Summary
Mutations in PINK1, a serine/threonine kinase linked to familial early onset Parkinsonism, compromise mitochondrial integrity and metabolism and impair AKT signaling. As the activation of a naïve T cell requires an AKT-dependent reorganization of a cell’s metabolic machinery, we sought to determine if PINK1 deficient T cells lack the ability to undergo activation and differentiation. We show that CD4+ T cells from PINK1 knockout mice fail to properly phosphorylate AKT upon activation, resulting in reduced expression of the IL-2 receptor subunit CD25. Following, deficient IL-2 signaling mutes the activation-induced increase in respiratory capacity and mitochondrial membrane potential. Under polarization conditions favoring the development of induced regulatory T cells, PINK1−/− T cells exhibit a reduced ability to suppress bystander T cell proliferation despite normal FoxP3 expression kinetics. Our results describe a critical role for PINK1 in integrating extra-cellular signals with metabolic state during T cell fate determination, and may have implications for the understanding of altered T cell populations and immunity during the progression of active Parkinson’s disease or other immunopathologies.
Keywords: Peripheral T cell differentiation, regulatory T cells, T cell signaling, AKT signaling, Parkinson’s disease
Introduction
The activation of a naïve T cell requires a re-organization of its metabolic machinery to support rapid proliferation and differentiation, coordinating both mitochondrial and cytosolic signaling pathways. During the initial 24 hours post-activation, a T cell shifts from generating ATP via the citric acid cycle and mitochondrial oxidative phosphorylation to aerobic glycolysis. The integration of signals from the TCR, co-stimulation and cytokines re-shapes cellular metabolism through downstream effectors including the serine/threonine kinase AKT (Reviewed in [1]).
PTEN-induced kinase 1 (PINK1) is a ubiquitously expressed serine/threonine kinase found at both the mitochondrion and in the cytosol. In the absence of PINK1’s kinase activity, individuals develop hereditary, early-onset Parkinson’s disease (PD) [2]. PINK1 has a central role in mitophagy, a specialized form of autophagy that degrades depolarized mitochondria and protects a cell from the reactive oxygen species. Healthy mitochondria with intact mitochondrial membrane potential (Δψm) import PINK1 to the inner mitochondrial membrane, where it undergoes proteolysis [3]. Upon a decrease in Δψm and subsequent reduction of mitochondrial protein import, PINK1 accumulates on the outer mitochondrial membrane where it recruits the E3 ubiquitin ligase parkin, initiating mitophagy [4].
Nutrient sensors and extracellular stimuli have the ability to link cellular metabolism and CD4+ T cell lineage plasticity (reviewed in [5]). Pro and anti-inflammatory T cell subsets have different energy requirements and a cell’s fate decision can be altered by manipulating its nutritional milieu [6],[7]. Induced regulatory T cells (iTregs), an anti-inflammatory subset of T lymphocytes, have lower glycolytic rates and higher amounts of lipid oxidation and accompanying increases in Δψm, versus pro-inflammatory T cells [6], consistent with the positive influence of AKT blockade on expression of FoxP3, the master transcriptional regulator of Tregs [8]. In an environment where iTregs must first increase and then maintain higher levels of oxidative phosphorylation, mitochondrial health and fidelity are of critical importance.
Therefore, we hypothesized that the development and function of iTregs would be altered in PINK1−/− T cells. Our data reveal that impaired T cell activation and IL-2 signaling in the absence of PINK1 results in FoxP3+ iTregs with diminished in vitro suppressor function.
