Abstract
There have been many exciting recent advances in our understanding of the molecular and cellular basis of itch. These discoveries cover diverse aspects of itch sensation, from the identification of new receptors to the characterization of spinal cord itch circuits. A common thread of these studies is they demonstrate that itch sensory signals are segregated from input for other somatosensory modalities, such as pain, touch, and thermosensation. This specificity is achieved by the expression of dedicated receptors and transmitters in a select population of sensory neurons which detect pruritogens. Further, recent studies show that itch specificity is maintained in a spinal cord circuit by the utilization of specific neurotransmitters and cognate receptors to convey input along a distinct cellular pathway.
Introduction
Itch is an unpleasant sensation produced by a disturbance of the skin that triggers a strong innate urge to scratch. The act of scratching in turn results in the removal of the upper epidermal layers of the skin which is thought to be a mechanism to dislodge potential parasites. In many animals a variety of ethologically common stimuli elicit the same scratch response suggesting that itch confers an evolutionary advantage. In the Western world invasion of the skin by invertebrate organisms is rare, but itch caused by many dermatological conditions and some systemic diseases can produce a type of itch which becomes debilitating. This type of pathological (chronic) itch appears to serve no purpose and can be particularly disturbing to patients, because of the paucity of effective treatments and its adverse impact on quality of life. Therefore at a clinical level and from a biological perspective, there has been a strong impetus to better understand the mechanisms by which itch sensation is produced. This review will highlight the discoveries in the neuroscience of itch-reception over the last 3 years.
Reception and peripheral coding of itch
Where itch starts, depends on your stand-point. For dermatologists, there has been a long standing recognition that the interaction of immune system and cells in the skin is critical for itch in most of the diseases with a dermatological origin (Figure 1). For instance, the detection of many parasites in the skin is performed by mast cells. Upon stimulation, mast cells release the well-known pruritogen, histamine and several chemical substances such as serotonin, serine proteases, and lipid mediators [1,2]. Other immune and skin cells also release itch-inducing compounds [3–5]. For neuroscientist, reception occurs at the level of peripheral sensory neurons. Most studies performed by neuroscientist concentrate on itch produced by chemical activation of itch sensitive neurons, but another poorly characterized itch stimulus is generated by a very specific types of mechanical stimulation [6]. This type of itch is produced by very transient low threshold mechanical stimulus, for example the stimulation produced by an insect landing on the skin. In addition, there is cross-talk between the neuronal and immune systems that potentiates responses for both [7]. Finally, for psychologists, some types of itch may be elicited by activation of central itch-circuits, such as, itch provoked by visual cues [8,9].
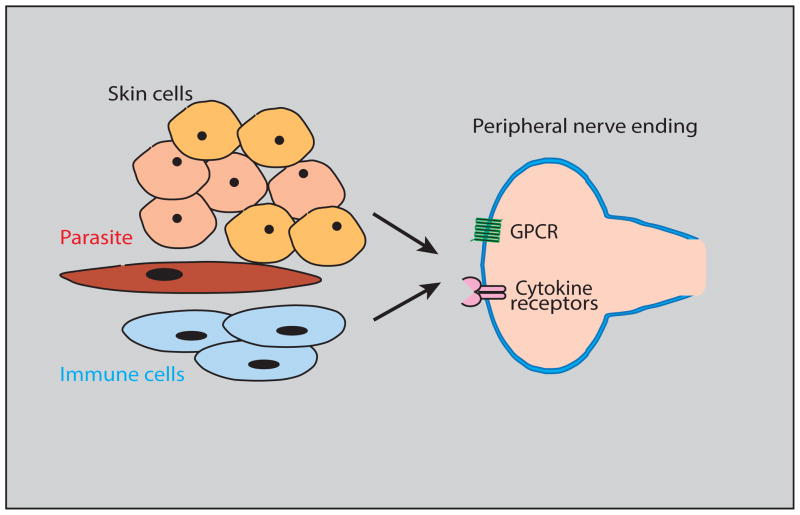
Figure 1.
Model depicting the cellular and molecular components responsible for the peripheral detection of itch stimuli.
The skin is a complex micro-environment containing multiple cell-types that collaborate to detect and either directly or indirectly stimulate pruciceptive neurons. Skin and immune cells as well as agents released from invading organisms activate sensors in the itch-responsive nerve fibers in the skin. Through different signaling cascades G protein-coupled receptors (GPCR) and cytokine receptors cause the cells in which they are expressed to be depolarized.
