Abstract
Objective
The purpose of this study was to examine the prevalence of posttraumatic stress symptoms (PTSS) in patients with renal cell carcinoma (RCC), the relationships and co-occurrence between PTSS, depressive, and other cancer-related symptoms, and the ability of a single-item distress question to identify patients with PTSS.
Methods
Patients with Stage I-IV RCC completed assessments of depressive symptoms (CES-D), PTSS (IES), cancer-related symptoms (MDASI), fatigue (BFI), and sleep disturbance (PSQI). We used the distress item on the MDASI as a distress screener and general linear model analyses to test study hypotheses.
Results
Of 287 patients (29% stage IV; 42% female; mean age=58 years), 46% (n=131) reported psychiatric symptoms with 15% (n=44) reporting comorbid clinical levels of depressive symptoms and PTSS; 24% (n=70) PTSS alone; and 6% (n=17) depressive symptoms alone. Controlling for age, gender, and stage, patients with comorbid depressive symptoms and PTSS reported more cancer-related symptoms (P<.0001), fatigue (P<.0001), and sleep disturbance (P=.0003) than those with PTSS alone and more cancer-related symptoms (P=.002) and fatigue (P=.09) than those with depressive symptoms alone. Sensitivity analyses revealed that 26.9% of negative cases on the distress item fell within the clinical range of the IES and 9.3% of negative cases met caseness on the CES-D.
Conclusions
PTSS occurred both independently as well as comorbid with depressive symptoms in patients with RCC. PTSS were correlated with overall cancer symptom burden. Single-item distress screening was less sensitive in detecting PTSS than depressive symptoms. Therefore, additional screening strategies are required in the clinical setting.
Keywords: Renal cell carcinoma, posttraumatic stress, depression, distress screening, cancer-related symptoms, psycho-oncology
INTRODUCTION
Anxiety, fear, and stress are ubiquitous reactions to the diagnosis and treatment of cancer, varying in intensity and expression depending on the individual patient. In the clinical setting, the psycho-oncologist routinely evaluates for both depression and anxiety, but until recently, most research has focused on depression alone. After the inclusion of “life-threatening illness” as a qualifying traumatic event for a diagnosis of posttraumatic stress disorder (PTSD) in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [1], the literature began to address the prevalence of PTSD and subthreshold posttraumatic stress symptoms (PTSS) in cancer [2-5]. Of note, the diagnosis of a life-threatening illness no longer meets Criterion A for a traumatic event warranting a diagnosis of PTSD in the newest edition of DSM (DSM-V) [6]. Rather, PTSS presenting in the context of cancer diagnosis and treatment would merit a diagnosis of adjustment disorder unless a sudden, catastrophic medical event occurred in the process. Nevertheless, treatment experiences endured by an individual with cancer may precipitate the expression or exacerbation of PTSS even in the absence of a DSM-V qualifying traumatic medical event.
Despite comparable prevalence estimates, the depth of our understanding of PTSS in the cancer setting lags behind what we know about depressive symptoms. One study found in a sample of 236 newly diagnosed Stage I-III breast cancer patients, 11% screened positive for major depression and 10% for PTSD, highlighting the need for further study of PTSS [7]. Of note, both depression and posttraumatic stress may occur in this population at subthreshold levels that have demonstrated clinical significance without meeting full diagnostic criteria. Up to this point, the literature has only minimally attempted to tease out whether PTSS occur as a distinct clinical entity from depressive symptoms. Understanding the rate of co-occurrence is of key importance because comorbidity may compound the level of emotional distress and require different treatment approaches. In addition, if depression becomes the sole focus of intervention, comorbid PTSS may remain unaddressed without a clear understanding of the co-occurrence of these symptoms.
In the oncology setting, concern for the identification of psychiatric symptoms is underscored by the connection that has been established in prior studies between depression and cancer-related symptoms (e.g., fatigue, sleep disturbance, pain, cognitive dysfunction) [8]. Activation of inflammatory responses regulated by the neuroendocrine system is described as a potential common physiological pathway linking these symptoms [9]. Recent studies reveal an association between inflammatory markers and PTSD as well [10]. An association between PTSS and cancer-related symptoms, occurring alone or as a compounding factor with comorbid depressive symptoms, has not been examined. These physiological alterations may even impact survival outcomes in an immune-responsive cancer like RCC, making it a particularly appropriate population for further study [11].
