Abstract
According to the World Health Organization (WHO), multiple drug-resistant (MDR) bacterial infection is a top threat to human health. Since bacteria evolve to resist antibiotics faster than scientists can develop new classes of drugs, the development of new materials which can be used, not only for separation, but also for effective disinfection of drug resistant pathogens is urgent. Driven by this need, we report for the first time the development of a nisin antimicrobial peptide conjugated, three dimensional (3D) porous graphene oxide membrane for identification, effective separation, and complete disinfection of MDR methicillin-resistant Staphylococcus aureus (MRSA) pathogens from water. Experimental data show that due to the size differences, MRSA is captured by the porous membrane, allowing only water to pass through. SEM, TEM, and fluorescence images confirm that pathogens are captured by the membrane. RT-PCR data with colony counting indicate that almost 100% of MRSA can be removed and destroyed from the water sample using the developed membrane. Comparison of MDR killing data between nisin alone, the graphene oxide membrane and the nisin attached graphene oxide membrane demonstrate that the nisin antimicrobial peptide attached graphene oxide membrane can dramatically enhance the possibility of destroying MRSA via a synergestic effect due to the multimodal mechanism.
Graphical Abstract
An antimicrobial peptide conjugated 3D graphene oxide membrane has the ability to separate, identify and disinfect MRSA from water.
Introduction
Even in the 21st century, multiple drug-resistant bacteria (MDRB) infection is one of the top three threats to human health 1–2. Since 1928, antibiotics have been responsible for saving countless human lives. However, due to the extensive use of antibiotics and the extraordinary genetic capacities of microbes, human pathogens have developed multiple mechanisms of resistance to all types of antibiotic used in clinical practice 1–6. The WHO predicts existing antibiotics can only be used for the next 1–2 decades 1–6. Since pathogens are evolving to resist antibiotics faster than scientists can develop new classes of antibiotic drugs 1–6, society is facing a very difficult battle against resistant pathogens in water and food supplies.
Recently several articles reported that antibiotic-resistant bacteria entering the aquatic environment through the wastewater discharge is a huge concern for human health 7–13. During the last few years several nanomaterial and polymer-based antimicrobial agents have been developed to tackle antibiotic resistant-bacteria problem5–6,9–11. Recently reported data12–13 indicate that wastewater treatment by ozonation, UV-irradiation, or photocatalysis in combination with different filtering techniques may be useful to eliminate microorganisms from sewage before discharging into surface waters. Now, society is facing an enormous problem, the nightmare of tackling antibiotic resistance bacteria. These challenges clearly indicate that an urgent need for the development of new materials which can be used for accurate separation and effective disinfection of multiple drug resistant pathogens exists. Driven by this urgent need, in the current manuscript, we report the development of an antimicrobial peptide conjugated, porous, graphene oxide membrane for identification, effective separation and complete disinfection of MDR pathogens from water as shown in Scheme 1.
Scheme 1.
Schematic representation showing the MDR pathogens separation and killing capability using nisin conjugated porous graphene oxide membrane.
Due to the low cost, ease of large scale production and remarkable properties, 2D graphene oxide holds great promise for daily-life applications 14–21. Several recent reports indicate that two-dimensional graphene oxide offer an exciting opportunity to develop a new class of membrane, which block all molecules or ions with a hydrated size larger than 9 Å22–33. Here we report for the first time that an antimicrobial peptide conjugated graphene oxide-based membrane with a pore size around 250 nm can not only block MDRB pathogens from water, but also has the capability to kill MDRB in contact, consequently acting as a disinfectant. In our design we have used nisin attached to a graphene oxide membrane, where nisin, a 34 amino acid residue peptide, acts as an antimicrobial. Nisin is usually used as a preservative in foods, approved by the WHO, and the FDA 34–38. Our experimental data show that nisin-conjugated porous membrane can be used as a versatile membrane to identify, remove and kill methicillin-resistant Staphylococcus aureus (MRSA) simultaneously. MRSA, a multidrug-resistant gram-negative bacteria, cause pneumonia, urinary tract, and bloodstream and skin infections which kill more than 19000 people every year in USA alone 1–6.