Specific nerve cells, called pruriceptors, are responsible for sensing a plethora of itch stimuli and express multiple types of receptor molecules. G protein-coupled receptors (GPCR) are a well characterized class of itch sensors that detect various itch-inducing chemicals. Among the GPCRs that have been reported to be expressed in sensory neurons are the H1 and H4 histamine, and the serotonin and PAR2 receptors [10–12]. In addition, the Mas related GPCRs, MrgprA3 and MrgprC11 are activated by the xenobiotic agent chloroquine and the endogenous compound Bam8-22 respectively [13]. Itch GPCRs trigger G-protein coupled signaling cascades through phospholipase C and Gβγ which ultimately mediate gating of TRP-ion channels [14–16].
New receptors for itch
Recently several non-GPCR itch receptors have been identified which either directly or indirectly lead to signaling cascades involving the phosphorylation of multiple intracellular proteins. These cytokine receptors were first characterized in immune cells and have recently been shown to have roles in pruriception [17]. The epithelial derived cytokine, thymic stromal lipoprotein (TSLP), is sensed by its receptor, TSLPR and IL7Ra, expressed in pruriceptors and was demonstrated to be an important pruritogen underlying certain types of dermatological itch [3]. The IL31Ra is expressed in a small subset of sensory neurons and is activated by the cytokine IL31 which is likely produced by keratinocytes and immune cells [4,5]. In addition, the Toll like receptors (TLR) have been shown to contribute to itch-sensation; however the expression of TLR receptors in pruriceptor neurons is still controversial [18]. There are probably other receptors for some other physiologically relevant itch-inducing agents which should be uncovered in the next few years as the full set of genes which pruriceptors express is characterized. It is important to note that some itch-inducing agents may not be completely specific pruritogens since they can also provoke other sensations because their receptors are likely expressed by a variety of sensory neurons. The sensation of itch can be distinguished from other sensations and it has been proposed that itch and pain can be differentiated from each other by the distinct coding properties of itch and pain neurons and their differential innervation of the skin [19]. The compound histamine is an example of an agent that is algesic and pruritic. Histamine can preferentially provoke itch when injected into outer dermal layers of the skin, but produces pain when injected into deeper layers. Recently the xenobiotic β-alanine has been suggested to activate the Mrgprd-receptor and cause an itch-like sensation [20]. Previously, it was shown that Mrgprd-expressing neurons are stimulated by the pain producing substance ATP and are required for noxious mechanosensation and thermoreception [21–23]. Since β-alanine can also cause burning and skin irritation in humans, it is tempting to speculate that this compound, like histamine, may trigger noxious responses rather than being a pure pruritogen.
Pruriceptor specificity
Recently, attempts have been made to define the specificity of pruriceptive neurons using molecular genetic and pharmacological strategies. Previously electrophysiological measurements had suggested that pruriceptors are a restricted population of sensory neurons, but indicated that these cells may not be selectively tuned for detection of itch stimuli. In contrast, recent molecular and pharmacological approaches suggest that there is, instead, considerable coding specificity. By manipulating the cells that express the MrgprA3 receptor, it has been shown that these neurons are required for detection of most itch stimuli and that their activation is sufficient to induce itch, but not pain-behavior [24]. This result restricts the neurons that are required and sufficient for itch and shows they are highly specific. However, because itch for many chemical substances was only partially lost when MrgprA3-neurons are ablated, it is still not clear that all itch receptors are expressed in this subset of cells. Similarly, targeted loading of the voltage gated sodium channels blocking agent QX-314 into histamine, and chloroquine/PAR2 responsive neurons, silenced itch, but not pain and did not change responses to mechanical stimuli [25]. Therefore, electrophysiological attempts to define the specificity of neurons do not agree with recent studies which paired molecular genetic ablation and pharmacological silencing with behavioral assays of itch. Future work should further help resolve these differences and establish definitively the coding properties of pruriceptive neurons.
Signal transmission and neural circuits for itch
The activation of peripheral sensory neurons is necessary for the feeling of itch, but it is not all that is required to produce the sensation of itch. Itch is in fact a neural correlate generated in the brain. Several recent studies have started to reveal some of the key early stages in the processing of itch information in the spinal cord. In particular, notable advances have been made in identifying several of the cellular and molecular substrates involved in the transmission and inhibition of itch.
