Abstract
Objective
The objectives were to: (1) describe the characteristics of a large ethnically/racially and geographically diverse population of adolescents with recent onset type 2 diabetes; and (2) assess effects of short-term diabetes education and treatment with metformin on clinical and biochemical parameters in this cohort.
Research Design and Methods
Descriptive characteristics were determined for subjects screened for TODAY who met criteria for diagnosis of type 2 diabetes (n=1092). Changes in clinical and biochemical parameters were determined for those who completed at least 8 weeks of the run-in phase of the trial, which included standardized diabetes education and treatment with metformin. Further analysis determined whether these changes differed according to treatment at screening.
Main Outcome Measures
Demographic, biochemical measurements, and anthropometrics at screening and changes over 8 weeks of run-in.
Results
Subjects screened for TODAY had a median age of 14 years and median hemoglobin A1c (HbA1c) of 6.9% (52 mmol/mol), 2/3 were female, and ethnic/racial minorities were overrepresented. Dyslipidemia and hypertension were common comorbidities. During run-in, HbA1c, body mass index, low-density lipoprotein cholesterol, triglycerides, and blood pressure significantly improved. Nearly all participants on insulin at screening were able to attain target HbA1c following insulin discontinuation.
Conclusions
Treatment with metformin and diabetes education provided short-term improvements in glycemic control and cardiometabolic risk factors in a large adolescent T2D cohort. Nearly all insulin-treated youth could be successfully weaned off insulin with continued improvement in glycemic control.
Keywords: insulin therapy, metformin, diabetes education
Introduction
Although it is still considered relatively uncommon in children, type 2 diabetes (T2D) has become recognized as a disease with increasingly significant impact in youth (1, 2), particularly among racial/ethnic minorities; T2D represents more than half of all newly diagnosed cases of diabetes in Black, Hispanic, Asian/Pacific Islander, and Native American youth aged 10-19 years (1, 3). The potential impact of the early development of T2D on future morbidity/mortality due to associated macro- and microvascular complications is significant (4).
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study was designed to evaluate three treatments for T2D in youth 10-17 years old: metformin, metformin + lifestyle intervention, and metformin + rosiglitazone. The intervention has been described in detail (5) and primary outcome results have been published (6). Prior to randomization, potential subjects participated in a run-in phase focused on establishing consistent dosing of metformin, delivering standard diabetes education, and weaning insulin in individuals who were receiving it at the time of screening. We previously compared the characteristics of those who successfully completed run-in and those who did not, including change in hemoglobin A1c (HbA1c) and body mass index (BMI) (7). The objectives of the current secondary analyses were to: (1) provide a more complete description of a large geographically and ethnically diverse cohort of youth with recent onset T2D receiving routine clinical care in the US, (2) assess the effect of short-term (8 weeks) standardized diabetes management, including education and treatment with metformin, on clinical and biochemical parameters, and (3) identify potential predictors of improvement in HbA1c and BMI during this short-term intervention.
Methods
Study Design
Details of screening for TODAY and the run-in phase were previously described (5, 7). Briefly, subjects were identified from the participating pediatric diabetes centers and partners. Public advertisements were also used, but resulted in very few identified participants. Pancreatic antibody-negative (glutamic acid decarboxylase-65 and tyrosine phosphatase) and c-peptide-positive youth ages 10-17 years who had < 2 years’ duration of T2D were eligible for inclusion in the study; therefore, T2D diagnosis was consistent with the recently published guidelines for diabetes classification in youth (8). At screening, diabetes medications were recorded and anthropometrics (height, weight, and BMI), blood pressure (BP), and physical examination were performed. BMI z-score was calculated based on age- and gender-stratified normative data. Family history of T2D and gestational diabetes was collected by questionnaire. Fasting blood samples were obtained for measurement of total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and c-peptide. All assays were performed at a centralized laboratory using standard biochemical assays (see www.todaystudy.org) (5). Since all participants were overweight or obese (BMI > 85th percentile for sex and age) and had diabetes, participants were classified as having metabolic syndrome if they also had either dyslipidemia (high total or low HDL cholesterol, according to sex-, race-, and age-based cut-offs) and/or hypertension (BP > 95th percentile for age, sex, and height). Elevated liver transaminases were defined as ≥ 1.5 times the upper limit of normal (ALT: female ≥ 75 and male ≥ 97.5; AST: female ≥ 69 and male ≥ 97.5 U/L).