Due to overwhelming evidence that anxiety and depression are prevalent, potentially treatable factors manifesting as distress in cancer patients, efforts have long been underway to utilize brief screening tools in the oncology setting to identify those with emotional distress, culminating in the development of the single item Distress Thermometer [12]. Following numerous validation studies, the National Comprehensive Cancer Network (NCCN) recommends this tool be used routinely for distress screening to identify patients in need of mental health referral and intervention at the time of cancer diagnosis, throughout treatment, and into survivorship [13]. Sensitivity of the Distress Thermometer employing a cutoff score of ≥4 (0-to-10 visual analogue scale) to detect the presence of psychological distress approximates 80% [14].
The Institute of Medicine of the National Academies of Sciences’ report “Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs” specifically points to the need to screen for the development of PTSS in the cancer setting [15]. A recent validation study of the Distress Thermometer in prostate cancer demonstrated high sensitivity in detecting PTSS when compared against the Impact of Event Scale-Revised (IES-R) [16]. However, an evaluation of the ability of single-item distress screening to identify PTSS compared to depressive symptoms in another study population has yet to be completed. This is an essential step in establishing a quick, practical screening tool for PTSS in the oncology clinic.
To better understand the impact of PTSS and our ability to detect its presence, the purpose of this study was to investigate the prevalence of PTSS, with and without comorbid depressive symptoms, in patients with renal cell carcinoma (RCC) and to examine the relationship between these symptoms and other cancer-related symptoms. RCC is a particularly suitable study population as it affects both genders, is immune-responsive, and has been the subject of few psychosocial studies to date. We hypothesized that PTSS will emerge as a distinct clinical entity, occurring both alone and along with comorbid depressive symptoms, and that PTSS will be independently correlated with higher cancer-related symptom burden, with comorbid PTSS and depressive symptoms having the highest cancer-related symptom burden. Second, this study examines the ability of a single-item distress question to identify PTSS and depressive symptoms. We hypothesized that this screening item would be less sensitive in identifying PTSS than depressive symptoms.
PATIENTS AND METHODS
Study Eligibility
The current study involves the baseline data of a randomized controlled trial evaluating the benefits of an expressive writing intervention on quality of life outcomes [17]. Patients with stage I-IV renal cell carcinoma, a Zubrod performance status [18] of ≤ 2, no serious intercurrent medical illness requiring hospitalization, and who were at least 18 years old and able to read, write, and speak English were eligible to participate in the study. Patients who were unable to provide consent, on immunosuppressive drugs, currently participating in psychotherapy, and/or had a history of primary or secondary immunodeficiency were excluded from the study.
Procedures
Research staff identified eligible patients and approached them during a pretreatment clinic visit with the patient’s surgeon or medical oncologist. After providing informed consent, patients completed baseline measures either approximately 1 week prior to their surgical procedure or at the time of their initial consult prior to receiving systemic treatment. Participants received a $20 gift card for completing the assessment. In the larger study, participants completed three additional assessments. The Institutional Review Board at MD Anderson Cancer Center approved the protocol.
Measures
Demographic and Medical Factors
Some demographic items (e.g., age, marital status, educational background) were included in the questionnaires. Medical data were extracted from patients' electronic medical records.
Psychiatric Risk Factors
Depressive Symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item self-report measure of depression that focuses on depressive feelings and behaviors over the past week, with higher scores representing worse symptoms [19]. A cutoff score of ≥16 indicates “caseness” warranting further psychological evaluation for clinical depression.
Posttraumatic Stress Symptoms were measured with the Impact of Events Scale (IES), a 15-item self-report scale assessing thought intrusion and avoidance during the past week [20]. Participants were instructed to “think about their kidney cancer” when answering the items. Higher scores represent greater intrusion and avoidance. A cutoff score of ≥19 has been identified as a clinically relevant screener for PTSD [21].
Cancer Symptoms
Overall cancer-related symptoms were assessed with the MD Anderson Symptom Inventory (MDASI) [22]. The MDASI consists of 13 core symptoms that are common across all cancer diagnoses and treatments. Patients rate symptom severity and interference with daily activities. Higher scores denote greater severity and interference. Importantly, because the purpose of the current study is to examine the associations between psychiatric and cancer-related symptoms, we excluded the “sad” and “distressed” items from the symptom severity subscale to reduce measurement confounds. Additionally, similar to a previous study, we used the “distressed” symptom severity item ranging from 0 (“not present”) to 10 (“as bad you can imagine”) as a single-item psychiatric symptom screener with a cutoff score of 4 [23].
