Abstract
Tissue localization of immune cells is critical to the study of disease processes in mouse models of human diseases. However, immunohistochemistry (IHC) for immune cell phenotyping in mouse tissue sections presents specific technical challenges. For example, CD4 and CD8 have been difficult to detect using IHC on formalin-fixed and paraffin-embedded (FFPE) mouse tissue, prompting alternative methods. We investigated the use of formalin free zinc-salt fixation (ZN) (Beckstead, 1994) and optimized IHC protocols for detecting a panel of immune cell-related markers (CD3, CD4, CD8, Foxp3, B220, F4/80, CD68, MHC class-I, MHC class-II and Gr-1). The IHC results for these markers were compared on mouse spleen tissue treated with: neutral buffered formalin (NBF) or ZN with or Zn without antigen retrieval (AR). Whereas CD4 and CD8 were not detected in NBF treated tissue, all markers were detected in ZN treated tissue without AR. Thus, the use of ZN treatment for IHC staining can be a good tool for studying immuno-reactive lesions in tissues.
Keywords: antigen retrieval, formalin fixation, immune cells, immunohistochemistry, zinc-salt fixation
Introduction
Tissue localization of immune cells is critical to the study of disease processes in mouse models of human diseases. For example, the role of immune cells in cancer suppression and progression depends on analysis of intratumoral versus peritumoral immune cell infiltrates, localized macrophage polarization and direct tumor cell—immune cell interactions(Coussens and Pollard, 2011). Antibody reagents useful in flow cytometry and western blot analyses do not always perform well in IHC, and immune cell phenotypes are defined primarily by cluster of differentiation (CD) markers, themselves originally defined by mouse monoclonal antibodies recognizing leukocyte surface epitopes. Use of mouse monoclonal antibodies on mouse tissue for IHC is difficult due to the need for anti-mouse secondary antibody detection. Cell surface epitopes are often more difficult for IHC detection due to relatively inadequate levels of target proteins and limited epitope access in conventionally FFPE tissue sections. Whereas the distribution of immune cells in tissue has been performed by IHC, not all immune cell markers can be detected in tissue section (Cardiff et al., 2013, Whiteland et al., 1995). For example, most of studies have shown that T-cell lineage markers, CD4 and CD8, were not detectable with IHC on NBF treated tissue. However, some studies have successfully detected these markers on tissues treated with zinc fixative (Beckstead, 1994, Hicks et al., 2006, Wester et al., 2003), paraformaldehyde (Tingstedt et al., 2003) or periodate-lysine-paraformaldehyde (Whiteland et al., 1995). Detecting other markers on tissue sections treated with different fixative reagents including NBF, ZN and paraformaldehyde was also performed previously, which showed that non-NBF fixatives have advantages in IHC (Mikaelian et al., 2004). In these fixatives, ZN has been especially suggested as an alternate fixative for mouse immune cell markers that previously been unable to stain for histology sections for CD4 and CD8 (Whiteland et al., 1995). In this study, we sought a practical solution to these problems and report the results of ZN fixation and optimized protocols for IHC for a panel of immune cell markers. Our results indicate that this ZN method is useful to detect immune cell related markers including CD4 and CD8, which will support studies to decipher the differences in normal and tumor microenvironments.
Materials and methods
Preparation of tissues from mice
Spleen was isolated from FVB/NJ (JAX Labs, Bar Harbor, ME) and used as positive control for some immune cell related markers. Mice were housed in a vivarium under NIH guidelines and all animal experiments followed protocols approved by the UC Davis Institutional Animal Care and Use Committee. Animals were fed LabDiet (PicoLab #5058; St. Louis, MO), ad lib water is autoclaved deionized -water and housed in a 12hr/12hr light-dark cycle at 21°C. Pathogenic agents are routinely monitored both by histopathological- and serogenic- profile (UC Davis mouse level2 serogenic profile: MHV, Sendai, PVM, MPV, MVM, M.pul and arth, TMEV (GDVII), Reo-3, LCM, Ectro, EDIM, MAD 1 and 2, MNV). Bacterial pathogens were tested on cecum or nasopharynx. Pinworms or fur mites were also checked. No pathogens were detected during this study.
Zinc-salt fixation
Tissues were cut into 2-3 mm slices and fixed in IHC zinc fixative solution(Beckstead, 1994) (BD Biosciences) for 24 hours at room temperature (RT). After rinsing with tap water for 45 min, tissues were dehydrated at RT for 45 min each with 70% ethanol, 95% ethanol 100% ethanol and xylene, respectively. Tissues were infiltrated in paraffin at 58°C for 45 min using a Sakura Tissue-Tek®IV Embedding center (Sakura, Mars, PA). Tissue sections were prepared by cutting at 4 μm and floated out on a water bath at 43°C and collected on coated glass slides (SuperFrost/Plus; Fisher Scientific, Pittsburgh, PA). The slides were dried at RT for overnight.