During the run-in period, participants and an identified and consenting adult family member were given one-on-one standardized diabetes education, including diabetes pathophysiology, medication action, lifestyle guidelines, diabetes self-care, and goal-setting (9). The metformin dose was titrated as tolerated to a maximum dose of 1000 mg twice daily, other oral diabetes medications were discontinued, and insulin was tapered with the goal of discontinuation. Laboratory studies, anthropometrics, and BP measurements were repeated at subsequent run-in visits. In order to successfully complete run-in, participants were required to demonstrate ≥ 80% medication adherence for at least 6 weeks, tolerate metformin at a dose of at least 500 mg twice daily, miss no more than two run-in visits, maintain a HbA1c of ≤ 8% (64 mmol/mol) for at least 2 months on metformin alone, and demonstrate mastery of basic diabetes education. The run-in period lasted from 2-6 months during which time participants had a minimum of 6 and maximum of 12 visits for mastery of diabetes education (9); the median time in run-in for all those screened was 2.4 months (25th and 75th percentiles = 2.0 and 3.0 months, respectively) (7). A total of 228 screened participants (24%) failed to meet criteria for randomization and their characteristics have been previously described (7). The study protocol was approved by the institutional review board of each participating institution. Parents of participants provided written informed consent and all children and adolescents provided assent according to local guidelines.
Statistical analyses
To describe characteristics of T2D in recently diagnosed youth in routine clinical care and before any TODAY interventions were undertaken (objective 1), all diabetes antibody-negative subjects screened for the TODAY study (n=1092) were included. To describe changes in subject characteristics during run-in, those who completed at least 8 weeks of run-in were included (n=814), regardless of whether they were ultimately randomized.
For comparisons between race/ethnicity groups and medication groups, Kruskal-Wallis tests were used for continuous variables and χ2 tests were used for categorical variables. Only non-Hispanic Whites, Blacks, and Hispanic Whites were included in the racial/ethnic comparisons, as subject numbers in other racial/ethnic groups were inadequate for meaningful analysis. For adjusted comparisons, p-values from the F-test are reported. McNemar’s test was used to compare the change in the proportion of participants with cardiometabolic risk factors from screening to the end of run-in. Linear models were used to evaluate potential predictors of change in HbA1c and BMI z-score during the run-in period (SAS PROC GLM, version 9.2, SAS Institute Inc., Cary, NC). The TODAY study was powered for the primary outcome only; the outcomes reported herein are considered exploratory. P-values < 0.05 are considered statistically significant with no adjustment for multiple testing.
Results
Descriptive characteristics
Characteristics of this cohort of youth with recently diagnosed T2D prior to any TODAY intervention can be found in Table 1, which represents all antibody negative youth screened in TODAY. The median duration of disease at the time of screening visit was 2 months. Briefly, subjects were in mid-late puberty and two-thirds were female. The median HbA1c was 6.9% (52 mmol/mol) (range 4.5-17.4 [26-167 mmol/mol]) and 60% of subjects had an HbA1c ≤ 7.5% (58 mmol/mol). Almost half (45%) were being treated with metformin alone, and most of the remainder was either not on medication (13.6%), on insulin alone (12.5%), or on a combination of insulin and metformin (25.7%). Very few (3%) were being treated with medications other than insulin and metformin and were grouped together in Table 1 due to small sample size. Median values for lipids, BP, and liver enzymes were normal. However, 60% had dyslipidemia and 17% had hypertension, while a smaller proportion (8.2%) had elevated liver enzymes. Over 65% of participants were characterized as having the metabolic syndrome.