Results and Discussion
Absence of PINK1 attenuates AKT activation in response to TCR stimulation
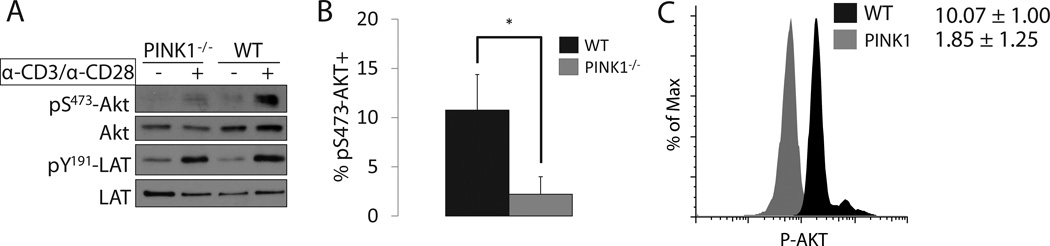
In addition to its integration into the mitochondrial membrane, PINK1 also localizes to the cytoplasm where it activates AKT [9],[10]. Previously, we demonstrated that PINK1−/− embryonic fibroblasts have reduced AKT phosphorylation in response to growth factor stimulation [10], so we reasoned a similar dysfunction could exist in T cells following TCR crosslinking [11]. After activating T cells isolated from peripheral lymph nodes (PLN), we found reduced pS437-AKT in PINK1−/− T cells versus their wild type counterparts, both by Western blot (Figure 1A) and flow cytometry (Figure 1B/1C). Because β-selection of αβ lymphocytes requires AKT, we immunophenotyped murine thymocytes and surprisingly found normal numbers and distribution of T cell precursors (Supplementary Figure 1), potentially due to functional compensation by other AKT isoforms [12] or the higher levels of mitochondrial content in thymocytes [13], rendering the cells resistant to impaired mitophagy.
Figure 1. TCR crosslinking of peripheral CD4+ T cells requires PINK1 for optimal AKT activation.
A) Western blot comparison of activated AKT (pS473-AKT) and total AKT in PLN cells after CD3/CD28 stimulation. Activated LAT (pY191-LAT) was included as a control to demonstrate equal TCR activation (n=3).
B) Percent of cells with activated AKT after stimulating PLN cells for 3 minutes with α-CD3 and α-CD28 antibodies. Cells were first gated for lymphocytes by forward and side scatter before CD4+ T cells were selected (n=4, Mean +/− SD).
C) Representative histogram of data in B. Mean fluorescence intensity (MFI) located to the right of the plot.
PINK1 is required for full early-activation and proliferation of T cells
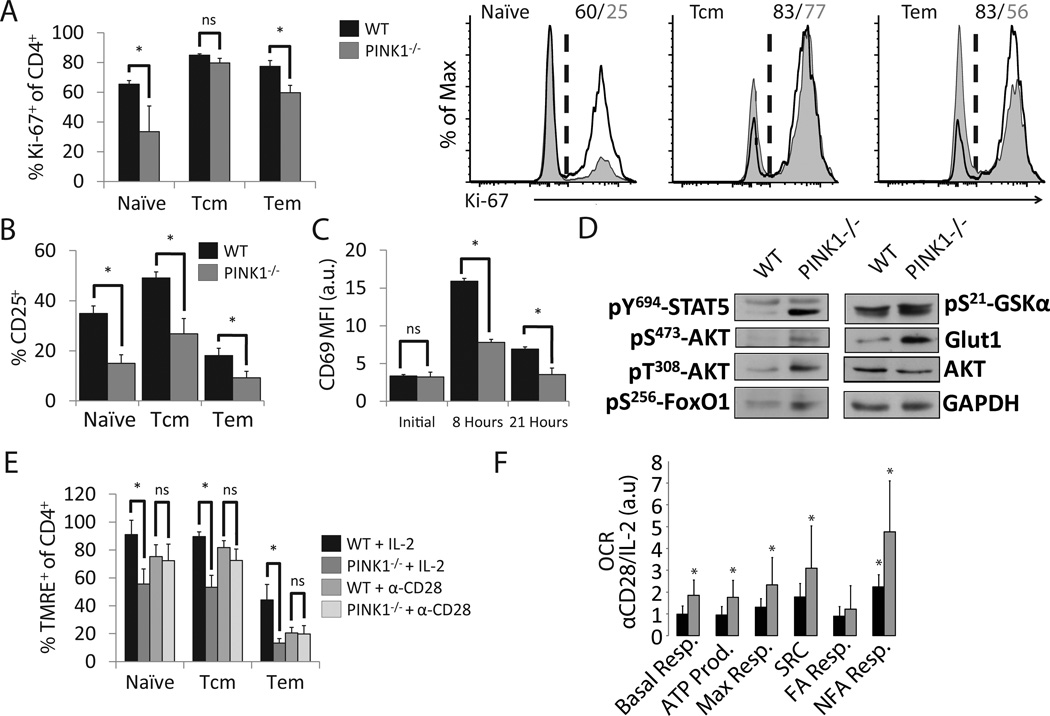
A defect of TCR signaling in cells lacking PINK1 foreshadowed effects on ensuing proliferation and activation. Indeed, stimulation of PINK1−/− T cells with α-CD3/α-CD28 resulted in fewer proliferating cells (Figure 2A). and low expression levels of early activation markers CD25 and CD69 (Figure 2B/2C) These changes were not associated with differences in production of IL-2, as synthesis was similar between groups (Supplementary Figure 2). When we analyzed the integrity of the