Results and Discussions
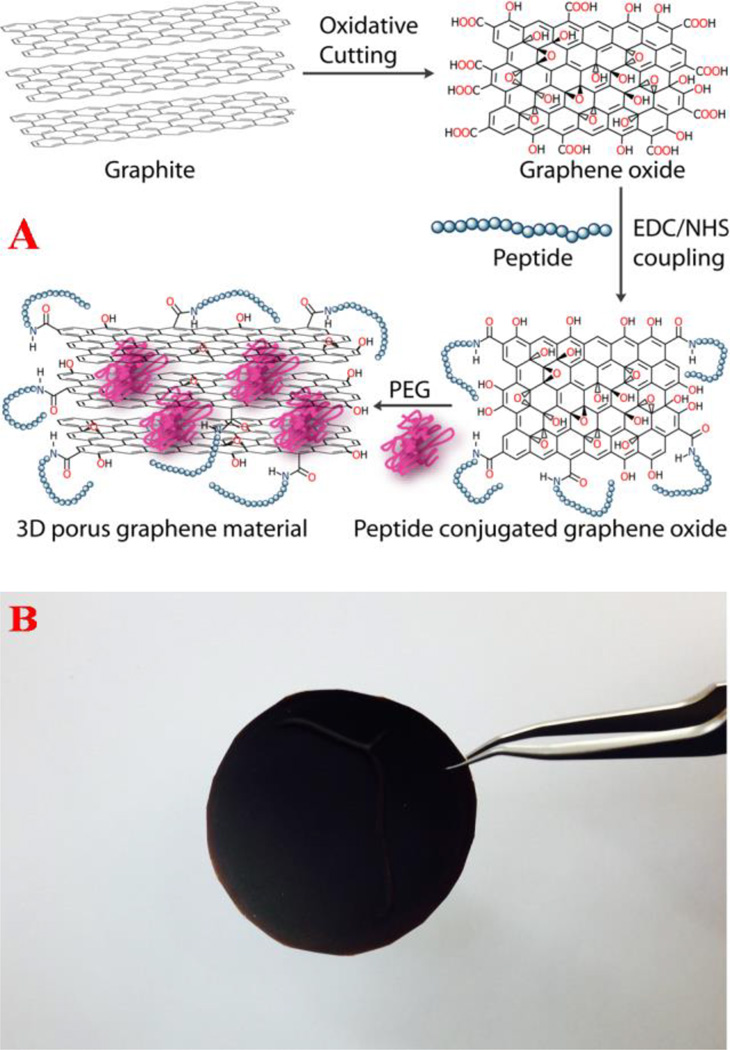
For highly efficient capture and disinfection, 3D graphene oxide foam based membranes with the antimicrobials peptide, nisin attached has been developed. As shown in Figure 1A, first 2D graphene oxide sheet were produced using a modified Hummers method as reported recently 17–19,32–33,39–40. Next, nisin was attached to the 2D graphene oxide via amide linkages using the coupling chemistry between -CO2H group of 2D graphene oxide and -NH2 group of nisin by the cross-linking agent EDC (1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide).
Figure 1.
A) Schematic representation showing the synthetic procedure for the formation of nisin antimicrobials peptide attached 3D graphene oxide. B) Photograph shows the foam membrane formed from graphene oxide membrane with antimicrobial nisin peptides attached.
In the final step, a 3D graphene oxide foam was developed by attached cross-linking the 2D graphene oxide using amine functionalized PEG and attaching the nisin antimicrobials peptide, as shown in Figure 1A. PEG was used to form the 3-D porous architecture by interconnecting the graphene oxide sheets and the antimicrobials peptide attached via amine linkages. The semi-solid formed was spin casted to yield the membrane with 5 X 5 cm2 size, as shown in Figure 1B.
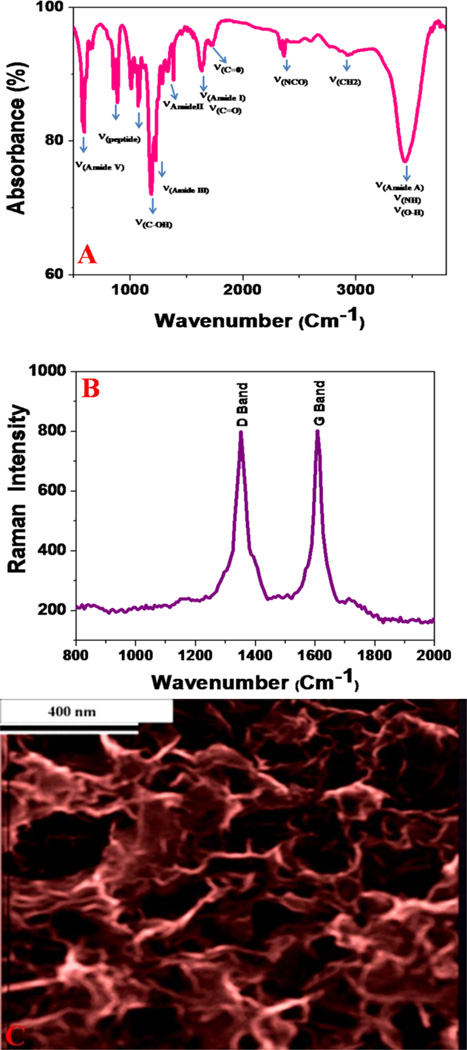
Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and high-resolution scanning electron microscope (SEM) were used to characterize the membrane, as reported in Figure 2. The FTIR spectrum of the nisin antimicrobials peptide attached graphene oxide membrane shows a very strong and broad band at ~3350 cm−1, which is due to the peptide Amide A band due to the N-H stretching vibration. This bond is very broad due to overlap of three different bands from peptide Amide A band, PEG amine band and -OH vibration from graphene oxide carboxyl group. The amide II band observed at ~1550 cm−1 is mainly due to the in-plane NH bending vibration from the peptide. Similarly, the peptide’s amide III and amide V bands are observed ~1250 cm−1 and ~650 cm−1 as shown in Figure 2A. The strong IR peak observed ~2300 cm−1 is due to the -NCO vibration, clearly indicates the formation of amides from peptide/PEG-NH2 groups and graphene oxide-CO2H group. Also, the carbonyl (–C=O) stretch observed at ~1725 cm−1 correspond to unreacted carboxylic acid groups of graphene oxide.