Table 1.
Continuous and categorical measures at screening by race/ethnicity for all youth screened
| Race/Ethnicity | p-value* | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | White | Black | Hispanic | ||||||||||||||
| CONTINUOUS MEASURES | N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | |
| Age (yr) | 1092 | 14 | 13 | 16 | 207 | 14 | 13 | 16 | 397 | 14 | 12 | 16 | 377 | 14 | 13 | 16 | 0.1341 |
| Duration of T2D at screening (mo) | 1047 | 2 | 1 | 6 | 201 | 2 | 1 | 5 | 379 | 2 | 1 | 7 | 361 | 2 | 1 | 7 | 0.4313 |
| BMI (kg/m2) | 1085 | 34.9 | 30.4 | 39.9 | 207 | 34.4 | 30.4 | 39.2 | 390 | 35.6 | 31.5 | 40.8 | 377 | 34.3 | 29.8 | 39.4 | 0.0026 |
| BMI Z-score | 1085 | 2.4 | 2.1 | 2.6 | 207 | 2.3 | 2.0 | 2.6 | 390 | 2.4 | 2.2 | 2.6 | 377 | 2.4 | 2.0 | 2.6 | 0.0034 |
| C-peptide (ng/mL) | 1087 | 3.8 | 2.6 | 5.2 | 207 | 4.1 | 2.8 | 5.4 | 395 | 3.3 | 2.4 | 4.4 | 376 | 4.0 | 2.8 | 5.6 | <0.0001 |
| HbA1c (%) | 1087 | 6.9 | 6.0 | 8.9 | 207 | 6.3 | 5.8 | 7.6 | 395 | 7.2 | 6.2 | 9.6 | 376 | 7.0 | 6.0 | 8.8 | <0.0001 |
| Total cholesterol (mg/dL) | 1087 | 157.0 | 135.0 | 179.0 | 207 | 162.0 | 137.0 | 194.0 | 395 | 155.0 | 134.0 | 176.0 | 376 | 155.0 | 135.0 | 178.0 | 0.0521 |
| Triglycerides (mg/dL) | 1087 | 107.0 | 73.0 | 156.0 | 207 | 124.0 | 85.0 | 207.0 | 395 | 84.0 | 64.0 | 117.0 | 376 | 122.5 | 87.0 | 181.0 | <0.0001 |
| LDL (mg/dL) | 1087 | 92.0 | 74.0 | 111.0 | 207 | 96.0 | 74.0 | 116.0 | 395 | 93.0 | 77.0 | 114.0 | 376 | 122.5 | 87.0 | 181.0 | 0.0047 |
| HDL (mg/dL) | 1087 | 39.0 | 33.0 | 46.0 | 207 | 37.0 | 31.0 | 43.0 | 395 | 42.0 | 36.0 | 48.0 | 376 | 38.0 | 32.0 | 45.0 | <0.0001 |
| Systolic BP (mm Hg) | 1056 | 114.0 | 107.0 | 122.0 | 201 | 115.5 | 106.5 | 124.5 | 381 | 115.0 | 109.0 | 123.0 | 367 | 112.0 | 105.0 | 120.0 | <0.0001 |
| Diastolic BP (mm Hg) | 1056 | 68.0 | 62.0 | 74.0 | 201 | 68.5 | 62.0 | 75.5 | 381 | 68.5 | 62.5 | 74.0 | 367 | 66.5 | 61.0 | 73.5 | 0.0308 |
| CATEGORICAL MEASURES | N | % | N | % | N | % | N | % | p-value* | ||||||||
| Sex | 0.0424 | ||||||||||||||||
| F | 703 | 64.4 | 129 | 62.3 | 270 | 68.0 | 224 | 59.4 | |||||||||
| M | 389 | 35.6 | 78 | 37.7 | 127 | 32.0 | 153 | 40.6 | |||||||||
| Diabetes medications | 0.0030 | ||||||||||||||||
| No diabetes medications | 149 | 13.6 | 38 | 18.4 | 40 | 10.1 | 48 | 12.7 | |||||||||
| Insulin alone | 137 | 12.5 | 22 | 10.6 | 60 | 15.1 | 45 | 11.9 | |||||||||
| Metformin alone | 495 | 45.3 | 101 | 48.8 | 155 | 39.0 | 185 | 49.1 | |||||||||
| Insulin and metformin | 281 | 25.7 | 39 | 18.8 | 133 | 33.5 | 93 | 24.7 | |||||||||
| Other | 30 | 2.7 | 7 | 3.4 | 9 | 2.3 | 6 | 1.6 | |||||||||
| HbA1c category (n=1087) | <0.0001 | ||||||||||||||||
| HbA1c = < 7.5% | 655 | 60.0 | 155 | 74.9 | 211 | 53.1 | 223 | 59.2 | |||||||||
| HbA1c > 7.5% | 432 | 39.6 | 52 | 25.1 | 184 | 46.3 | 153 | 40.6 | |||||||||
|
Dyslipidemia by laboratory value
(n=1087) |
655 | 60.0 | 141 | 68.1 | 220 | 55.4 | 241 | 63.9 | 0.0052 | ||||||||
| Low HDL (n=978) | 239 | 21.9 | 47 | 22.7 | 89 | 22.4 | 103 | 27.3 | 0.2354 | ||||||||
| High LDL (n=1087) | 402 | 36.8 | 89 | 43.0 | 151 | 38.0 | 125 | 33.2 | 0.0590 | ||||||||
| High triglycerides (n=1087) | 292 | 26.7 | 82 | 39.6 | 48 | 12.1 | 133 | 35.3 | <0.0001 | ||||||||
| BP > =95%tile (n=1086) | 193 | 17.7 | 44 | 21.3 | 79 | 19.9 | 51 | 13.5 | 0.0191 | ||||||||
| LFT category (n=1087) | <0.0001 | ||||||||||||||||
| <1.5 X ULN | 997 | 91.3 | 183 | 88.4 | 385 | 97.0 | 334 | 88.6 | |||||||||
| ≥1.5 X ULN | 90 | 8.2 | 24 | 11.6 | 10 | 2.5 | 42 | 11.1 | |||||||||
| Metabolic syndrome (n=1092) | 714 | 65.4 | 150 | 72.5 | 254 | 64.0 | 251 | 66.6 | 0.1095 | ||||||||
Statistics presented are median, 1st quartile, and 3rd quartile.