Immunohistochemistry
Sections were deparaffinized in three times changes of xylene for 5 min each, followed by three times changes of 100% ethanol for 2 min each. They were rehydrated through 95% and 70% ethanol to tap water, then antigen retrieval (AR) procedure was performed with a Decloaking Chamber (Biocare Medical, Concord, CA) with citrate buffer (10mM sodium citrate, pH6) for 45min constantly heating at 125 °C at 15 p.s.i. using a digital decloaking chamber (Biocare Medical LLC, Concord, CA), if it is required (see Table 2). Tissue section was washed in EnVison™ FLEX wash buffer (Dako, Carpinteria, CA) for 2 min followed by blocking with a 10 min incubation in 10% goat serum in PBS (pH 7.4). Goat serum was used because any antibody used in this study was not raised in the species. The primary antibody (listed in Table 1) was diluted in 0.5% bovine serum albumin (BSA) in PBS (pH 7.4) and incubated with tissue sections for 1 hour at RT. The slides were then rinsed twice with EnVision™ FLEX wash buffer (Dako) for 5 min each. Immunohistochemical reaction was performed by using a VECTASTAIN Elite ABC kit (PK-6100; Vector Laboratories, Burlingame, CA) with biotinylated anti-rabbit or anti-rat IgG (BA-9400 and BA-9401 respectively; Vector Laboratories) following manufacturer's protocol. Shortly, the slides were incubated with a biotinylated anti-rabbit at 1:1000 dilution or an anti-rat IgG at 1:500 dilution in 5% goat serum in PBS (pH7.4) for 60min. Then sections were washed twice with PBS for 5 min each followed by incubating with VECTASTAIN Elite ABC reagent for 30min. After the slides were washed twice with PBS, samples were then incubated in freshly prepared 0.1% 3,3’-diaminobenzidine (DAB) solution (Dako) following the manufacturer's directions. After incubation with DAB for less than 5min to prevent background staining, the sections were rinsed in tap water for 2min. Counterstaining was performed with Mayer's hematoxylin for 30 seconds, then rinsed in tap water for 2min. The slides were dehydrated in 70% and 95% ethanol, then three times changes of 100% ethanol and xylene for 2 min each and mounted with Clear Mount (American MasterTech Scientific, Lodi, CA). If an automated procedure was desired, a Dako Autostainer Plus (Dako) was programmed and operated with the following : a set of a VECTASTAIN Elite ABC kit with biotinylated anti-rat IgG for detecting CD4 and CD8, HRP labelled polymer anti-rabbit (K4003; Dako) for other markers, respectively. Counterstaining was performed with Liquid DAB+ Substrate Chromogen System (K3468; Dako). The procedures for the Autostainer Plus were performed by applying same protocols as described in manual immunohistochemistry. Images were taken either by the Aperio Leica ScanScope XT (Leica Biosystems) or Zeiss Axioskop with Zeiss AxioCam color CCD camera (Carl Zeiss) using the x20 objective lens.
Table 2.
The summary of detecting immune cell related markers in tissues treated with NBF or ZN with or without antigen retrieval
| Markers | Antigen retrieval | result of IHC | |
|---|---|---|---|
| NBF | ZN | ||
| CD3 | - | n.d. | strong |
| + | strong | weak | |
| CD4 | - | no signal | strong |
| + | no signal | no signal | |
| CD8 | - | no signal | strong |
| + | no signal | no signal | |
| B220 | - | n.d. | strong |
| + | strong | strong | |
| Foxp3 | - | no signal | no signal |
| + | strong | strong | |
| F4/80 | - | n.d. | weak |
| + | strong | strong | |
| CD68 | - | no signal | strong |
| + | no signal | no signal | |
| MHC class-I | - | no signal | strong |
| + | no signal | no signal | |
| MHC class-II | - | no signal | strong |
| + | no signal | weak | |
| Gr-1 | - | n.d. | strong |
| + | no signal | weak | |
Table 1.