IL-2 signaling pathway, our results showed impaired phosphorylation of STAT5, AKT [14] and FOXO1a [15], but not GSK-3β, and reduced expression of GLUT1 [16] (Figure 2D), suggesting that some, but not all of the conventional AKT pathway is less active. Since the AKT-mammalian target of rapamycin (mTOR) axis is a known regulator of mitochondrial cellular metabolism, we used the fluorescent dye TMRE and showed that activated PINK1−/− T cells have reduced Δψm (Figure 2E). A comparative mitochondrial stress test of wild type and PINK1−/− CD4+ T cells demonstrated directly that activation by TCR crosslinking combined with CD28 co-stimulation had a similar effect on T cell metabolic state in PINK1 deficient and sufficient cells. Conversely, PINK1−/− cells had a significant metabolic deficit upon IL-2 treatment, and display a high α-CD28/IL-2 ratio of key metabolic metrics (Figure 2F and Supplementary Figure 3A). Addition of the CPT-I transferase inhibitor etomoxir had little relative effect on respiratory capacity, suggesting that non-fatty acid metabolism is dysregulated, potentially associated with reduced GLUT1 expression (Figure 2F and Supplementary Figure 3B).
Figure 2. Reduced activation of CD4+ T in PINK1−/− mice.
A) Percentages of proliferating CD4+ LN T cells from PINK1−/− mice were assessed by Ki-67 expression after 48 hours of α-CD3/α-CD28 stimulation (left). Representative histogram (right) (n=4, Mean +/− SD).
B) Percentage of CD25+ cells of CD4+ T cells stimulated as in Figure 2A (n=3, Mean +/− SD).
C) MFI of surface CD69 on CD4+ T cells activated as in Figure 2A for the indicated time points (n=3, Mean +/− SD).
D) Western blot of components of the IL-2 and AKT signaling pathways after culture with α-CD3 and IL-2 for 48 hours.
E) Δψm was measured by flow cytometric analysis of TMRE after 48 hours of the indicated stimulus. (n=3, Mean +/− SD).
F) Ratio of oxygen consumption rate (OCR) between cells cultured with α-CD28 or IL-2 for 48 hours as a direct measure of oxidative phosphorylation. Derived parameters are as follows, based on regions described in Supplementary Figure 3A: Basal resp. = A–E, ATP Prod. = A–B, max resp. = C–E, spare respiratory capacity (SRC) = C–A, fatty acid (FA) resp. = C–D, Non-FA resp. = D–E, * indicates where p < 0.05 between α-CD28 and IL-2 values within a genotype, (n=4, Mean +/− SD).