P-value for race/ethnicity from χ2 square test for White, Black, and Hispanic race/ethnicity groups only.
Race/ethnicity comparisons are shown in Table 1. Black youth had lower triglycerides and higher HDL compared with non-Hispanic Whites. There was no difference in BP between Blacks and non-Hispanic Whites, but systolic BP (SBP) was slightly higher in both Black and non-Hispanic White youth compared with Hispanics. Hispanic participants had the lowest BP and LDL cholesterol and the strongest family history of T2D (data not shown). Non-Hispanic Whites were the least likely to be on treatment, the least likely to be on insulin, and the most likely to have an HbA1c ≤ 7.5% (58 mmol/mol). There were no significant race/ethnicity differences in the prevalence of metabolic syndrome.
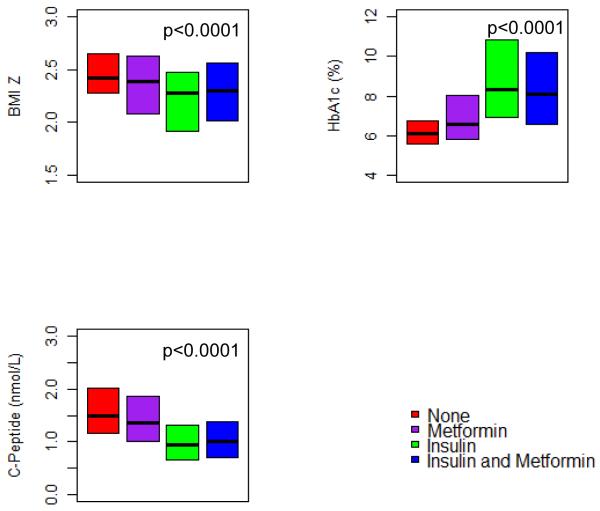
Subjects on insulin at screening had a lower c-peptide, a higher HbA1c, were less overweight, and were more likely to be racial/ethnic minorities (Figure 1). There were no statistically significant or clinically relevant differences in lipid profiles or BP by treatment at screening (data not shown).
Figure 1.
Baseline weight status, HbA1c, and c-peptide by diabetes treatment at screening. Median baseline BMI z-score, HbA1c and c-peptide for all screened subjects with type 2 diabetes and by treatment at screening. Bottoms and tops of the boxes represent the 25th and 75th percentiles, respectively.
Changes from screening to completion of run-in
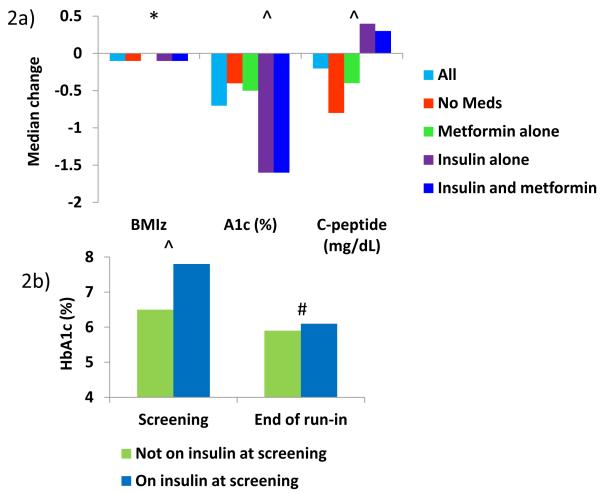
Metabolic parameters and BMI significantly improved in those who completed at least 8 weeks of standardized diabetes management (Figure 2a). Of the 330 participants who were on insulin at screening and who completed 8 weeks of run-in, all but 36 (11%) could be weaned from insulin and some of these 11% failed run-in for other reasons (e.g., poor compliance with visits). Most notably, median HbA1c decreased by 0.7% (7.7 mmol/mol, p<0.0001) for all subjects who completed at least 8 weeks of run-in. After the subjects who were still on insulin at the completion of run-in were removed, this improvement in HbA1c persisted (Figure 2b). No subject weaned off of insulin experienced metabolic decompensation or occurrence of ketoacidosis.
Figure 2.
Median change in BMI z-score, HbA1c, and c-peptide from screening to end of run-in by diabetes treatment at screening.