Spinal cord itch-circuit
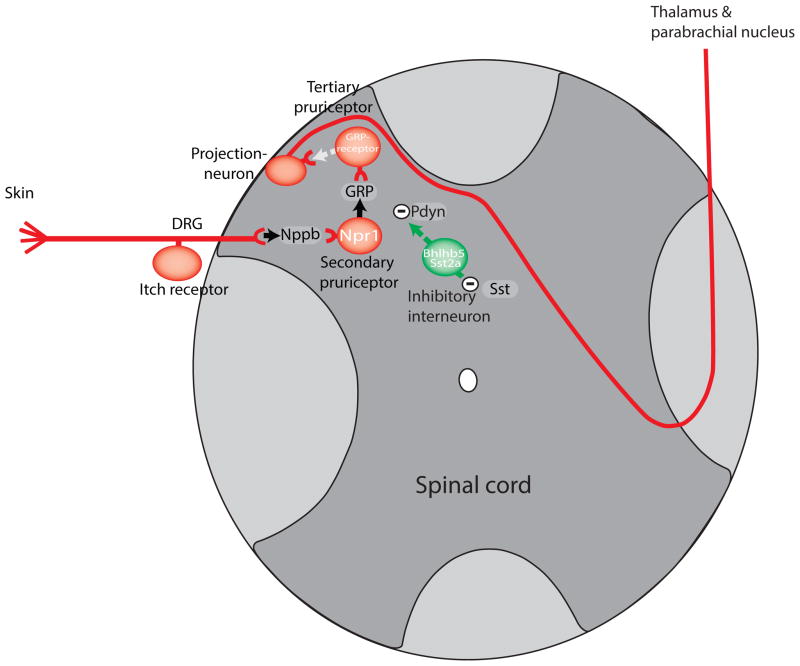
It is generally believed that glutamate is a primary neurotransmitter of itch. Post-synaptic input from itch sensory afferents can be blocked by inotropic glutamate ion-channel antagonists and all sensory neurons types have the appropriate cellular machinery to generate synaptic stores of glutamate supporting glutamate as a transmitter used by pruriceptors [26–28]. Since, elimination of glutamate from pruriceptors does not abolish itch [27,28], pruriceptors must contain other transmitter(s). It was initially hypothesized that the neuropeptide, gastrin releasing peptide (GRP), was a co-transmitter employed by pruriceptors based on immunological detection of GRP in pruriceptors [29,30]. However, recent results cast doubt on GRP-staining in pruriceptive neurons [31–35]. In particular, it is hard to reconcile the almost complete lack of RNA for GRP in pruriceptors with this peptide contributing significantly to itch transmission by DRG-neurons [36]. Whereas GRP expression is extremely low or absent in sensory neurons, a recent report revealed that another peptide, Natriuretic peptide b (Nppb), is highly expressed in these cells [32]. Further, Nppb (also called BNP) was shown to be co-expressed with itch-receptors and signaling components which mediate itch-sensation. Importantly, Nppb was demonstrated to be required for itch responses in mice. Corroborating Nppb as a transmitter of itch signals, ablating spinal cord neurons that express the Nppb-receptor, Npr1 (Figure 2) attenuates itch behavior. Additionally, intrathecal injection (into the spinal cord) of Nppb elicits scratching. While evidence for GRP being the primary transmitter for itch has been waning, there is strong evidence that GRP itself has an important contribution in transmission of itch-signals downstream of Nppb. Concordant with this concept, GRP-receptor deficient mice, GRP-antagonists, and mice in which neurons that express the GRP-receptor are ablated display greatly reduced responses to administration of histamine and Nppb, but blocking Nppb-evoked scratching does not alter GRP-induced itch-behavior [32]. Also consistent with this linear model of itch transmission, GRP is co-expressed with Npr1 in the spinal cord neurons (Figure 2). This model provides the most parsimonious explanation of the available data, and fits well with other more recent data which shows that elimination of spinal GRP-expressing interneurons abolishes itch-behavior [37,38]. How these neuropeptide transmitters exert their effects in the spinal cord is still an open question. An attractive concept, taken from other areas of the nervous system, is that these neuropeptide transmitters, which exert their effects on metabotropic receptors, may serve as modulators of the tone within the spinal cord rather than acting like the more traditional small molecule neurotransmitters glutamate, glycine and GABA. At this stage, the molecular and cellular steps beyond the neurons expressing GRPR are still poorly understood. Various data suggest that TacR1-expressing spinothalamic projection neurons in lamina I of the dorsal horn of the spinal cord transmit signals to the parabrachial nucleus and thalamus (Figure 2) [39,40]. Electrophysiological data suggest that restricted lamina I projection neurons respond to itch stimuli, but these cells are also excited by painful stimuli [41,42]. Therefore, it is unclear how itch and pain signals are distinguished as sensory information moves into the brain. Exact details of the neurons within the brain activated by itch are also obscure.
Figure 2.
Schematic of the itch spinal cord circuit.
