oncol. 2007;14:1638–1643. doi: 10.1245/s10434-006-9316-1. [DOI] [PubMed] [Google Scholar]
- 2.Morrow M, Schmidt R, Cregger B, Hassett C, Cox S. Preoperative evaluation of abnormal mammographic findings to avoid unnecessary breast biopsies. Arch Surg. 1994;129:1091–1096. doi: 10.1001/archsurg.1994.01420340105021. [DOI] [PubMed] [Google Scholar]
- 3.Alexander HR, Candela FC, Dershaw DD, Kinne DW. Needle-localized mammographic lesions: results and evolving treatment strategy. Arch Surg. 1990;125:1441–1444. doi: 10.1001/archsurg.1990.01410230035006. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MJ, Gamagami P, Colburn WJ, et al. Nonpalpable breast lesions: diagnosis with slightly overpenetrated screen-film mammography and hook wire-directed biopsy in 1,014 cases. Radiology. 1989;171:633–638. doi: 10.1148/radiology.171.3.2717734. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm MC, Edge SB, Cole DD, de Paredes E, Frierson HF., Jr Nonpalpable invasive breast cancer. Ann Surg. 1991;213:600–603. doi: 10.1097/00000658-199106000-00010. discussion 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer facts & figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 7.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853: a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 9.Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45:536–543. doi: 10.1080/02841860600681569. [DOI] [PubMed] [Google Scholar]
- 10.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 11.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 12.Rakovitch E, Pignol JP, Chartier C, et al. The management of ductal carcinoma in situ of the breast: a screened population-based analysis. Breast Cancer Res Treat. 2007;101:335–347. doi: 10.1007/s10549-006-9302-0. [DOI] [PubMed] [Google Scholar]
- 13.Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vicini FA, Beitsch PD, Quiet CA, et al. First analysis of patient demographics, technical reproducibility, cosmesis, and early toxicity: results of the American Society of Breast Surgeons MammoSite breast brachytherapy trial. Cancer. 2005;104:1138–1148. doi: 10.1002/cncr.21289. [DOI] [PubMed] [Google Scholar]
- 15.Benitez PR, Streeter O, Vicini F, et al. Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ: a phase II clinical study. Am J Surg. 2006;192:427–433. doi: 10.1016/j.amjsurg.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Beitsch PD, Wilkinson JB, Vicini FA, et al. Tumor bed control with balloon-based accelerated partial breast irradiation: incidence of true recurrences versus elsewhere failures in the American Society of Breast Surgery MammoSite® Registry Trial. Ann Surg Oncol. 2012;19:3165–3170. doi: 10.1245/s10434-012-2489-x. [DOI] [PubMed] [Google Scholar]
- 17.Shah C, McGee M, Wilkinson JB, et al. Clinical outcomes using accelerated partial breast irradiation in patients with ductal carcinoma in situ. Clin Breast Cancer. 2012;12:259–263. doi: 10.1016/j.clbc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Park SS, Grills IS, Chen PY, et al. Accelerated partial breast irradiation for pure ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2011;81:403–408. doi: 10.1016/j.ijrobp.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Parrett BM, Schook C, Morris D. Breast reduction in the irradiated breast: evidence for the role of breast reduction at the time of lumpectomy. Breast J. 2010;16:498–502. doi: 10.1111/j.1524-4741.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 21.Khansa I, Colakoglu S, Curtis MS, et al. Postmastectomy breast reconstruction after previous lumpectomy and radiation therapy: analysis of complications and satisfaction. Ann Plast Surg. 2011;66:444–451. doi: 10.1097/SAP.0b013e3182166b81. [DOI] [PubMed] [Google Scholar]
- 22.Cordeiro PG, Snell L, Heerdt A, McCarthy C. Immediate tissue expander/implast breast reconstruction after salvage mastectomy for cancer recurrence following lumpectomy/irradiation. Plast Reconstr Surg. 2012;129:341–350. doi: 10.1097/PRS.0b013e318205f203. [DOI] [PubMed] [Google Scholar]
- 23.Chadha M, Feldman S, Boolbol S, Wang L, Harrison LB. The feasibility of a second lumpectomy and breast brachytherapy for localized cancer in a breast previously treated with lumpectomy and radiation therapy for breast cancer. Brachytherapy. 2008;7:22–28. doi: 10.1016/j.brachy.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Trombetta M, Julian T, Bhandari T, Werts ED, Miften M, Parda D. Breast conservation surgery and interstitial brachytherapy in the management of locally recurrent carcinoma of the breast: the Allegheny General Hospital experience. Brachytherapy. 2008;7:29–36. doi: 10.1016/j.brachy.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Trombetta M, Julian TB, Werts DE, et al. Long-term cosmesis after lumpectomy and brachytherapy in the management of carcinoma of the previously irradiated breast. Am J Clin Oncol. 2009;32:314–318. doi: 10.1097/COC.0b013e31818af0b9. [DOI] [PubMed] [Google Scholar]
- 26.Forman DL, Chiu J, Restifo RJ, Ward BA, Haffty B, Ariyan S. Breast reconstruction in previously irradiated patients using tissue expanders and implants: a potentially unfavorable result. Ann Plast Surg. 1998;40:360–363. doi: 10.1097/00000637-199804000-00007. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 27.Habel LA, Achacoso NS, Haque R, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast Cancer Res. 2009;11:85. doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punglia RS, Schnitt SJ, Weeks JC. Treatment of ductal carcinoma in situ after excision: would a prophylactic paradigm be more appropriate? J Natl Cancer Inst. 2013;105(20):1527–1533. doi: 10.1093/jnci/djt256. [DOI] [PubMed] [Google Scholar]
- 30.Punglia RS, Burstein HJ, Weeks JC. Radiation therapy for ductal carcinoma in situ: a decision analysis. Cancer. 2012;118(3):603–611. doi: 10.1002/cncr.26293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soeteman DI, Stout NK, Ozanne EM, Greenberg CC, Hassett MJ, Schrag D, et al. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J Natl Can Inst. 2013;105(11):774–781. doi: 10.1093/jnci/djt096. [DOI] [PMC free article] [PubMed] [Google Scholar]