Figure 2.
A) FTIR spectrum shows the existence of amide I, amide II, amide III and amide V bands, which clearly indicate the presence of peptides on the 3D graphene oxide membrane. The stretches of –CO, -OH, -CN, and –NH groups due to the graphene oxide and PEG can also be seen. B) Raman spectrum clearly indicates the presence of D and G bands in nisin antimicrobials peptide attached graphene oxide membrane. C) SEM image of nisin antimicrobials peptide attached graphene oxide membrane shows the 3D structure with pore sizes of 200–350 nm.
The Raman spectrum from membrane shown in Figure 2B clearly shows the D-band ~1340 cm−1 and a G-band ~ 1620 cm−1.11–20 The strong D band indicates that the degree of graphene oxide modification is high. The high-resolution scanning electron microscope (SEM) image in Figures 2C shows an interconnected 3-D network with a pore size of 200–350 nm. Using nitrogen adsorption analysis via the Brunauer–Emmett–Teller (BET) method, the specific surface area for the membranes was 523 m2 g−1, and the pore volume was 0.480 cm3 g−1. From BET analysis, the pore size distribution indicated an average pore diameter of 280 nm.
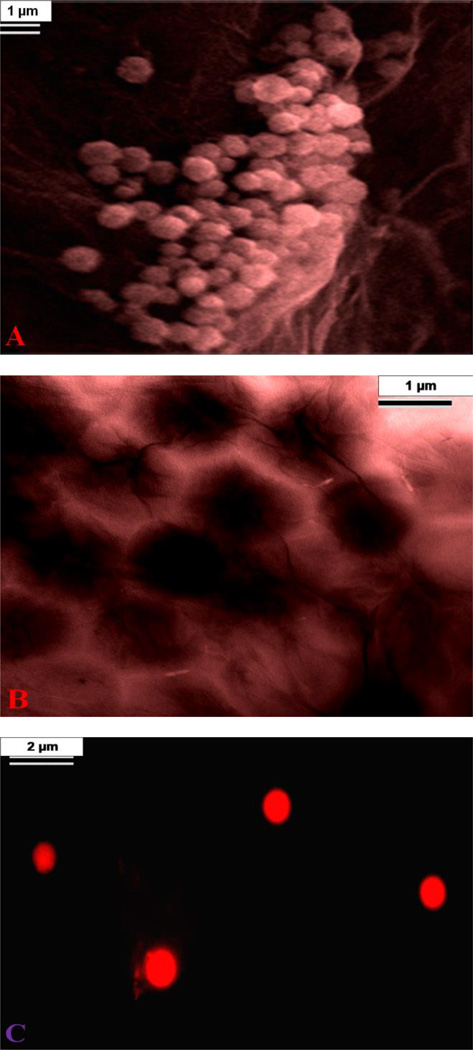
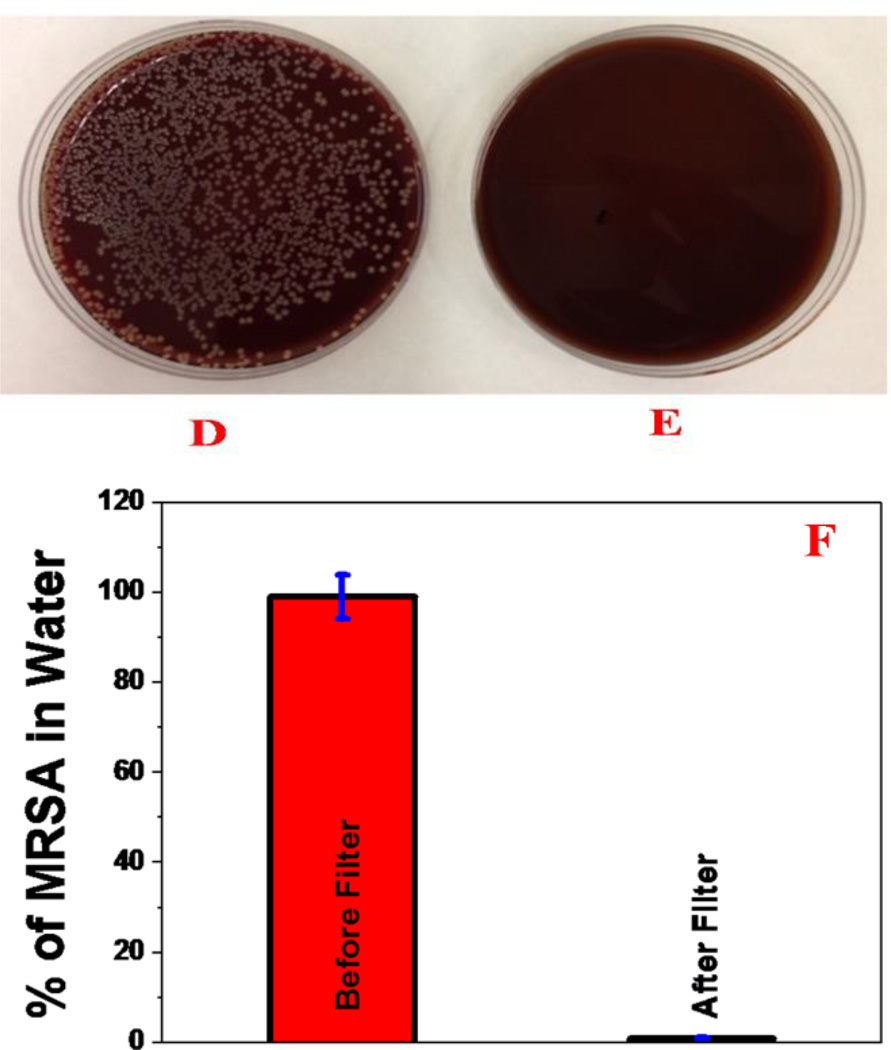
To understand, whether the developed nisin antimicrobials peptide attached graphene oxide membranes can be used for the removal of MRSA from water, 8.9 × 106 colony-forming units (CFU)/mL of MRSA were used to contaminate 100 mL of drinking water. After 90 minutes of gentle shaking, the infected water sample was filtered using the membrane. After filtering, the removal efficiency was measured using reverse transcription polymerase chain reaction (RT-PCR) technique 42, as well as colony plating technique on LB agar in water sample, as shown in Figure 3. SEM, TEM and fluorescence imaging techniques were used to characterize the MRSA separated by the membrane. Experimental measurement using colony plating technique and RT-PCR, as shown in Figures 3D, 3E and 3F, clearly show that about 100% of MRSA was removed by the membrane. The high efficiency of MRSA removal by the membrane