Fatigue was measured with the Brief Fatigue Inventory (BFI), a nine-item questionnaire asking participants to rate the severity of their fatigue in the last 24 hours and how much it interfered with their lives [24]. Higher scores represent worse fatigue.
Sleep disturbance. The Pittsburgh Sleep Quality Index (PSQI), an 18-item self-rated questionnaire was used to assess sleeping problems over the past month [25]. The instrument includes seven subscales and a total score. We report here on the total score. Higher scores represent greater problems with sleep.
Data Analyses
All analyses were performed with SAS (9.2.2 version; Cary, NC). We calculated descriptive statistics to characterize the sample. To test our main hypothesis, we factored participants into four psychiatric symptom groups using clinical cutoff scores of CES-D ≥16 and IES ≥19: comorbid PTSS and depressive symptoms; PTSS symptoms alone; depressive symptoms alone; and neither PTSS nor depressive symptoms. Then, using separate general linear model (GLM) analyses and planned contrast comparisons, we examined the association between the psychiatric symptom groups and each cancer symptom index (e.g., overall cancer symptoms, fatigue, and sleep disturbance). Because age, gender, and stage at diagnosis have been associated with patient-reported outcomes in RCC, we included these factors as a priori covariates in all main analyses [26].
To explore if a single-item distress question is sensitive to identify not only patients with elevated depressive symptoms but also PTSS, we examined the correspondence between positive cases based on the MDASI distressed item (score of ≥4 on a 0-10 scale) and the CES-D and the IES clinical cutoff scores (scores of ≥16 and ≥19, respectively).
RESULTS
Sample Characteristics and Descriptive Results
We approached 761 eligible patients, of whom 355 consented to participate in the study, with 68 withdrawing prior to any assessment leaving a sample size of 287 participants. Consenters withdrew because of death in family (n=2); too busy (n=3), changed their mind (n=24) or were unable to be reached after repeated attempts (n=39). Patients who refused to provide consent for participating in the expressive writing intervention expressed a general lack of interest (n=326), schedule conflicts (n=69) or physical limitations (e.g., tremors, carpal tunnel syndrome; n=11). Chi-square tests comparing demographic and medical characteristics study completers versus non-completers revealed no significant differences except for education (χ2=14.63, P=.005); completers were more likely to have had higher education compared to non-completers.
Demographic and medical characteristics of the sample are shown in Table 1. Patients were predominately White (79%), married (71%), well-educated (50% had some college education or more), employed (62%) and had a mean age of 58 years. Women and patients with advanced disease were well represented (42% and 46%, respectively). Table 2 presents means, standard deviations, and correlations of study variables. As expected, there was a significant correlation between all the measures.
Table 1.
Participant Demographics and Clinical Characteristics
| Variable | N=287 |
|---|---|
| Mean age, years ±SD (range) | 58.1±9.8 (31-84) |
| Male, n (%) | 169 (58.3) |
| Ethnicity, n (%) | |
| White/Caucasian | 228 (78.6) |
| Hispanic/Latino | 30 (10.3) |
| African-American/B1ack | 9 (3.1) |
| Asian/Pacific Islander | -- |
| Native American | 6 (2.1) |
| Other | 9 (3.1) |
| Missing | 8 (2.8) |
| Marital Status, n (%) | |
| Married | 206 (71%) |
| Highest Level of Education, n (%) | |
| Some college or higher | 136 (49.1) |
| Income, n (%) | |
| 50,000 ≤ | 37 (13.1) |
| 50,000 ≥ | 233 (82.6 |
| Missing | 20 (7.1) |
| Employment Status, n (%) | |
| Full-time | 157 (54.7) |
| Part-time | 21 (7.4) |
| Unemployed | 9 (3.2) |
| Retired | 93 (32.4) |
| Missing | 10 (3.5) |
| Group Stage a n (%) | |
| I | 99 (34.4) |
| II | 36 (12.5) |
| III | 51 (17.7) |
| IV | 82 (28.6) |
| Missing | 19 (6.6) |
| Time Since Diagnosis, days ±SD (range) |
82.5 ± 60.72 (0-273) |
| Surgery, n (%) | |
| Yes | 195 (70.3) |
| Systemic Treatment, n (%) | |
| Yes | 103 (37.2) |
| Cell Type, n (%) | |
| Clear cell | 215 (80.2) |
Note:
Group stage is based on the 2002 TNM staging of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) as suggested by Ng et al.[41] Abbreviations: EW, emotional writing; NW, neutral writing.