Induced regulatory T cell development and function requires PINK1
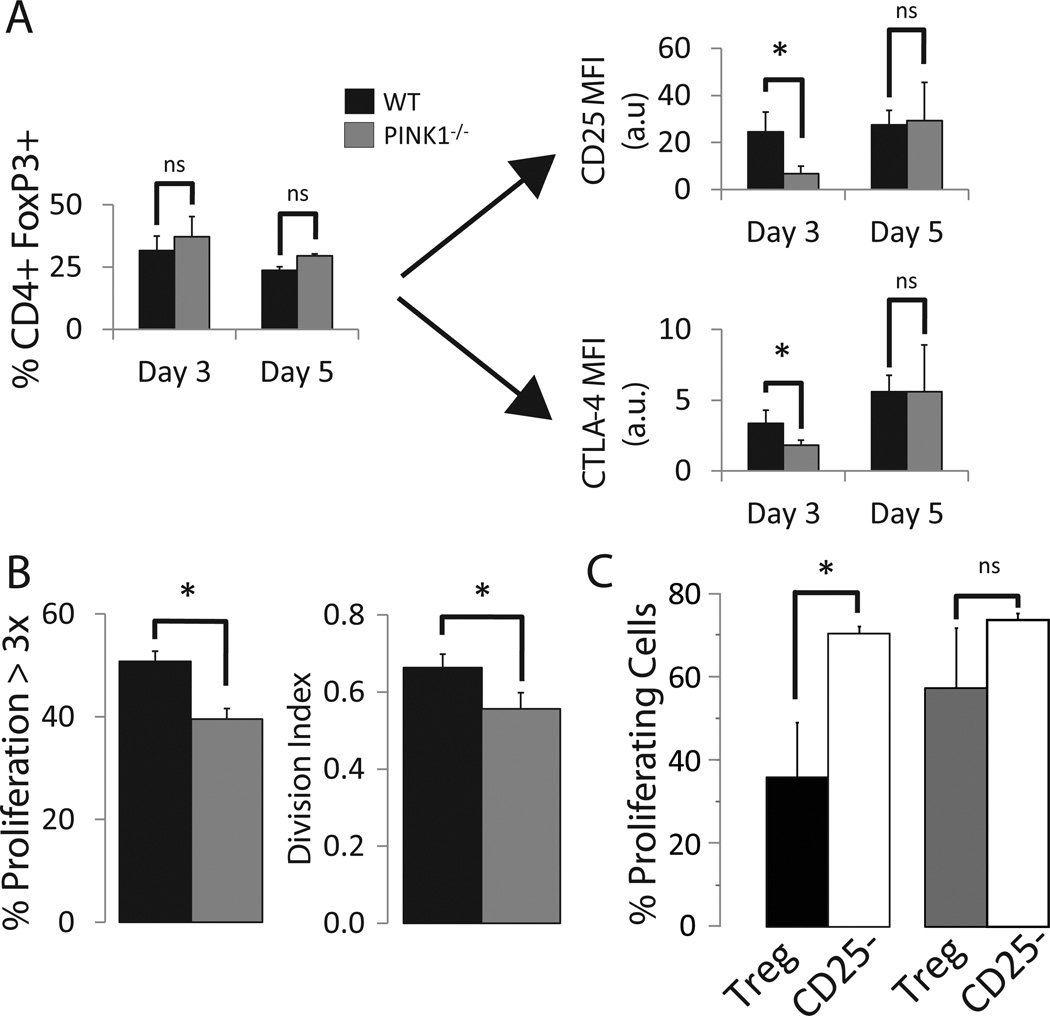
We and others have demonstrated the crucial role of the AKT pathway in the conversion of conventional T cells into suppressor iTregs [17]–[19]. Since decreased IL-2 receptor and AKT signaling oppose each other in the decision of a T cell to acquire an iTreg phenotype, we asked how PINK1−/− T cells would react to iTreg polarization. PINK1−/− T cells exhibited a delay in the surface expression of CD25 and CTLA-4 (Figure 3A). Yet, the knockout T cells never faltered in FoxP3 expression, (Figure 3A). In association with these results, these PINK1−/− T cells proliferated less, which is of particular interest as the acquisition of suppressor ability is associated with multiple rounds of proliferation [20],[21] (Figure 3B). When we measured the suppressor ability of PINK1 sufficient or deficient iTregs (Supplementary Figure 4), wild type, but not PINK1−/− iTregs were able to dampen the proliferation of the co-cultured, CFSE labeled target cells, indicating an uncoupling of FoxP3 expression and suppressor function in vitro (Figure 3C and Supplementary Figure 5). The deletion of PINK1 seemed to only affect the acquisition of anti-inflammatory activity, as Th17-inducing medium induced the simultaneous expression of both RORγT and IL-17 in deficient cells (Supplementary Figure 6) despite reduced GLUT1 expression.