is due to the fact that the size of MRSA is about .6-1 micron, where as the pore size of membrane is about .28 microns. As a result, water only can pass through the porous membrane. The SEM and TEM images reported in the Figure 3A & 3B, clearly shows that MRSA is attached to the surface of the membrane. For better understanding, fluorescence imaging was performed. For imaging purpose, fluorescent Cy5.5-modified peptide was attached to the 3D membrane. The fluorescence image in Figures 2C shows that the MRSA is attached on the surface of the 3-D membrane after MRSA-infected water sample was filtered.
Figure 3.
A) SEM image demonstrating capture of MRSA by 3D graphene oxide based membrane. B) TEM image demonstrating capture of MRSA by 3D graphene oxide based membrane. C) Fluorescence image shows the presence of MRSA separated by membrane. D, E) Colonies of MRSA demonstrating amount of live bacteria, (D) before filtration and (E) after filtration using 3D graphene oxide based membrane. F) Graph shows MRSA removal efficiency using 3D graphene oxide membrane. Reverse transcription polymerase chain reaction (RTPCR) was used to quantify the amount of MRSA present.
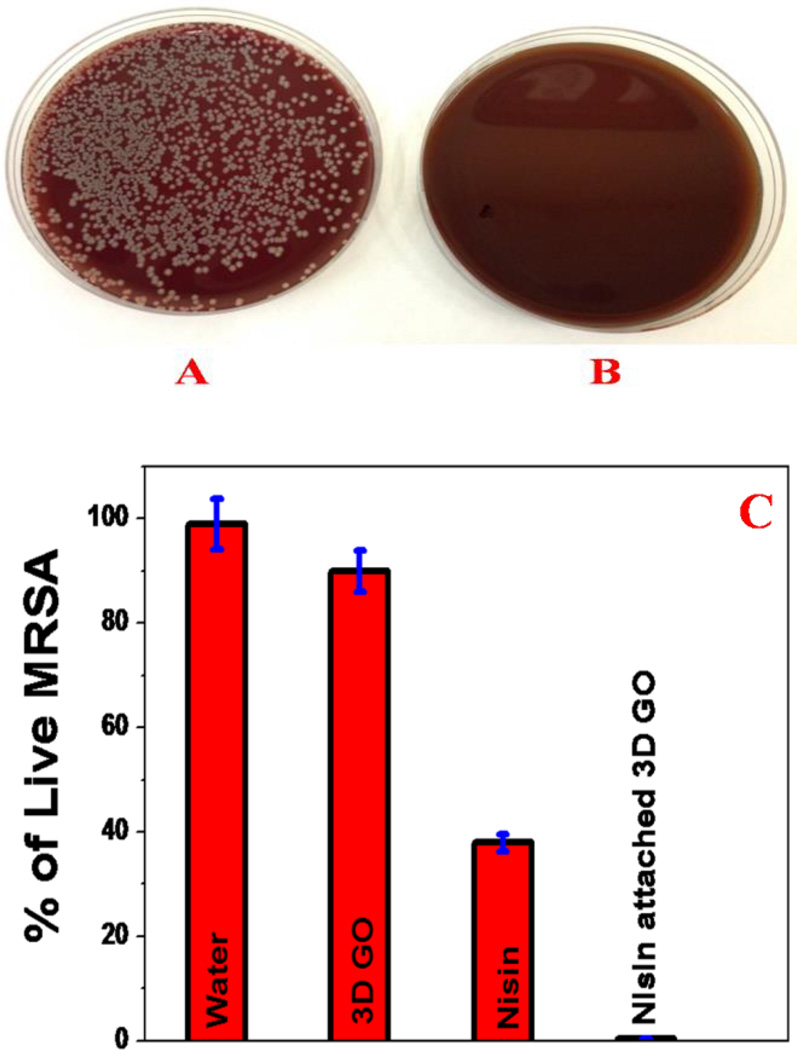
Since MRSA is resistant to several antibiotics such as penicillin, amoxicillin, oxacillin, methicillin, mupirocin, the ability of the membrane able to disinfect MRSA after separation to prevent spreading is very important. To determine whether the MRSA captured by nisin antimicrobials peptide attached graphene oxide membrane is alive or dead, the membrane surface was washed thoroughly using 100 mL of water and the amount of live MRSA was estimated using colony plating. Also, to find out whether the presence of nisin antimicrobials peptide is necessary to kill MRSA, the same experiment was performed with a 3D graphene oxide membrane without the nisin antimicrobials peptide attached. As shown in Figure 4B, almost 100% of MRSA was killed when we used nisin antimicrobials peptide attached membrane. On the other hand, most of the MRSA was alive when an unmodified graphene oxide membrane was used.