Table 2.
Means, Standard Deviations, Ranges, and Correlation Coefficients for Study Variables
| Variable | Mean ± SD (range) | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|
| 1. |
CESD
(n=283) |
10.7 ± 9.3 (0-45) | ||||
| 2. |
IES
(n=255) |
18.7 ± 15.1 (0-62) | .56* | |||
| 3. |
MDASI-tot≠
(n=281) |
1.5 ± 1.5 (0-6.6) | .63* | .48* | ||
| 4. |
BFI
(n=283) |
2.6 ± 2.2 (0-9) | .55* | .38* | .80* | |
| 5. |
PSQI
(n=240) |
6.9 ± 4.1 (0-18) | .46* | .36* | .52* | .49* |
Note.
p<0.001; CES-D, Center for Epidemiological Studies-Depression Scale; IES, Impact of Events Scale; MDASI-tot, MD Anderson Symptom Inventory-Total Scale; BFI, Brief Fatigue Inventory PSQI, Pittsburgh Sleep Quality Index;
excluding the “sadness” and “distress” items;
Regarding psychiatric symptom grouping, 15.2% were identified as having comorbid PTSS and depressive symptoms; 24.1% PTSS alone; 5.9% depressive symptoms alone; and 54.8% scored below scale cutoffs for PTSS and depressive symptoms.
Psychiatric and Cancer-Related Symptoms
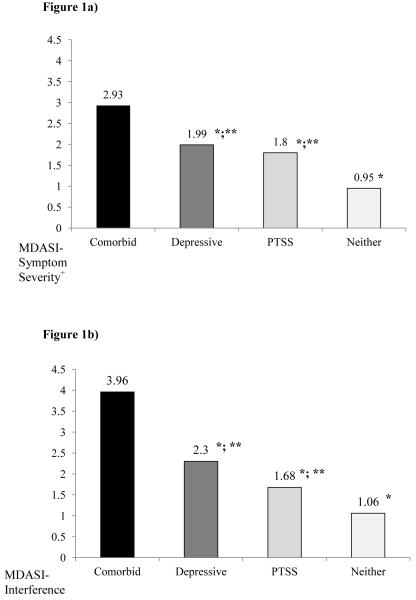
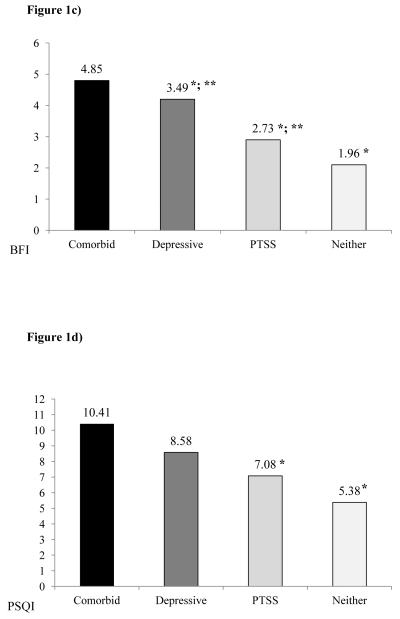
Least square means (adjusted for age, gender, and stage) by psychiatric symptom grouping are presented in Figure 1a-d.
Figure 1.
Least square means for the associations between the four psychiatric groups of comorbid symptoms (comorbid), posttraumatic symptoms alone (PTSS), depressive symptoms alone (depressive), or neither; and a) cancer-related symptoms severity (MDASI-Symptom Severity), cancer-related symptoms interference (MDASI-Interference), fatigue (BFI), and sleep disturbances (PSQI). +, denotes excluding “sadness” and “distress” items. *, denotes a significant difference from the comorbid group at P<0.05; **, significant difference from the neither PTSS nor depressive symptom group at P<0.05.