2a) Median change in BMI z-score, HbA1c, and c-peptide for all subjects who completed at least 8 weeks of run-in. Changes in these parameters based on treatment at screening are also shown. P-values represent overall significance. *P<0.05, ^P<0.0001
2b) Median HbA1c at screening and after 8 weeks of run-in for those not on insulin at screening vs. those on insulin at screening, excluding subjects who were still on insulin at the end of run-in. #P=0.02,^P<0.0001
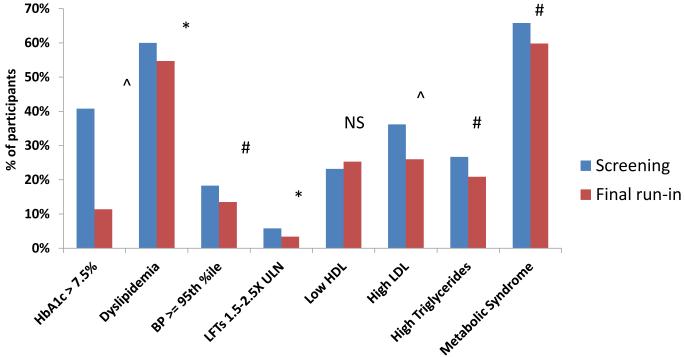
During run-in, the proportion of subjects with a HbA1c > 7.5% (58 mmol/mol) also decreased from 40.8% to 11.4% (p<0.0001), and there were small, but statistically significant, improvements in BMI z-score (Fig 2a), total (−8.88 mg/dL [−0.23 mmol/L], p < 0.0001) and LDL (−5.02 mg/dL [−0.13 mmol/L], p < 0.0001) cholesterol, triglycerides (−9.73 mg/dL [−0.11 mmol/L], p < 0.0001), systolic BP (−1.0 mm Hg, p = 0.039), and diastolic BP (−1.0 mm Hg, p = 0.002). Again, these improvements remained significant after removing the subjects that remained on insulin at the completion of run-in. There were also statistically significant improvements in the proportion of subjects with dyslipidemia, hypertension, and elevated liver enzymes (Figure 3), and the total number meeting the definition of metabolic syndrome fell from 536 (65.8%) to 487 (59.8%) (p=0.0027).
Figure 3.
Change in proportion of subjects with cardiometabolic risk factors from screening to end of run-in.
Proportion of those who completed at least 8 weeks of run-in with poor glycemic control or other metabolic abnormalities. *P<0.05, #P<0.01,^P<0.0001
The changes in clinical parameters were compared between those on no medication, metformin alone, insulin alone, and combined metformin/insulin treatment. There were only small differences in improvement in BMI z-scores between groups, but those who were not on treatment at screening showed the most weight loss during run-in (Figure 2a). The greatest improvements in HbA1c were seen in the groups who were on insulin at screening (p < 0.0001) (Figure 2a). This difference remained significant after removing those who were unable to be weaned from insulin by the completion of run-in (p < 0.0001) (Figure 2b). Those on insulin at screening also had a significant increase in fasting c-peptide during the run-in period (p < 0.0001) (Figure 2a). Differences in change in HbA1c by prescreening treatment were no longer statistically significant after adjustment for baseline HbA1c (p = 0.24), but difference in change in c-peptide remained significant (p < 0.0001). Those who were not on medication at screening showed the greatest improvement in triglycerides (−30.97 mg/dL [−0.35 mmol/L], p < 0.05) and HDL (+1.93 mg/dL [+0.05 mmol/L], p < 0.01). There were no other differences in change in lipids based on prescreening treatment.
Predictors of change during run-in
In regression analyses, higher HbA1c at screening was associated with greater improvement in HbA1c during the run-in period (p<0.001), whereas higher HDL (p = 0.001), DBP (p = 0.03), triglycerides (p = 0.01), and a history of in utero exposure to gestational diabetes (p = 0.007) were associated with less improvement in HbA1c during the run-in period. HbA1c at screening had by far the greatest effect size on improvement in HbA1c during the run-in period. Higher triglycerides (p = 0.01), older age (p = 0.03), and higher BMI (p < 0.0001) at screening were associated with continued increase in BMI z-score. Black (p < 0.0001), Hispanic (p = 0.0002), and American Indian (p = 0.006) race/ethnicity was also significantly associated with gain in BMI z-score.
Discussion
The cohort of subjects screened for the TODAY study is a large group of racially and geographically diverse children with T2D. This screening population provides an opportunity to better understand the clinical characteristics of US youth with T2D early in the course of their disease, prior to any study interventions. As previously reported in randomized subjects (10), all were overweight or obese, two-thirds were female, and racial/ethnic minorities were disproportionately represented. We also report here that, at a median disease duration of 2 months, nearly half of youth in routine clinical care were being treated with metformin alone, while approximately 25% were being treated with metformin and insulin in combination, and 12% with insulin alone. However, it has not been previously reported that nearly all youth with recently diagnosed T2D who are treated with insulin, even those with high HbA1c at presentation, could be rapidly weaned off and still attain lower HbA1c levels. Furthermore, with intensive follow-up, education specific for T2D, and titration of metformin to a maximally tolerated dose, these youth also showed improvement in other metabolic parameters.