Figure 4.
Colonies of MRSA demonstrating amount of live bacteria after filtration by membrane, A) only 3D graphene oxide based membrane, B) nisin antimicrobials peptide attached 3D graphene oxide based membrane. C) Amount of live MRSA when treated with only 3D graphene oxide, only nisin and nisin attached 3D graphene oxide.
The observed very high killing efficiency by membrane in the presence of nisin antimicrobials peptide can be due to the several facts. First, several reports 34–38 indicate that nisin inhibits bacterial cell-wall synthesis by the binding of the N-terminal AB-ring fragment to lipid II. After binding to lipid II, the C-terminus of nisin inserts into the phospholipid membrane and, as a result, the collapse of a vital ion gradients occurs, ultimately resulting in MRSA cell death. Another possibility is that 3D graphene oxide can kill MRSA. The mechanism for killing MRSA by 3D graphene oxide is by mechanically wrapping of MRSA, as shown in SEM and TEM image in Figures 2A & 2B. This wrapping may cause induce membrane stress by disrupting and damaging cell membranes until cell lysis occurs, as previously reported39–41.
To understand which mechanism is really responsible for MRSA killing, the same experiment was performed using only 3D graphene oxide without nisin. Similarly, to find out how much MRSA is killed by nisin peptide only, nisin was added directly to the water. As shown in Figures 4C, nisin is able to kill about 60% of MRSA, whereas 3D graphene can kill only 8% MRSA. This experimental data demonstrate clearly that nisin attached graphene oxide exhibit synergistic killing effect, causing almost 100% of MRSA to be killed. This synergistic mechanism is due to the fact that 3D porous graphene oxide helps to trap MRSA which causes bacterial membrane stress. This condition allows nisin to bind more easily with MRSA via N-terminal to lipid II and C-terminal with phospholipid membrane. As a result, MRSA collapses more easily. The experimental data clearly indicate that the multimodal mechanism by nisin antimicrobials peptide attached graphene oxide membrane can dramatically enhance the possibility of destroying MRSA due to the synergistic therapeutic effect.
The reported separation and killing efficiency for MRSA using nisin antimicrobial peptide conjugated porous 3D graphene oxide membrane is about 2–5% higher than the reported data by ozonation followed by a filter passage or UV disinfection followed by a filter passage 7–8,12–13. Since retention and reuse of membrane are keys for the water treatment technology, the removal capacity of nisin antimicrobial peptide conjugated porous 3D graphene oxide membrane for several reuse cycles when the concentration of MRSA was 106 CFU/mL was tested. The removal and killing capacity efficiency can be maintained for 7 cycles. Additional filtrations after 7 cycles show a decrease in efficiency.
Conclusion
In conclusion, in this manuscript we have reported the development of nisin antimicrobial peptide conjugated porous 3D graphene oxide membrane which has the capability to separate, identify and completely disinfect multidrug-resistant pathogens from water. We have shown that since the pore size of the membrane (~300 nm) is much smaller than MRSA (~1000 nm), only water can pass through the porous membrane when MRSA infected water sample was filtered by the membrane. MRSA pathogens were captured by the membrane, which had been confirmed by SEM, TEM and fluorescence images. Using RT-PCR and colony counting data, we have shown that almost 100% of MRSA were removed from the water sample and killed using nisin antimicrobial peptide conjugated membrane.