Overall cancer-related symptoms. Psychiatric symptom grouping was significantly associated with cancer-related symptom severity as measured by MDASI symptom severity subscale (F=25.65, P<0.0001). Contrast comparisons revealed that patients with comorbid PTSS and depressive symptoms had significantly worse overall cancer-related symptom severity than those with PTSS alone (F=34.74, P<0.0001), depressive symptoms alone (F=6.82, P<0.001) and neither PTSS nor depressive symptoms (F=73.70, P<0.0001). However, patients with PTSS alone did not significantly differ from those with depressive symptoms alone (F=2.64P=0.11). Additionally, those with PTSS alone (F=6.27, P<0.01) and those with depressive symptoms alone (F=10.45, P=0.001) reported significantly greater symptom severity than those with neither PTSS nor depressive symptoms (see Figure 1a).
Psychiatric symptom grouping was also significantly associated with cancer-related symptom interference (F=25.20, P<0.0001). Contrast comparisons revealed that patients with comorbid PTSS and depressive symptoms had significantly worse cancer-related symptom interference than those with PTSS alone (F=37.09, P<0.0001), depressive symptoms alone (F=9.87, P<0.01) and neither PTSS nor depressive symptoms (F=74.08, P<0.0001). Those with depressive symptoms alone did not report more interference than patients with PTSS alone (F=1.55, P=.21). Those with PTSS alone (F=4.89, P<.05) and those with depressive symptoms alone (F=7.05, P<0.01) reported significantly greater symptom severity than those with neither PTSS nor depressive symptoms (see Figure 1b).
Fatigue. Psychiatric symptom grouping was also significantly associated with BFI scores (F=22.49, P<0.0001). Based on contrast comparisons, patients with comorbid PTSS and depressive symptoms had significantly worse fatigue than those with PTSS alone (F=28.28P<.0001), depressive symptoms alone (F=5.81, P<0.05) and those without PTSS and depressive symptoms (F=64.47, P<.0001). In addition, those with depressive symptoms alone (F=9.36, P<0.01) and PTSS alone (F=6.80, P<0.01) had significantly worse fatigue than those with neither PTSS nor depressive symptoms. Patients with PTSS alone did not significantly differ from those with depression alone (F=2.00, P=0.16) regarding fatigue (see Figure 1c).
Sleep disturbance. Lastly, psychiatric symptom grouping was significantly associated with PSQI scores (F=11.32, P<0.001). Contrast comparisons revealed that patients with comorbid PTSS and depressive symptoms reported greater sleep disturbance compared to those with PTSS alone (F=13.71, P<0.001) and those without PTSS and depressive symptoms (F=31.54, P<0.0001), but not compared to those with depressive symptoms alone (F=2.08, P=0.15). Further, patients with PTSS alone did not significantly differ from those with depression alone (F=1.57, P=0.21) regarding sleep disturbance (see Figure 1d).
Single-Item Distress Screening
Sensitivity analyses revealed that 69.7% positive cases on the single-item distress question also scored within the clinical cutoff range of PTSS on the IES. However, 26.9% of negative cases on the distress item fell within the clinical range of the IES. Only 3.5% of positive distress cases scored below the clinical cutoff on the IES. Regarding depressive symptoms, 86.5% positive cases on the single-item distress screener met the caseness criterion of the CES-D, 9.3% of negative cases on the distress item actually met caseness on the CES-D, and only 4.1% of positive cases on the distress screener fell below the CES-D caseness criterion.
DISCUSSION
This study is the first to our knowledge to examine the association between PTSS and depressive symptoms and other cancer-related symptoms in the psychosocially understudied population of RCC patients. The first aim of the study was to investigate the prevalence of PTSS and depressive symptoms, occurring together and independently. The findings confirmed our hypothesis that PTSS are indeed prevalent in patients with RCC. Importantly, PTSS represent a clinical entity distinct from depressive symptoms, and they also co-occur with depression in a significant fraction of patients. Separating out PTSS from depressive symptoms was important since they may be otherwise indistinguishable when detected on single-item distress screening.