Figure 3. Reduced suppressor activity in PINK1−/− iTregs.
A) Flow cytometric analysis of CD4+ FoxP3+ T cells and their expression of CD25 and CTLA-4 (n=4, Mean +/− SD).
B) Expansion of PINK1−/− T cells in iTreg polarizing conditions. Percentage of CD4+ T cells having undergone 3 rounds of proliferation cultured in iTreg medium as assessed by CFSE dilution (left). Average number of divisions undergone per cell (division index, right) (n=3, Mean +/− SD).
C) CFSE labeled target cells at a ratio of 3:1 with either CD25hi iTregs or CD25− T cells were co-cultured for five days with α-CD3/α-CD28 stimulation. Graph represents the percentage of target cells which underwent >1 division, (n=3, Mean +/− SD). * = p-value < 0.05.
AKT engages in bi-directional communication with mTOR at the hub of several nutrient and energy sensing pathways, influencing metabolism [22] and iTreg development [19], particularly the IL-2 dependent acquisition of suppressor function [21]. In line with the reduced AKT signaling in the PINK1 knockouts, Tregs in mice with a T cell specific deficit in AKT-mTOR signaling adequately express FoxP3, but lack suppressor function. The resulting effect on protein translation by modulating mTOR targets 4E-BP1 and S6 kinase could explain the delayed surface expression of CD25 and CD69 reported above. Indeed, cells lacking PINK1 have reduced phosphorylation of ribosomal protein S6, the target of S6 kinase [10]. Perturbations in intracellular AMP/ATP concentrations or normoxia can also adjust the activation status of mTOR via AMP-activated protein kinase (AMPK), a negative regulator of mTOR both directly [23] and indirectly [24]. An investigation into intracellular signaling in PINK1 deficient cells in response to extracellular oxygen and nutrient availability, while beyond the scope of this paper, would be interesting, particularly as the AMPK activator metformin is currently prescribed to treat hyperinsulinemia.
Several studies have shown abnormalities in peripheral T-lymphocyte populations in sporadic PD [25]–[27]: CD4+ and CD8+ T cell infiltrates are present in the post-mortem human Parkinsonian brain, and CD4+ T cells directly contribute to dopaminergic neuron loss in the MPTP mouse model of PD [28]. The low frequency of lymphocytes behind the blood-brain-barrier during non-inflammatory conditions is inconsistent with the contribution of T cells to disease initiation in PD. Yet, calcium induced cell death of substantia nigra neurons during disease activates resident microglia to secrete cytokines, inviting T cells to infiltrate and potentially provoke disease progression. With an eye toward therapeutics, mice with reduced numbers of CD4+ CD25+ Tregs showed increased microgliosis and dopaminergic neuron death in response to MPTP, whereas adoptive transfer of Tregs protected against neuron loss [29].
Although others have suggested links between PINK1 and type 2 diabetes [30], cancer [31] and psychiatric dysfunction [32], there exists no epidemiologic correlation between PINK1 loss and immune dysregulation, potentially related to the relative rarity of PINK1 mutations (1%–9% of early onset PD) [33] or to concomitant effects on pro-inflammatory cell activation as seen in Raptor−/− mice [21]. The failure of the PINK1−/− mice to develop a scurfy-like autoimmune syndrome could be the result of incomplete elimination of suppressor function in vivo or the normal development of thymic-derived natural Tregs. However, we would expect these mice to have an exacerbated reaction to a subsequent inflammatory challenge. Indeed, the effect of PINK1 loss on T cell subset differentiation via metabolism or mTORC2 activation [9] could each skew the T cell response, [6], [34] resulting in reduced immunosurveillance or autoimmunity. As mitochondrial dysfunction is not only present in familial PINK1 deficiency but also a hallmark of sporadic PD, metabolic defects may likewise account for altered T-cell subpopulations and function in sporadic PD. If so, elucidating T cell function in the absence of PINK1 may also help to unravel the role of T cells in the progression of sporadic PD. Further investigation of T cell function in PINK1 mutation carriers may be warranted in parallel with studies in PINK1-deficient mice.