A previous report (7) described changes in HbA1c and BMI during the run-in phase of TODAY, but focused on a comparison of those subjects who could be randomized and those who could not. The goal of the current analysis was to evaluate the effectiveness of initial treatment with metformin and standardized diabetes education in recently diagnosed youth with T2D by examining all subjects who completed the minimum run-in period, whether or not they were randomized. Furthermore, we analyzed here changes in metabolic risk factors not included in the previous report. Finally, in contrast to the primary outcome of the TODAY trial, which addresses the sustained response to treatment, the current analysis demonstrates the initial metabolic improvement that can be achieved early in the course of the disease.
Cardiometabolic disease risk differed according to race/ethnicity. As previously described in obese children with or without T2D, Blacks had lower triglyceride and higher HDL levels than did non-Hispanic and Hispanic Whites (11, 12). Black adolescents have also been shown to have lower insulin sensitivity than their obese White counterparts, but to have relatively more robust insulin secretion in the face of insulin resistance (13-15). Therefore, the lower fasting c-peptide seen in the Black subjects in this cohort is unexpected. We previously reported a lack of racial/ethnic difference in insulin secretion and disposition index as estimated from oral glucose tolerance testing in the TODAY cohort (16), suggesting that the lower fasting c-peptide in Black participants at screening may have been due to the higher prevalence of insulin use (causing c-peptide suppression). Hypertension is known to be more common in Black adults than in other race/ethnicity groups (17), and some, but not all, studies suggest that this racial difference emerges in youth (18-20). However, the Black adolescents screened for TODAY did not have a higher median BP or prevalence of hypertension than non-Hispanic Whites or Hispanics. Elevated liver enzymes were more common in Hispanic youth, as expected, since fatty liver disease has been reported to be more common in obese Hispanic adolescents than in Blacks and non-Hispanic Whites (21). Although there were race/ethnicity differences in the specific cardiometabolic risk factors, the overall prevalence of metabolic syndrome was not different among race/ethnicity groups.
At the time of screening, 38% of the population was being treated with insulin alone or in combination with metformin. Even though insulin-treated subjects had the highest HbA1c levels at screening, nearly all (90%) of these subjects were able to be weaned off insulin and still had reduced HbA1c levels by the end of run-in. Furthermore, based on the fact that the proportion of those who successfully complete run-in and were on insulin (40.5%) at screening was slightly higher than those who failed run-in (31.5%), being on insulin at baseline was not a barrier to successfully meeting randomization criteria. No cases of metabolic decompensation or ketoacidosis occurred with weaning of insulin. Indeed, among all the subjects who entered run-in, including those who remained on unchanged treatment with metformin, there were improvements in HbA1c, BMI, BMI z-score, percent overweight, LDL, triglycerides, and BP. These improvements remained significant after those who were unable to be weaned from insulin by the end of run-in were excluded from analyses. Therefore, the improvements seen were not biased by the small subset that remained on insulin after 8 weeks and, therefore, may have had better metabolic control. These results indicate that treatment with metformin monotherapy is almost always successful in improving metabolic control even in youth who require initial brief treatment with insulin. These findings support the recent guidelines for treatment of T2D in youth, which conclude that many patients with T2D can be weaned from insulin therapy (22).
The finding that nearly all patients can be either initially treated with metformin alone or weaned onto metformin after a brief treatment with insulin may appear to conflict with the primary results of TODAY, which showed a progressive loss of metabolic control in many youth during long-term treatment with metformin (6), and other studies showing rapid β-cell decline in T2D (23). However, the analysis here and the primary results of TODAY are addressing different periods in the course of youth-onset T2D. Taken together, the results of TODAY screening, run-in, and primary outcome suggest that most youth can be initially treated successfully with metformin alone and that, when insulin is initially required due to elevated HbA1c, insulin therapy can be brief and weaned rapidly. While approximately half of these youth eventually required reinitiation of insulin due to rising HbA1c later in the course of the disease, it is just as important to note that the other half of participants in TODAY maintained durable metabolic control without the addition of insulin. Therefore, these data support early weaning of insulin to avoid overtreatment of those who will maintain control on oral therapy. However, while insulin may not be necessary to maintain metabolic control early in the course of diabetes, further studies are required to evaluate whether early temporary treatment with insulin might have other benefits, such as preservation of β-cell function in the long term (24). Analysis is underway to determine whether insulin use at screening predicted eventual need for addition of insulin later in the trial.