Our reported disinfection data with only nisin, only the graphene oxide membrane and the nisin attached graphene oxide membrane demonstrate that the nisin attached graphene oxide membrane can dramatically enhance the possibility of destroying MRSA via synergistic effect. 3D graphene oxide helps to trap MRSA which causes membrane stress. This condition allows nisin to bind easily with MRSA via N-terminal to lipid II and C-terminal with phospholipid membrane, and as a result the nisin attached porous graphene oxide membrane kills almost 100 % of the bacteria. Though we are in relatively early stage of development of antimicrobial peptide conjugated 3D graphene oxide based porous membrane, we believe that the reported membrane has enormous potential for the separation and disinfection of different pathogens. Vigorous research needs to be conducted to find a cost effective process for large scale development of graphene oxide membranes and methods to improve the long-term performance of the membrane for water and wastewater treatment before it can be used for technological application. Continuous collaboration between scientists and engineer will open up a new possibility for rapid removal, identification and highly efficient disinfections of drug resistant MRSA from environmental samples.
Experimental
All chemicals were purchased from Fisher Scientific and Sigma-Aldrich, including graphite, nisin, KMnO4, PEG, NaNO3, sulfuric acid and ethylene glycol. MRSA bacteria and MRSA growth media were purchased from the American Type Culture Collection (ATCC, Rockville, MD).
Development of nisin antimicrobial peptide attached 2D Graphene Oxide
For developing 2D graphene oxide, modified Hummers reported method was used for graphite exfoliation by strong oxidizing agents, as reported before and shown in Figure 1A17–19,32–33. In brief, 1.5g of graphite powder was treated for 25 minutes with 1.5g of NaNO3 in 50 mL of H2SO4 and 4g of KMnO4, without changing the temperature. Next, we have continued the reaction for 30 minutes and a thick paste was obtained. After that, we filtered and re-dispersed the obtained graphene oxide in 100 mL of water and sonicated for several hours for exfoliation. The graphene oxide carboxylic acid using the amine group of nisin via EDC (1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide) cross-linking. Finally, high-resolution JEM-2100F transmission electron microscope (TEM) instrument, IR and Raman spectroscopy were used to characterize nisin antimicrobial peptide attached 2D graphene oxide.
FTIR Measurement
To find out the chemical composition of nisin antimicrobial peptide attached 2D graphene oxide, Thermo Nicolet Nexus 870 Fourier Transform Infrared Spectrometer (FTIR) equipped with three beam splitters was used.
Development of nisin antimicrobial peptide attached porous graphene oxide membranes
To develop nisin antimicrobial peptide attached 3D porous graphene oxide, a 3D graphene oxide foam was first developed from nisin antimicrobial peptide attached 2D graphene oxide using PEG as a cross-linking agent as shown in Figure 1A. For this purpose, 10 mL of nisin antimicrobial peptide attached 2D graphene oxide was added with 20 mg of PEG and then it was sonicated for 5 min. After 5 min of sonication, samples were kept under a hood in an oil bath at about 60–70 °C for 60 minutes. The resulting semi-solid 3D graphene oxide foam was used to develop 5 × 5 cm2 membranes using spin casting, as shown in Figure 1B.
Nisin antimicrobial peptide attached 3D graphene oxide membrane characterization using TEM, SEM, and EDX
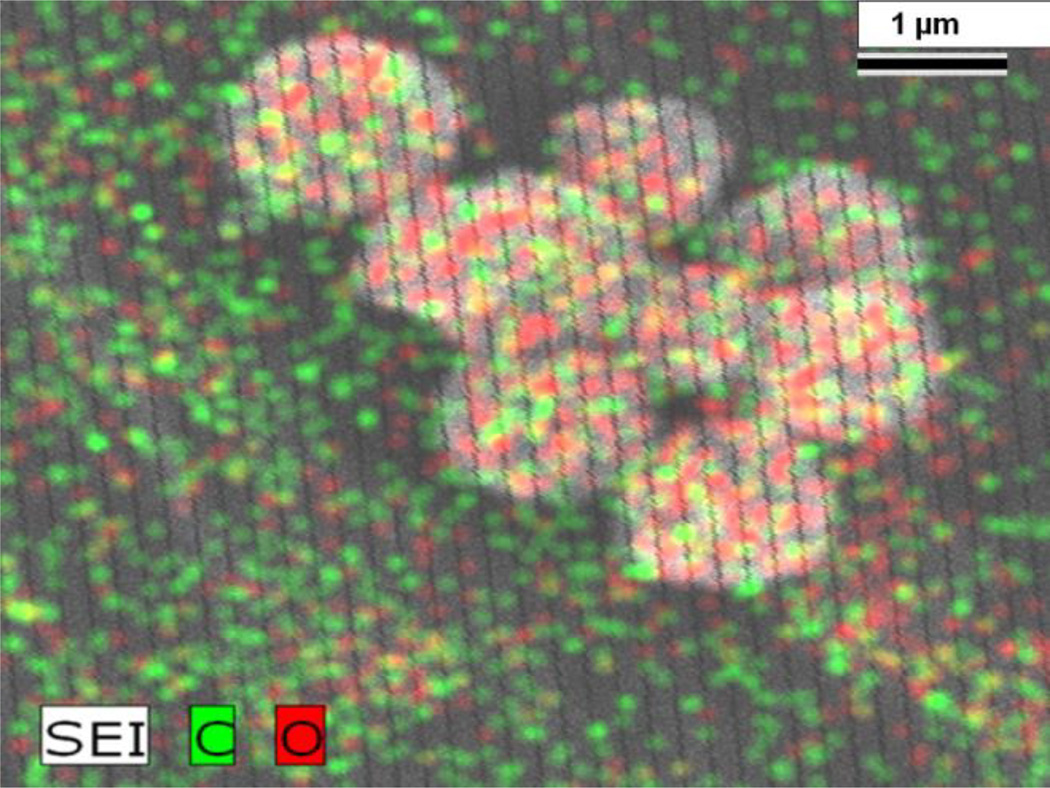
Nisin antimicrobial peptide attached 2D graphene oxide 2-D and 3-D graphene oxide architectures were characterized using ultra-high resolution field emission scanning electron microscopy (FE-SEM HITACHI) and a JEOL 2010-F microscope (TEM) using 200kV of applied voltage. The SEM was coupled with a BF/DF Duo-STEM detector and EDX spectroscopy (Bruker). Figure 5 shows the EDX mapping of MRSA captured 3D graphene oxide membrane.