The list of antibodies used for detecting immune cell related markers.
| Antigen | Major cells expressing antigen | Antibody | clone | Source | product number |
|---|---|---|---|---|---|
| CD3 | T-cells | rabbit polyclonal | Dako | A0452 | |
| CD4 | HelperTcells (Th cells) | rat monoclonal | RM4-5 | BD Biosciences | 553043 |
| CD8 | Subsets of T-cells, Cytotoxic T cells (CTL), most thymocytes and NK cells | rat monoclonal | 53-6.7 | Biolegend | 100701 |
| Foxp3 | Regulatory T cells and effector T-cells | rat monoclonal | FJK-16S | e-Bioscience | 14-5773 |
| B220 | B-cells, subsets of T-cells, NK cells and dendritic cells | rat monoclonal | RA3-6B2 | BD Biosciences | 557390 |
| F4/80 | Histiocytes/macrophages | rat monoclonal | CI:A3-1 | AbD Serotec | MCA497 |
| CD68 | Histiocytes, myeloid cells and mast cells | rat monoclonal | FA-11 | Biolegend | 137001 |
| MHCI | Target for CD8+ CTL | rabbit monoclonal | EP1395Y | Abeam | ab52922 |
| MHCII | Target for CD4+Th cells | rat monoclonal | M5/114 | Abeam | abl39365 |
| Gr1 | Granulocytes, Neutrophils and Monocytes | rat monoclonal | RB6-8C5 | Biolegend | 108401 |
Note. Most of ‘Major cells expressing antigen’ is adapted from Rehg et al. ,Toxicological Pathology (2012). For GR-1, we followed Fleming et al., The Journal of Immunology (1993) and Ribechini et al., European Journal of Immunology (2009).
Results
Comparison of immune cell related markers between NBF and ZN treated mouse spleen tissues
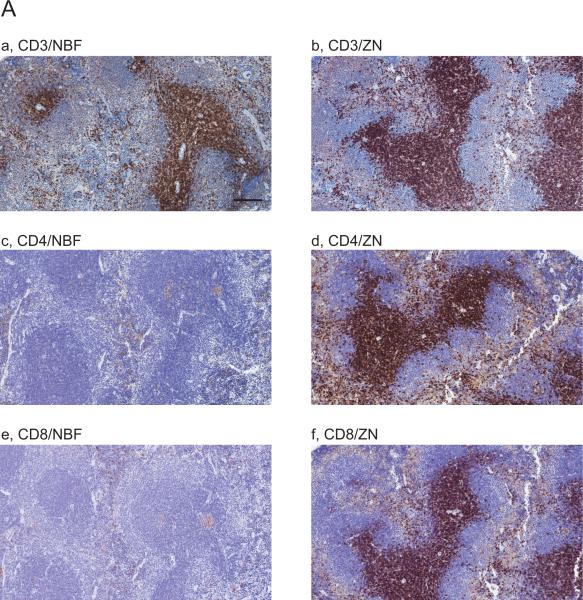
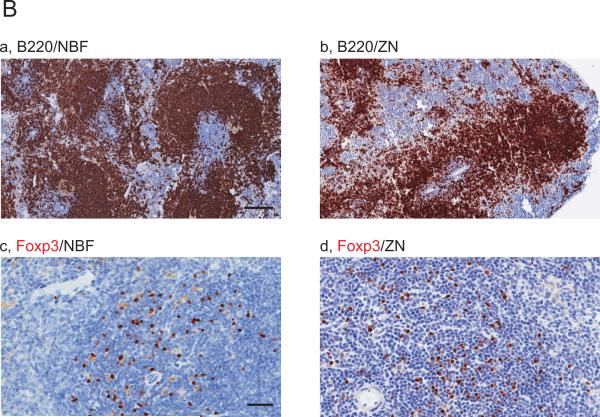
We performed IHC on mouse spleen tissues treated with NBF and ZN to detect CD3, CD4, CD8, B220 and Foxp3 (a list for all antibodies tested in this work is indicated in Table 1). Whereas positive staining of CD4 and CD8 in periarteriolar lymphoid sheaths (PALS) were not seen in NBF treated tissue sections as expected (Fig.1Ac,e), CD3 (Fig 1Aa) and Foxp3 (Fig.1Bc) in PALS and B220 in lymphoid follicles (Fig.1Ba) were successfully detected. On the other hand, IHC staining for these markers on ZN treated spleen sections were all positive (Fig.1Ab,d,f and Bb,d). These results indicate that ZN fixation on tissue samples is useful to detect lymphocytic lineage markers.
Figure 1. Comparison of immune cell related markers between NBF and ZN treated mouse spleen tissues.