While clinically relevant improvements in median values for cardiometabolic risk factors were not observed during run-in, there was a 5% decrease in the proportion of participants with hypertension and overall dyslipidemia, a 6% decrease in hypertriglyceridemia and metabolic syndrome, and a 10% decrease in participants with elevated LDL; this represents an overall reduction in the proportion of subjects with clinically significant metabolic risk. Diabetes patients who present with initial metabolic instability may gain weight when this instability is corrected due to improved carbohydrate utilization; however, even in the face of improvements in metabolic control of T2D, BMI z-scores significantly decreased in our cohort. Since many of these improvements were seen in subjects who did not change treatment during run-in, i.e., who were already on only metformin at screening, at least some of the effect can be attributed to intensive health care team contact and diabetes education. This is important in light of the failure of lifestyle intervention to show added benefit over metformin alone during the main TODAY trial, suggesting a difference between the short-term effects of initial diabetes education and the outcome of a sustained lifestyle intervention. On the other hand, the observation that increases in BMI z-scores were predicted by older age and Black, Hispanic, and American Indian race/ethnicity underscores the special challenges faced by these groups of patients.
Subjects screened for the TODAY study were not randomly drawn from a population-based sample, but were mostly recruited from pediatric academic medical center practices with the general study eligibility in mind. However, no efforts to prescreen participants were encouraged and the fact that 228 of the 927 (24%) participants who entered run-in could not ultimately be randomized suggests that the cohort was not preselected for characteristics of success. In addition, attempts by participating TODAY Study sites to identify patients being cared for at other medical practices or through public advertisement yielded only a handful of screened participants, suggesting that youth with T2D are being generally cared for at diabetes centers and not in the primary care setting (25). Furthermore, the clinical characteristics of the TODAY screening population are similar to those of youth with T2D in the population-based SEARCH for Diabetes in Youth (SEARCH) Study and other sources (1, 3). Therefore, the population recruited for TODAY likely reflects the general population of U.S. youth with T2D. It is possible that the cardiometabolic improvements presented herein largely occurred in those who were successfully randomized during run in, as 86% of those who entered run-in were ultimately randomized. However, the fact that such a significant proportion were able to be randomized, given the stringent study entry criteria, indicates that standardized diabetes education and metformin alone can be successful in newly-diagnosed youth with T2D.
The participants screened for the TODAY study represent a large and diverse group of youth with T2D. Therefore, their characteristics provide broadly applicable insight into the presentation and initial management of youth with newly diagnosed T2D in the US. Furthermore, the results of the run-in phase of the TODAY Study demonstrate that nearly all adolescents with T2D, including those with a high HbA1c requiring brief treatment with insulin at diagnosis, can be safely and successfully treated with metformin alone and demonstrate clinically relevant improvements in metabolic risk factors when treatment is combined with focused diabetes education. Further analysis of TODAY data is underway to discriminate those subjects who may be at risk for early loss of metabolic decompensation after initial stabilization on metformin from those expected to maintain durable control on oral therapy.
Acknowledgments
This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi-Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.
The following individuals and institutions constitute the TODAY Study Group (* indicates principal investigator or director):
CLINICAL CENTERS Baylor College of Medicine: S. McKay*, B. Anderson, C. Bush, S. Gunn, M. Haymond, H. Holden, K. Hwu, S.M. Jones, S. McGirk, B. Schreiner, S. Thamotharan, M. Zarate Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms (deceased), A. Davis, A. Haider, S. Huestis, C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan, D. Rogers Children’s Hospital Los Angeles: M. Geffner*, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, J. Sanchez, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda Children's Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, K. Gralewski, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi Children's Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti Columbia University Medical Center: R. Goland*, R. Cain, I. Fennoy, D. Gallagher, P. Kringas, N. Leibel, R. Motaghedi, D. Ng, M. Ovalles, M. Pellizzari, R. Rapaport, K. Robbins, D. Seidman, L. Siegel-Czarkowski, P. Speiser Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, M. Malloy, K. Milaszewski, L. Orkin, A. Rodriguez-Ventura Massachusetts General Hospital: D. Nathan*, L. Bissett, K. Blumenthal, L. Delahanty, V. Goldman, A. Goseco, M. Larkin, L. Levitsky, R. McEachern, K. Milaszewski, D. Norman, B. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, V. Kociela, T. Whelan, B. Wolff State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief University of Colorado Denver: P. Zeitler* (Steering Committee Chair), N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, A. Hull, M. Kelsey, G. Klingensmith, K. Nadeau, T. Witten University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, J. Smith, S. Sternlof, R. Swisher University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters Washington University School of Medicine: N. White*, A. Arbeláez, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, M. Mignosa, P. Rose, A. Syme, W. Tamborlane
COORDINATING CENTER George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long, L. Pyle
PROJECT OFFICE National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S. Marcovina*, J. Chmielewski, M. Ramirez, G. Strylewicz DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang Diet Assessment Center (University of South Carolina): M. Nichols*, E. Mayer-Davis, Y. Liu Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, D. Laughlin, G. Leibach, C. Massmann, M. Mills, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren, A. Vannucci
OTHER Centers for Disease Control: P. Zhang State University of New York at Buffalo: L. Epstein University of Florida: J. Silverstein
Footnotes
Disclosures
The authors do not have any related conflicts of interest to report. Megan Kelsey wrote the first draft of the manuscript and did not receive any payment for doing so. Each of the authors has seen and approved the current version. Drs. Zeitler, Tamborlane, Geffner, and White contributed to data interpretation, discussion, and editing of the manuscript. Dr. Pyle performed the data analysis and contributed to discussion and editing of the manuscript. Cindy Guandalini contributed to the discussion and data interpretation. The study was sponsored by the National Institute of Diabetes, Digestive Disease and Kidney Diseases. Representatives of the sponsor participated in the study design and data analysis. No honorarium was conferred to anyone to produce this manuscript.