Figure 5.
EDX mapping shows presence of C and O in MRSA captured 3D graphene oxide membrane membranes.
Bacteria sample preparation
MRSA pellet were purchased from the ATCC and then cultured using ATCC protocol as instructed. Initially, supplied pellet of MRSA was rehydrated on 5 to 6 ml of Bacto tryptic soy broth (BD) and incubated at 37°C for 24 hours. After that, a single colony of MRSA from tryptic agar plate was inoculated into 10 ml of Tryptic Soy Broth for 12 hours. From the stock solution of bacteria, we have diluted several times to vary the concentration of MRSA from 103–107 CFU (colony forming unit)/mL.
Fluorescence Imaging
For fluorescence imaging of MRSA, an Olympus IX71 inverted confocal fluorescence microscope was used to detect the Cy5 attached peptide. 630 nm light was used as an excitation source and SPOT Insight digital camera has been used for fluorescence collection. Olympus DP capture software has been used for data processing.
Determination of the percentage of live bacteria
After removal, the MRSA bacteria were transferred to colony-countable plates and incubated for 24 h at 37°C. The colony number for each plate was counted with a colony counter (Bantex, Model 920 A).
Acknowledgements
Dr. Ray is grateful for NSF-PREM grant # DMR-1205194 for their generous funding.
Notes and References
- 1. [date of access 10/12/2013]; http://www.cdc.gov/drugresistance/threat-report-2013/, Threat Report. 2013
- 2. [date of access 10/12/2013]; http://www.who.int/mediacentre/factsheets/fs194/en/, Antimicrobial Resistance.
- 3.Nikaido H. Annu. Rev. Biochem. 2009:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer MOA, Dantas G, Church GM. Science. 2009;325:1128–1131. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray PC, Khan SA, Singh AK, Senapati D, Fan Z. Chem. Soc. Rev. 2012;41:3193–3201. doi: 10.1039/c2cs15340h. [DOI] [PubMed] [Google Scholar]
- 6.Fan Z, Senapati D, Khan SA, Singh AK, Hamme A, Yust B, Sardar D, Ray PC. Chem.—Eur. J. 2013;19:2839–2847. doi: 10.1002/chem.201202948. [DOI] [PubMed] [Google Scholar]
- 7.C-Cáceres W, Melgarejo A, C-Lluch M, Stoll C, Lucena F, Jofre J, Muniesa M. Environ. Sci. Technol. 2014;48:7602–7611. doi: 10.1021/es501851s. [DOI] [PubMed] [Google Scholar]
- 8.Gadipelly C, P-González A, Yadav GD, Ortiz I, Ibáñez R, Rathod VK, Marathe KV. Ind. Eng. Chem. Res. 2014;53:11571–11592. [Google Scholar]
- 9.Ghosh S, Chakraborty P, Saha P, Acharya S, Ray M. RSC Adv. 2014;4:23251. [Google Scholar]
- 10.Qu X, Alvarez PJJ, Li Q. Water Res. 2013;47:2931–2946. doi: 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 11.Grinberg O, Natan M, Lipovsky A, Varvak A, Keppner H, Gedanken A, Banin E. J. Mater. Chem. B. 2015;3:59. doi: 10.1039/c4tb00934g. [DOI] [PubMed] [Google Scholar]
- 12.Lüddeke F, He S, Gallert C, Winter J, Güde H, Loffler H. Water Res. 2015;69:243–251. doi: 10.1016/j.watres.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Michael I, Hapeshi E, Michael C, Varela AR, Kyriakou S, Manaia CM, Fatta-Kassinos D. Water Res. 2012;46:5621–5634. doi: 10.1016/j.watres.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Novoselov KS, Falko VI, Colombo L, Gellert PR, Schwab MG, Kim KA. Nature. 2012;490:192–200. doi: 10.1038/nature11458. [DOI] [PubMed] [Google Scholar]
- 15.Chung C, Kim YK, Shin D, Ryoo SR, Hong BH, Min DH. Acc. Chem. Res. 2013;46:2211–2224. doi: 10.1021/ar300159f. [DOI] [PubMed] [Google Scholar]
- 16.Chou SS, De M, Luo J, Rotello VM, Huang J, Dravid VP. J. Am. Chem. Soc. 2012;134:16725–16733. doi: 10.1021/ja306767y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Z, Kanchanapally R, Ray PC. J. Phys. Chem. Lett. 2013;4:3813–3818. [Google Scholar]