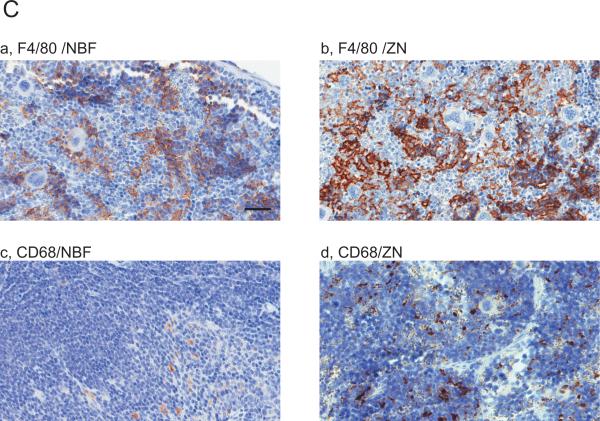
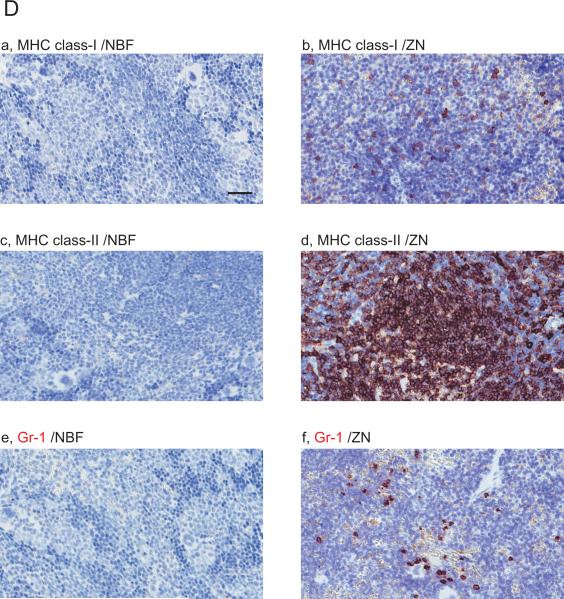
Images are showing IHC with 10 immune cell related markers on NBF or ZN treated murine spleen tissue sections. (A and B) IHC images are indicating the staining with antibody for (A) (a,b) CD3, (c,d) CD4, (e,f) CD8, (B) (a,b) B220 and (c,d) Foxp3, respectively. Whereas CD4 and CD8 positive cells were not detected on NBF treated spleen tissues, the IHC on ZN treated tissue sections shows staining positive cells. Scale bar indicates 200μm (a-h) and 50 μm (i-j). (C and D) IHC images are indicating the staining with antibody for (C) (a,b) F4/80, (c,d) CD68 and (D) (a,b) MHC class-I, (c,d) MHC class-II, (e,f) Gr-1, respectively. IHC with MHC class-I, -II and Gr-1 did not detect staining positive cells on NBF treated tissue sections. However, all markers were detected on ZN tissue sections. These indicate that ZN treatment on tissue is better for detecting various cell surface markers. Counterstaining is performed with hematoxylin. Whereas all NBF treated tissues were treated with antigen retrieval, most of ZN treated tissues were not treated except for Foxp3 and F4/80. Scale bar indicates 50 μm. Please also see Table 2 for the requirement of antigen retrieval.
Other immune cell related markers (F4/80, CD68, MHC class-I, MHC class-II and Gr-1) were also tested (Fig.1C and D). As far as we tested, whereas F4/80 (Fig.1Ca) and CD68 (Fig.1Cc) were positively detected in IHC on NBF treated tissue sections, the staining of these markers were much clearer on ZN treated samples (Fig.1Cb,d). Staining positive cells for MHC class-I (Fig.1Da), MHC class-II (Fig.1Dc) and Gr-1 (Fig.1De) were not detected in NBF treated tissue sections with antibody sets indicated on Table 1. In contrast, IHC for these markers on ZN treated tissue section detected marker positive cells (Fig.1Db,d,f). These results suggest that ZN treatment on tissue is also useful to study various immune cell related markers in addition to lymphocytes. We also validated the necessity of antigen retrieval (AR) procedure in IHC on both NBF- and ZN-treated tissues (Table 2). NBF treated tissue needed AR procedure to detect marker proteins. However, marker positive cells were clearly detected in most of ZN treated tissues without AR, except for F4/80 and Foxp3. We also observed that IHC signal on ZN treated tissue with AR were weaker than the condition without AR (Table 2; data not shown).