References
- 1.Dabelea D, Bell RA, D'Agostino RB, Jr., Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA: The Journal of the American Medical Association. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. The Journal of Pediatrics. 1996;128:608–15. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 3.Group SfDiYS, Liese AD, D'Agostino RB, Jr., Hamman RF, Kilgo PD, Lawrence JM, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–8. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 4.Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, et al. Long-Term Complications and Mortality in Young-Onset Diabetes: Type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–9. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group TS, Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine. 2012;366:2247–56. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laffel L, Chang N, Grey M, Hale D, Higgins L, Hirst K, et al. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes. 2012;13:369–75. doi: 10.1111/j.1399-5448.2011.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig ME, Jefferies C, Dabelea D, Balde N, Seth A, Donaghue KC. Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2014;15(Suppl 20):4–17. doi: 10.1111/pedi.12186. [DOI] [PubMed] [Google Scholar]
- 9.Grey M, Schreiner B, Pyle L. Development of a diabetes education program for youth with type 2 diabetes. Diabetes Educ. 2009;35:108–16. doi: 10.1177/0145721708325156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of Adolescents and Youth with Recent-Onset Type 2 Diabetes: The TODAY Cohort at Baseline. Journal of Clinical Endocrinology & Metabolism. 2011;96:159–67. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001-2006. Archives of pediatrics & adolescent medicine. 2009;163:371–7. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr. 2006;149:809–16. doi: 10.1016/j.jpeds.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 13.Bacha F, Gungor N, Lee S, Arslanian SA. Type 2 diabetes in youth: are there racial differences in beta-cell responsiveness relative to insulin sensitivity? Pediatr Diabetes. 2012;13:259–65. doi: 10.1111/j.1399-5448.2011.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–9. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 15.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–21. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 16.Bacha F, Pyle L, Nadeau K, Cuttler L, Goland R, Haymond M, et al. Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes. 2012;13:376–83. doi: 10.1111/j.1399-5448.2011.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 18.Cruz ML, Huang TT, Johnson MS, Gower BA, Goran MI. Insulin sensitivity and blood pressure in black and white children. Hypertension. 2002;40:18–22. doi: 10.1161/01.hyp.0000019972.37690.ef. [DOI] [PubMed] [Google Scholar]
- 19.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic variation in cardiovascular disease risk factors among children and young adults: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. JAMA. 1999;281:1006–13. doi: 10.1001/jama.281.11.1006. [DOI] [PubMed] [Google Scholar]
- 20.Rosner B, Prineas R, Daniels SR, Loggie J. Blood pressure differences between blacks and whites in relation to body size among US children and adolescents. American journal of epidemiology. 2000;151:1007–19. doi: 10.1093/oxfordjournals.aje.a010129. [DOI] [PubMed] [Google Scholar]
- 21.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. JPediatrGastroenterolNutr. 2006;43:413–27. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 22.Copeland KC, Silverstein J, Moore KR, Prazar GE, Raymer T, Shiffman RN, et al. Management of Newly Diagnosed Type 2 Diabetes Mellitus (T2DM) in Children and Adolescents. Pediatrics. 2013;131:364–82. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 23.Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of beta-cell function in obese youth with type 2 diabetes. Pediatr Diabetes. 2013;14:106–11. doi: 10.1111/j.1399-5448.2012.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. The New England journal of medicine. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 25.Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, et al. Type 2 diabetes in the child and adolescent. Pediatr Diabetes. 2014;15(Suppl 20):26–46. doi: 10.1111/pedi.12179. [DOI] [PubMed] [Google Scholar]