The initial stages of the itch-circuit in the spinal cord are characterized by the expression of neuropeptides and the post-synaptic expression of their cognate receptors. Initially Nppb is released from primary puriceptive neurons (black arrow) and activates Npr1-expressing spinal cord neurons. In turn, Npr1-neurons release GRP which activates tertiary GRPR-expressing cells. The GRPR-neurons then directly or indirectly, (broken white arrow) activate projection neurons which send projections to higher brain centers. Inhibitory Bhlhb5-interneurons (green) express Sst2a receptors and can be hyperpolarized by somatostatin. The Bhlhb5-cells are thought to release the kappa-opioid dynorphin in response to activation by primary afferent DRG stimulation. Dynorphin causes inhibition of the excitatory (red) itch pathway.
Inhibition of itch
Importantly, scratch responses are limited; too much scratching elicits pain which, in turn inhibits itch. In this way, itch and pain are closely tied to each other with a unidirectional feed-forward inhibitory path from pain to itch which functionally prevents skin trauma. A clue to how this inhibition might happen came from genetically eliminating the glutamate transporter, VGlut2, from subsets of sensory neurons [27,28]. The genetically manipulated mice used in these studies exhibit spontaneous itch and display hyper-reactivity to pruritic compounds, and have greatly reduced responses to algesic stimuli. It has been suggested this result was caused by the release of a tonic inhibition from pain-sensing neurons which normally silence itch. Building on this model of itch inhibition, mice lacking Bhlhb5 inhibitory spinal cord neurons exhibit the same phenotype as animals in which VGlut2 is lost from nociceptors, suggesting Bhlhb5-cells might be part of the cellular substrate where tonic input originates [43] (Figure 2). This arm of the itch pathway has recently been advanced by showing that Bhlhb5-neurons can be directly activated by the itch-counter stimuli capsaicin, mustard, and menthol and that Bhlhb5-cells contain the itch relieving neuropeptide dynorphin [44]. In addition, Bhlhb5-neurons can be hyperpolarized by the neuropeptide somatostatin [44]. However, it is not clear what the exact role of somatostatin is in this case, since somatostatin can be found in multiple types of spinal cord interneurons and has been suggested to have many potential roles in the spinal cord and DRG [45]. Lastly, paradoxically the ablation of dynorphin inhibitory interneuron did not alter itch behavioral responses suggesting that the inhibitory pathway may be redundant or, that this pathway is more complex than our current view [46].
Summary and future directions
The past few years have seen some notable discoveries in many different areas of the neuroscience of itch which have revealed some of the critical steps in detection as well as downstream steps in neuronal transmission. In the area of signal transmission, recent molecular data suggest a remarkably specific and simple model of how itch is encoded. Having said this, there is still much apparent complexity in the spinal cord and in the brain that remains to be understood. Among the most controversial questions in the field is whether itch, along its neuro-axis, uses dedicated lines or some sort of patterning method to code this unique sensation. Given the great interest, recent exciting new discoveries, and the advent of novel methodologies [46–48], the prospects is good that in the next few years more important advances will be made in our understanding of itch.
Highlights.
New itch receptors identified that detect pruritogens released from the skin.
Molecular studies demonstrate itch sensory neurons are selectively tuned.
Novel itch sensory neurons neurotransmitter, Nppb, is required for itch.
Itch is encoded by a dedicated spinal cord circuit.
Dynophin, released by inhibitory spinal cord neurons can reduce itch.
Acknowledgments
I am grateful to Santosh Mishra and Hans Jürgen Solinski for their critical readings on this manuscript. This work was supported by the intramural program of the NIH NIDCR.