Concluding remarks
Ultimately, the results presented here on the control of iTreg differentiation and function via the mitochondrial and cytoplasmic positioning of PINK1 identifies the molecule as a potential etiologic agent for immunopathology and as a putative target for Parkinson’s disease therapy via its effects on T cell metabolism and cell fate determination
Materials and Methods
Mice
6–8 week old PINK1 knockout mice were generated on a 129/Sv background [35] before crossing with C57BL/6 for at least 10 generations, and are now considered to be on a mixed background. All mouse work was approved by the Institutional Animal Care and Use Committee at the University of Kentucky..
T cell isolation, enrichment and culture conditions
Isolated CD4+ lymph node T cells (StemCell Technologies) resuspended at a concentration of 2×106 cells/mL in RPMI with 10% FBS (Gibco), antibiotics and 50 µM β-mercaptoethanol (Sigma-Aldrich). IL-2 (1 ng/mL) and TGF-β (2 ng/mL) were added for iTreg differentiation. Cells were incubated for 6 days in 96 well tissue culture plates coated with 1 µg/mL plate-bound α-CD3 (Clone: 145-2C11, Bio X Cell).
in vitro T cell activation
To a single cell suspension of 106 LN CD4+ T cells, 1 µg of α-CD3 antibody with or without 500 ng of α-CD28 (clone: 37.51, eBioscience) antibody was cross-linked with 2 µg of α-IgG for 3 minutes at 37°C.
Flow cytometry
Single cell suspensions were stained with fluorescently labeled antibodies recognizing CD4, CD8, CD62L, CD44 (eBioscience) and CD25 (Miltenyi Biotec). For detection of intracellular targets, cells were fixed and permeabilized with the FoxP3 / Transcription Factor Staining Buffer Set and stained with labeled FoxP3 or CTLA-4 antibodies (eBioscience). Cells to be used for intracellular cytokine staining were cultured with GolgiStop (BD Biosciences), PMA (50 ng/mL) and ionomycin (500 ng/mL) for 5 hours before staining. To evaluate Δψm, cells were incubated with 30 nM TMRE (Invitrogen) for 30 minutes in RPMI at 37°C before immediate analysis. Cytometry was performed on a LSR II flow cytometer (BD Biosciences) and data was analyzed with FlowJo (Tree Star, Inc.).
Treg suppressor assay
Treg suppressor assay was performed as previously described [20]. We used labeled LN T cells from C57/BL6 mice as target cells, and Mouse T-Activator CD3/CD28 Dynabeads (Gibco) as a stimulus.
Metabolic Analysis
Metabolic analysis was performed on an XF-96 bioanalyzer (Seahorse Bioscience) according to manufacturer’s protocol. When indicated, 1 µM oligomycin, 1.5 µM carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP), 200 mM etomoxir (Sigma-Aldrich) and 1 µM each of rotenone/antimycin A were added to the assay medium. Derived parameters were calculated as described ([36], Figure 3 and Supplementary Figure 3A).
Statistical analysis
Unpaired, two-tailed student’s t-tests were used to determine statistical significance between two groups of data. A p-value of < 0.05 was considered significant.
Supplementary Material
Acknowledgements
This work was supported in part by a grant from Parkinson Schweiz to H.B. We thank Anthony Sinai for his important role in the initiation for this study and for helpful discussions, Greg Bauman and Jennifer Strange for technical assistance, and Alan Kaplan and Michael Kilgore for thoughtful comments. The University of Kentucky Flow Cytometry and Cell Sorting Core Facilityis supported in part by the Office of the Vice President for Research, the Markey Cancer Center and a grant from the NIH Shared Instrument Program (S10 RR026827-01A1).