- 18.Pramanik A, Chavva SR, Fan Z, Sinha S, Nellore BP, Ray PC. J. Phys. Chem. Lett. 2014;5:2150–2154. doi: 10.1021/jz5009856. [DOI] [PubMed] [Google Scholar]
- 19.Pramanik A, Fan Z, Reddy SC, Sinha SS, Ray PC. Sci. Rep. 2014:4. doi: 10.1038/srep06090. Article No. 6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightcap IV, Kosel TH, Kamat PV. Nano Lett. 2010;10:577–583. doi: 10.1021/nl9035109. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y-H, Chang K-H, Li J-M, Simon P, Tang J, Torad NL, Hu C-C, Yamauchi Y. Chem. Eur. J. 2014;20:13838–13852. doi: 10.1002/chem.201403649. [DOI] [PubMed] [Google Scholar]
- 22.Nair RR, Wu HA, Jayaram PN, Grigorieva IV, Geim AK. Science. 2012;335:442–444. doi: 10.1126/science.1211694. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Song Z, Wei N, Shi L, Mao Y, Ying Y, Sun L, Xu Z, Peng X. Nat. Commun. 2013;4:3979. doi: 10.1038/ncomms3979. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Xu Z, Gao C. Adv. Funct. Mater. 2013;23:3693–3700. [Google Scholar]
- 25.Shen J, Liu G, Huang K, Jin W, Lee K-R, Xu N. Angew. Chem., Int. Ed. 2015;54:578–582. doi: 10.1002/anie.201409563. [DOI] [PubMed] [Google Scholar]
- 26.Smith ZP, Freeman BD. Angew. Chem., Int. Ed. 2014;53:10286–10288. doi: 10.1002/anie.201404407. [DOI] [PubMed] [Google Scholar]
- 27.Huang K, Liu G, Lou Y, Dong Z, Shen J, Jin W. Angew. Chem., Int. Ed. 2014;53:6929–6932. doi: 10.1002/anie.201401061. [DOI] [PubMed] [Google Scholar]
- 28.Sun P, Zhu M, Wang K, Zhong M, Wei J, Wu D, Xu Z, Zhu H. ACS Nano. 2013;7:428–437. doi: 10.1021/nn304471w. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Song Z, Zhang X, Huang Y, Li S, Mao Y, Ploehn HJ, Bao Y, Yu M. Science. 2013;342:95–98. doi: 10.1126/science.1236686. [DOI] [PubMed] [Google Scholar]
- 30.Joshi RK, Carbone P, Wang FC, Kravets VG, Su Y, Grigorieva IV, Wu HA, Geim AK, Nair RR. Science. 2014;343:752–754. doi: 10.1126/science.1245711. [DOI] [PubMed] [Google Scholar]
- 31.Mi B. Science. 2014;343:740–742. doi: 10.1126/science.1250247. [DOI] [PubMed] [Google Scholar]
- 32.Fan Z, Yust B, Nellore BOV, Sinha SS, Kanchanapally R, Crouch RA, Pramanik A, Reddy SC, Sardar D, Ray PC. J. Phys. Chem. Lett. 2014;5:3216–3221. doi: 10.1021/jz501402b. [DOI] [PubMed] [Google Scholar]
- 33.Nellore BPV, Kanchanapally R, Pramanik A, Sinha SS, Chavva SR, Hamme A, II, Ray PC. Bioconjugate Chem. 2015 doi: 10.1021/bc500503e. Articles ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao D, Davidson PM, Zhong Q. J. Agric. Food Chem. 2011;59:7393–7404. doi: 10.1021/jf200774v. [DOI] [PubMed] [Google Scholar]
- 35.Knerr PJ, van der Donk WA. Annu. Rev. Biochem. 2012;81:479–505. doi: 10.1146/annurev-biochem-060110-113521. [DOI] [PubMed] [Google Scholar]
- 36.Hsu STD, Breukink E, Tischenko E, Lutters MAG, de Kruijff B, Kaptein R, Bonvin AMJJ, van Nuland NAJ. Nat. Struct. Biol. 2004;11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 37.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. Science. 2006;313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 38.Rink R, Wierenga J, Kuipers A, Kluskens LD, Driessen AJM, Kuipers OP, GN Moll GN. Appl. Environ. Microbiol. 2007;73:5809–5816. doi: 10.1128/AEM.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sofer Z, Pumera. M. Chem. Eur. J. 2014;20:13838–13852. [Google Scholar]
- 40.Akhavan O, Ghaderi E. ACS Nano. 2010;4:5731–5736. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- 41.Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H, Zhou R. Nat. Nanotechnol. 2013;8:594–601. doi: 10.1038/nnano.2013.125. [DOI] [PubMed] [Google Scholar]
- 42.Grisold AJ, Leitner E, Muhlbauer G, Marth E, Kessler HH. J. Clin. Microbiol. 2002;40:2392–2397. doi: 10.1128/JCM.40.7.2392-2397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]