The application of various scales and cutoff scores has produced a wide range of PTSD and PTSS prevalence estimates in the literature. This study revealed prevalence of PTSS on the higher end of that range reported for other cancer populations (3-45%) [3, 4]. Almost half of the participants scored above scale cutoffs for PTSS and/or depressive symptoms, with nearly 40% meeting the case criterion for PTSS. One potential explanation is our selection of a lower scale cutoff for the IES than other studies in order to capture subthreshold PTSS that may not meet full criteria for PTSD [27, 28]. A recent study on oncology outpatients examining prevalence of PTSD and partial PTSD reported similar prevalence as the current study [3]. Compared to previous studies, depressive symptom prevalence estimates appear similar at 21.5% [29].
The second aim of the study was to examine the association between PTSS and depressive symptoms, occurring independently or together, and cancer-related symptoms. We hypothesized that comorbid PTSS and depressive symptoms would be associated with higher overall cancer-related symptoms, fatigue, and sleep disturbance, and this was indeed the result of our analysis. The group with comorbid PTSS and depressive symptoms had the strongest association with cancer-related symptoms compared to all other groups, with the exception of sleep disturbance when compared to the depressive symptoms alone group. In addition, having PTSS and depressive symptoms alone was also associated with worse overall symptoms than those patients reporting neither. These findings may be attributable to heightened inflammation and other neuroendocrine dysregulation in those patients with comorbid psychiatric symptomatology. Previous studies have shown an impact of PTSS on quality of life and functional status [2, 3, 7, 30], but none to date have examined the association with cancer-related symptoms nor teased out PTSS from depressive symptoms in the analysis.
Given the significant association between PTSS and cancer symptom burden when occurring alone or when comorbid with depressive symptoms, and in light of the growing use of single-item distress screening in oncology settings, the second part of the study aimed to examine the ability of such single-item screening to identify PTSS. Numerous previous studies have validated the Distress Thermometer against structured psychiatric interviews and psychiatric scale assessments [31], generally evaluating its ability to detect “distress” defined as a mix of psychiatric symptomatology including symptoms of depression and anxiety. As hypothesized, the current study revealed a lower sensitivity for detection of PTSS (70%) compared to depressive symptoms (87%). This resulted in nearly a 27% false-negative rate for PTSS cases. These results are similar to those reported in a study of presurgical breast cancer patients (sensitivity of 71% for PTSD and 96% for depression) [7]. However, our results contrast with another study reporting the sensitivity of the baseline Distress Thermometer against the IES-R of 86.1% in prostate cancer patients [16]. The difference could be due to the fact that our study utilized a lower cutoff score for PTSS and had a significantly higher percentage of distressed participants. Also, our study did not employ the Distress Thermometer itself, but rather the distress item on the MDASI. However, our depression sensitivity is similar to or better than those already established in the Distress Thermometer literature [14], suggesting that the distress item we used sufficed as a reasonable substitute for depression screening.
There are several potential implications of these findings. Cancer outcomes, including the cancer-related symptoms described in this study, and even cancer recurrence and survival, could be negatively impacted if PTSS remain untreated. Growing evidence suggests that depressive symptoms are associated with decreased survival in cancer overall, although debate remains in the literature around this issue [32, 33]. One particular study in advanced renal cell carcinoma identified a link between depression and survival outcomes in this immune-responsive cancer [11]. PTSS may similarly influence cancer outcomes via direct effects on key physiological pathways, as suggested by work in animal cancer models demonstrating “stress” promoting tumor progression and metastasis through adrenergic stimulation [34, 35]. Individuals with PTSD have neuroendocrine dysregulation resulting in immune system alterations and increased circulating inflammatory markers, and they are at heightened risk for coronary atherosclerosis, mortality in cardiovascular disease, insulin resistance, and metabolic syndrome [36]. Finally, similar to depression, PTSS may be associated with negative health behaviors, such as smoking, alcohol consumption, increased rates of obesity, and medication nonadherence, further compounding the risk for negative medical outcomes [37, 38].
These findings underscore the importance of identifying PTSS in oncology clinics and capturing this state in psychosocial research, as this symptom spectrum has thus far been relatively neglected in spite of its high prevalence. Although single-item distress screening is of tremendous value due to its efficiency and validity in identifying distressed cancer patients, this study shows that as many as 25% of patients with significant PTSS may not be identified with this method of screening. Short scales like the 4-item self-administered Primary Care PTSD Screen (PC-PTSD) may be more useful in identifying PTSS in an oncology setting than the DT alone and can be given at key points during the treatment trajectory, particularly around the time of diagnosis [2, 39]. Additional methods for identifying patients with PTSS need to be validated and employed in oncology, especially for high risk populations, such as veterans, minorities, young and lower socioeconomic status patients [27, 28].