Footnotes
Conflict of interest
The author declares a potential competing interest. The author is an applicant on a patent to develop methods for treatment of itch.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521–1534. doi: 10.1016/j.jaci.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 3[*].Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. For many years dermatologists knew that TSLP was a component involved in itch, this report provided evidence for the neurogenic mechanisms involved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4[*].Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. This study presents clear data that shows that the cytokine receptor for IL31 is expressed in itch-responding primary neurons and functionally causes itch-behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, Loren AW, Dentchev T, Wysocka M, Yosipovitch G, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133:2783–2785. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 6.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd DM, Hall E, Hall S, McGlone FP. Can itch-related visual stimuli alone provoke a scratch response in healthy individuals? Br J Dermatol. 2013;168:106–111. doi: 10.1111/bjd.12132. [DOI] [PubMed] [Google Scholar]
- 9.Feneran AN, O’Donnell R, Press A, Yosipovitch G, Cline M, Dugan G, Papoiu AD, Nattkemper LA, Chan YH, Shively CA. Monkey see, monkey do: contagious itch in nonhuman primates. Acta Derm Venereol. 2013;93:27–29. doi: 10.2340/00015555-1406. [DOI] [PubMed] [Google Scholar]
- 10.Rossbach K, Nassenstein C, Gschwandtner M, Schnell D, Sander K, Seifert R, Stark H, Kietzmann M, Baumer W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience. 2011;190:89–102. doi: 10.1016/j.neuroscience.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Kim DK, Kim HJ, Kim H, Koh JY, Kim KM, Noh MS, Kim JJ, Lee CH. Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur J Pharmacol. 2008;579:390–394. doi: 10.1016/j.ejphar.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Reddy VB, Shimada SG, Sikand P, Lamotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130:1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulay JL, O’Shea JJ, Paul WE. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19:159–163. doi: 10.1016/s1074-7613(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–1589. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogorzala LA, Mishra SK, Hoon MA. The Cellular Code for Mammalian Thermosensation. J Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24[**].Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. An elegant molecular genetics study which establishes that distinct subsets of sensory neurons are dedicated receptors for pruritic stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25[**].Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–918. doi: 10.1038/nn.3404. This study uses pharmocological manipulation to demonstate that when specific cell types are silenced they produce selective itch-behavioral deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27[*].Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. This report, together with [28], for the first time showed a direct cross-talk between pain and itch. It provided strong evidence that glutamate is required for pain signal transduction and in the absence of pain afferent input, itch-sensation is potentiated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28[*].Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. See reference above, independently, the authors of this paper showed that mice can be made hypersensitive to prurtic stimulation by eliminating the glutamate transporter in subsets of sensory neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 30[*].Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. Key paper in which the authors demonstrate the important contribution of gastrin releasing peptide to itch-signal transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32[**].Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. Important study that reveal the new peptide neurotransmitter, Nppb, is critcal for transmission of itch-behavior from peripheral sensory neurons and defined the initial steps in the spianl itch-circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2014 doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez-Mecinas M, Watanabe M, Todd AJ. Expression of gastrin-releasing peptide by excitatory interneurons in the mouse superficial dorsal horn. Mol Pain. 2014;10:79. doi: 10.1186/1744-8069-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solorzano C, Villafuerte D, Meda K, Cevikbas F, Braz J, Sharif-Naeini R, Juarez-Salinas D, Llewellyn-Smith IJ, Guan Z, Basbaum AI. Primary afferent and spinal cord expression of gastrin-releasing Peptide: message, protein, and antibody concerns. J Neurosci. 2015;35:648–657. doi: 10.1523/JNEUROSCI.2955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goswami SC, Thierry-Mieg D, Thierry-Mieg J, Mishra S, Hoon MA, Mannes AJ, Iadarola MJ. Itch-associated peptides: RNA-Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord. Mol Pain. 2014;10:44. doi: 10.1186/1744-8069-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38[*].Xu Y, Lopes C, Wende H, Guo Z, Cheng L, Birchmeier C, Ma Q. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci. 2013;33:14738–14748. doi: 10.1523/JNEUROSCI.5512-12.2013. Rigorous study that delineated the neurons and developmental pathways of several classes of spinal cord interneurons in somatosensation including cells required for itch-sensation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trivedi M, Bergasa NV. Serum concentrations of substance P in cholestasis. Ann Hepatol. 2010;9:177–180. [PubMed] [Google Scholar]
- 41.Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43[*].Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. Early study which for the first time addresed the neurons involved in processing pruritic sensory information in the spinal cord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44[**].Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. Significant study that molecularly defined the cellular substrate and molecular players involved in itch inhibtion in the spinal cord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi TJ, Xiang Q, Zhang MD, Barde S, Kai-Larsen Y, Fried K, Josephson A, Gluck L, Deyev SM, Zvyagin AV, et al. Somatostatin and its 2A receptor in dorsal root ganglia and dorsal horn of mouse and human: expression, trafficking and possible role in pain. Mol Pain. 2014;10:12. doi: 10.1186/1744-8069-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. Identification of Spinal Circuits Transmitting and Gating Mechanical Pain. Cell. 2014;159:1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zampieri N, Jessell TM, Murray AJ. Mapping sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus. Neuron. 2014;81:766–778. doi: 10.1016/j.neuron.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams SC, Deisseroth K. Optogenetics. Proc Natl Acad Sci U S A. 2013;110:16287. doi: 10.1073/pnas.1317033110. [DOI] [PMC free article] [PubMed] [Google Scholar]