Discussion
The study of a variety of immune cells within normal and tumor tissues from patients or animal models can elucidate the involvement of immune cells in tumor progression or regression. However, limitations using current formalin-based fixation and standard IHC techniques prevent proper analysis of target immune cells or proteins in tissue samples. Previous reports indicate that IHC detection of CD4 and CD8 in formalin fixed tissues was unsuccessful perhaps due to epitope masking of antibody recognition (Beckstead, 1994, Cardiffet al., 2013, Hicks et al., 2006, Wester et al., 2003). Here we demonstrated that IHC on ZN treated tissues successfully allowed for visualizing 10 different immune cell related markerscompared to NBF treated tissues. As far as we observed cellular morphology from H&E stained tissues, ZN treated tissue exhibited marginal shrinkage, which might be due to its hypertrophic effects encountered within the dehydration procedure (data not shown). Since the ZN buffer formulation used in this study is formalin-free, ZN treatment on tissue might have less cross-linking reactivity compared to cross linking formalin, consequently generating better epitope exposure of target proteins in tissues when compared with NBF treated tissues. For this reason, ZN treated tissue might not need AR treatment for IHC on various immune markers. In fact, IHC for detecting CD4 and CD8 markers did not need AR treatment in this study. However, detecting some other markers (Foxp3 and F4/80) needed AR treatment for IHC, suggesting that detecting each marker in ZN treated tissue section may need optimization for to verify if AR treatment is necessary or required. Our results further demonstrated that IHC on ZN treated tissue could be performed even with antibodies which are not suitable for general NBF fixed tissues. These information of method and antibodies indicated on this work might shed a light on new applications for histological analysis for diagnosis and basic research with any other antibodies.
We predicted that tumors cancer model mice might be enriched for immune cells populations. The comparison between different cancer model mice or human tissue biopsies in different cancer progression series to decipher the involvement of immune cells and its lineages will be necessary in the future. Since IHC with chromogenic detection is limited and immune cells have various lineages with complex cell surface markers, future analyses will need an ability to detect multiple markers at once which could be performed by a multiplexed-IHC methods(Angelo et al., 2014).
Acknowledgement
This project was funded by grants from the National Cancer Institute's Mouse Models of Human Cancers Consortium (U01 CA141582, U01 CA141541, U01 CA105490-01). The author's wish to thank Ms. Judith Walls and Herlina Sugandha for providing excellent histology support.
Abbreviation
- AR
antigen retrieval
- CD
cluster of differentiation
- FFPE
formalin-fixed paraffin-embedded
- IHC
immunohistochemistry
- LN
lymph node
- MHC
major histocompatibility complex
- NBF
neutral buffer formalin
- ZN
zinc salt fixation
References
- Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, Levenson RM, Lowe JB, Liu SD, Zhao S, Natkunam Y, Nolan GP. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead JH. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem. 1994;42:1127–1134. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Hubbard NE, Engelberg JA, Munn RJ, Miller CH, Walls JE, Chen JQ, Velasquez-Garcia HA, Galvez JJ, Bell KJ, Beckett LA, Li YJ, Borowsky AD. Quantitation of fixative-induced morphologic and antigenic variation in mouse and human breast cancers. Lab Invest. 2013;93:480–497. doi: 10.1038/labinvest.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks DJ, Johnson L, Mitchell SM, Gough J, Cooley WA, La Ragione RM, Spencer YI, Wangoo A. Evaluation of zinc salt based fixatives for preserving antigenic determinants for immunohistochemical demonstration of murine immune system cell markers. Biotech Histochem. 2006;81:23–30. doi: 10.1080/10520290600725375. [DOI] [PubMed] [Google Scholar]
- Mikaelian I, Nanney LB, Parman KS, Kusewitt DF, Ward JM, Naf D, Krupke DM, Eppig JT, Bult CJ, Seymour R, Ichiki T, Sundberg JP. Antibodies that label paraffin-embedded mouse tissues: a collaborative endeavor. Toxicol Pathol. 2004;32:181–191. doi: 10.1080/01926230490274335. [DOI] [PubMed] [Google Scholar]
- Tingstedt JE, Tornehave D, Lind P, Nielsen J. Immunohistochemical detection of SWC3, CD2, CD3, CD4 and CD8 antigens in paraformaldehyde fixed and paraffin embedded porcine lymphoid tissue. Vet Immunol Immunopathol. 2003;94:123–132. doi: 10.1016/s0165-2427(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Wester K, Asplund A, Backvall H, Micke P, Derveniece A, Hartmane I, Malmstrom PU, Ponten F. Zinc-based fixative improves preservation of genomic DNA and proteins in histoprocessing of human tissues. Lab Invest. 2003;83:889–899. doi: 10.1097/01.lab.0000074892.53211.a5. [DOI] [PubMed] [Google Scholar]
- Whiteland JL, Nicholls SM, Shimeld C, Easty DL, Williams NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313–320. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]