In RCC patients identified with PTSS, it would be helpful to understand more about the events or treatment-related experiences that triggered the development of PTSS. This could enable providers to identify preventative strategies or provide additional support during potentially traumatizing time points over the treatment course. Future research aimed at examining the efficacy of newly developed or existing treatments for PTSD in RCC patients should also include any impact on associated depressive and other cancer-related symptoms. Clinical psycho-oncologists are reminded by this study to evaluate referred patients for these symptoms with specific questioning regarding risk factors and history of trauma. From the psychiatric literature we know that PTSD is associated with substance abuse and increased risk of suicidal ideation [40]. Patients with posttraumatic stress symptoms often require a tailored intervention approach by both the psychotherapist and psychopharmacologist.
It is important to note several limitations of this study. First, this study is a secondary analysis of data acquired for an expressive writing intervention that excluded participants currently receiving psychological (non-pharmacological) interventions. Although selection bias could have affected the results either direction, with distressed patients being more or less interested in participating in the study, the exclusion of those seeking psychotherapy may strengthen the conclusions since associations were found without data from patients already seeking treatment, presumably for psychiatric symptoms. As patients with lower level of education were more likely to withdraw from the study, our sample may potentially reveal a selection bias regarding education and our results may not generalize towards less educated patients. Self-administered rating scale scores were utilized instead of structured clinical interviews, and low but validated cutoffs were chosen for the IES and single distress item. However, this sacrifice of diagnostic accuracy was purposeful in order to include subthreshold symptomatology. Again, the fact that significant correlations were still found between psychiatric and cancer-related symptoms strengthens the conclusions. The data analyzed in this study represent a one-time, cross-sectional assessment; therefore the long-term trajectory of PTSS and depressive symptoms cannot be described from these results. Moreover, eligible patients
Although used in numerous studies of PTSD in cancer, the IES assesses only two of the now four symptom clusters defined in DSM-V PTSD diagnostic criteria, intrusion and avoidance. Whereas one of the strengths of this study is the relatively under-examined RCC population, this takes away from the generalizability of the findings. In addition, the demographic profile of the participants was largely white, older, and middle socioeconomic class. On the other hand, unlike previous studies with similar large samples of patients with the same cancer diagnosis (breast, prostate), both genders were represented in this study.
In conclusion, this study of posttraumatic and depressive symptoms in a large sample of RCC patients within six months of diagnosis revealed PTSS as a distinct, prevalent clinical entity occurring independently and comorbid with depressive symptoms. A relationship between all psychiatric symptoms groups and cancer-related symptoms was revealed, and the potential association of untreated PTSS on other cancer outcomes was described. Single-item distress screening was less sensitive in detecting PTSS then depressive symptoms, and additional screening strategies are recommended.
Acknowledgments
Grant Support: This research was supported in part by NCI grant R01CA090966 (PI, Lorenzo Cohen, Ph.D.)