Abbreviations
- PINK1
PTEN-induced kinase 1
- Δψm
mitochondrial membrane potential
- iTreg
induced regulatory T cell
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- TMRE
tetramethylrhodamine methyl ester
- CFSE
carboxyfluorescein succinimidyl ester
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s disease
- mTOR
mammalian target of rapamycin
- AMPK
AMP-activated protein kinase
- FCCP
Carbonilcyanide p-triflouromethoxyphenylhydrazone
- OCR
Oxygen consumption rate
- ECAR
Extracellular acidification rate
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nature immunology. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 2.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S, Ali Z, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science (New York, N.Y.) 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 3.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. The Journal of cell biology. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vives-Bauza C, Zhou C, Huang Y, Cui M, De Vries RLA, Kim J, May J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill LAJ, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 6.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of immunology (Baltimore, Md.: 1950) 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight Za, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami J, Yamada H, Kataoka K, et al. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. The Journal of biological chemistry. 2011;286:7182–7189. doi: 10.1074/jbc.M110.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akundi RS, Zhi L, Büeler H. PINK1 enhances insulin-like growth factor-1-dependent Akt signaling and protection against apoptosis. Neurobiology of disease. 2012;45:469–478. doi: 10.1016/j.nbd.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafont V, Astoul E, Laurence A, Liautard J, Cantrell D. The T cell antigen receptor activates phosphatidylinositol 3-kinase-regulated serine kinases protein kinase B and ribosomal S6 kinase 1. FEBS letters. 2000;486:38–42. doi: 10.1016/s0014-5793(00)02235-3. [DOI] [PubMed] [Google Scholar]
- 12.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pua HH, Guo J, Komatsu M, He Y-W. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. Journal of immunology (Baltimore, Md.: 1950) 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 14.Lockyer HM, Tran E, Nelson BH. STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. Journal of immunology (Baltimore, Md.: 1950) 2007;179:5301–5308. doi: 10.4049/jimmunol.179.8.5301. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, et al. Novel Foxo1-dependent transcriptional programs control Treg cell function. Nature. 2012:0–7. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 17.Reneer MC, Estes DJ, Vélez-Ortega AC, Norris A, Mayer M, Marti F. Peripherally induced human Regulatory T cells uncouple Kv1.3 activation from TCR-associated signaling. European journal of immunology. 2011:3170–3175. doi: 10.1002/eji.201141492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reneer MC, Marti F. The balancing act of AKT in T cells. Frontiers in Biology. 2012 [Google Scholar]
- 19.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 20.Ellis GI, Reneer MC, Vélez-Ortega AC, McCool A, Martí F. Generation of Induced Regulatory T Cells from Primary Human Naïve and Memory T Cells. Journal of Visualized Experiments. 2012:8–12. doi: 10.3791/3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013 doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 23.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism & related disorders. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Bas J, Calopa M, Mestre M, Molleví DG, Cutillas B, Ambrosio S, Buendia E. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. Journal of neuroimmunology. 2001;113:146–152. doi: 10.1016/s0165-5728(00)00422-7. [DOI] [PubMed] [Google Scholar]
- 27.Calopa M, Bas J, Callén A, Mestre M. Apoptosis of peripheral blood lymphocytes in Parkinson patients. Neurobiology of disease. 2010;38:1–7. doi: 10.1016/j.nbd.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. The Journal of clinical investigation. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi Y, Fan Y, He L, Liu W, Wen X, Zhou S, Wang X, et al. Novel role of aquaporin-4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson’s disease. Aging cell. 2011;10:368–382. doi: 10.1111/j.1474-9726.2011.00677.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, Petrovic N, et al. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21:3653–3665. doi: 10.1096/fj.07-8520com. [DOI] [PubMed] [Google Scholar]
- 31.Berthier A, Navarro S, Jiménez-Sáinz J, Roglá I, Ripoll F, Cervera J, Pulido R. PINK1 displays tissue-specific subcellular location and regulates apoptosis and cell growth in breast cancer cells. Human pathology. 2011;42:75–87. doi: 10.1016/j.humpath.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Hatano Y, Li Y, Sato K, Asakawa S, Yamamura Y, Tomiyama H, Yoshino H, et al. Novel PINK1 mutations in early-onset parkinsonism. Annals of neurology. 2004;56:424–427. doi: 10.1002/ana.20251. [DOI] [PubMed] [Google Scholar]
- 33.Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harbor perspectives in medicine. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature immunology. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akundi RS, Huang Z, Eason J, Pandya JD, Zhi L, Cass WA, Sullivan PG, et al. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PloS one. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.