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
REFERENCES
- 1.Diagnostic and Statistical Manual of Mental Disorders DSM-IV-Text Revision. 4th American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Wachen JS, et al. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014 doi: 10.1002/pon.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold JI, et al. The relationship between posttraumatic stress disorder, mood states, functional status, and quality of life in oncology outpatients. J Pain Symptom Manage. 2012;44(4):520–31. doi: 10.1016/j.jpainsymman.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Kangas M, Henry JL, Bryant RA. Posttraumatic stress disorder following cancer. A conceptual and empirical review. Clin Psychol Rev. 2002;22(4):499–524. doi: 10.1016/s0272-7358(01)00118-0. [DOI] [PubMed] [Google Scholar]
- 5.Shelby RA, Golden-Kreutz DM, Andersen BL. PTSD diagnoses, subsyndromal symptoms, and comorbidities contribute to impairments for breast cancer survivors. J Trauma Stress. 2008;21(2):165–72. doi: 10.1002/jts.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th. American Psychiatric Association; Washington, DC: 2013. [DOI] [PubMed] [Google Scholar]
- 7.Hegel MT, et al. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006;107(12):2924–31. doi: 10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 8.Lee BN, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–92. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 9.Miller AH, et al. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–82. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraly SA, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423–31. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen L, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7(8):e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth AJ, et al. Rapid screening for psychologic distress in men with prostate carcinoma. Cancer. 1998;82(10):1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Snowden A, et al. The clinical utility of the distress thermometer: a review. Br J Nurs. 2011;20(4):220–7. doi: 10.12968/bjon.2011.20.4.220. [DOI] [PubMed] [Google Scholar]
- 14.Grassi L, et al. Screening for distress in cancer patients. Cancer. 2012 [Google Scholar]
- 15.Holland J, Weiss T. The new standard of quality cancer care: integrating the psychosocial aspects in routine cancer from diagnosis through survivorship. Cancer J. 2008;14(6):425–8. doi: 10.1097/PPO.0b013e31818d8934. [DOI] [PubMed] [Google Scholar]
- 16.Chambers SK, et al. The validity of the distress thermometer in prostate cancer populations. Psychooncology. 2014;23(2):195–203. doi: 10.1002/pon.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milbury K, et al. Randomized controlled trial of expressive writing for patients with renal cell carcinoma. J Clin Oncol. 2014;32(7):663–70. doi: 10.1200/JCO.2013.50.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zubrod CG, Frei E, Brindley C, Gold GL, Shnider B, Oviedo R, Gorman J, Jones R, Jr, Colsky UJJ, Chalmers T, Ferguson B, Dederick M, Holland J, Selawry O, Regelson W, Lasagna L, Owens AH., Jr Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chron Dis. 1960;11:17–33. S.M. [Google Scholar]
- 19.Radloff L. The CES-D Scale: A new self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;(1):385–401. [Google Scholar]
- 20.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Wohlfarth TD, et al. Screening for Posttraumatic Stress Disorder: an evaluation of two self-report scales among crime victims. Psychol Assess. 2003;15(1):101–9. doi: 10.1037/1040-3590.15.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Cleeland CS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Jones D, et al. Screening for depressed mood in patients with cancer using the MD anderson symptom inventory: investigation of a practical approach for the oncologist. J Oncol Pract. 2014;10(2):e95–e102. doi: 10.1200/JOP.2013.001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendoza TR, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Stark D, et al. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. J Clin Oncol. 2002;20(14):3137–48. doi: 10.1200/JCO.2002.08.549. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor M, et al. How traumatic is breast cancer? Post-traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. Br J Cancer. 2011;104(3):419–26. doi: 10.1038/sj.bjc.6606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vin-Raviv N, et al. Racial disparities in posttraumatic stress after diagnosis of localized breast cancer: the BQUAL study. J Natl Cancer Inst. 2013;105(8):563–72. doi: 10.1093/jnci/djt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirl WF, Roth AJ. Diagnosis and treatment of depression in cancer patients. Oncology (Williston Park) 1999;13(9):1293–301. discussion 1301-2, 1305-6. [PubMed] [Google Scholar]
- 30.Amir M, Ramati A. Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: a preliminary research. J Anxiety Disord. 2002;16(2):195–206. doi: 10.1016/s0887-6185(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8(4):487–94. doi: 10.6004/jnccn.2010.0035. [DOI] [PubMed] [Google Scholar]
- 32.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 33.Giese-Davis J, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–20. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 35.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28(26):4094–9. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25(1):6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Dedert EA, et al. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39(1):61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kronish IM, et al. Posttraumatic stress disorder and medication nonadherence in patients with uncontrolled hypertension. JAMA Intern Med. 2014;174(3):468–70. doi: 10.1001/jamainternmed.2013.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French-Rosas LN, Moye J, Naik AD. Improving the recognition and treatment of cancer-related posttraumatic stress disorder. J Psychiatr Pract. 2011;17(4):270–6. doi: 10.1097/01.pra.0000400264.30043.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer RJ, et al. Clinical correlates of suicidal thoughts in patients with advanced cancer. Am J Geriatr Psychiatry. 2012;20(4):327–36. doi: 10.1097/JGP.0b013e318233171a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng CS, et al. Renal cell carcinoma: diagnosis, staging, and surveillance. AJR Am J Roentgenol. 2008;191(4):1220–32. doi: 10.2214/AJR.07.3568. [DOI] [PubMed] [Google Scholar]