Abstract
Biomonitoring for manganese (Mn) exposure is important due to its potential to cause adverse health effects. In this study, we investigate how different sample preparation methods (simple dilution, digestion, volumetric, gravimetric), calibration protocols (aqueous, blood-based, standard additions), and instrumental techniques affect Mn method bias and analytical imprecision. The techniques used included graphite furnace atomic absorption spectrometry (GFAAS), dynamic reaction cell inductively coupled plasma mass spectrometry (DRC-ICP-MS), and sector field (SF-) ICP-MS. We analyzed NIST SRM 1643e Trace Elements in Water and SRM 1598a Inorganic Constituents in Animal Serum (both certified for Mn), and SRM 955c Toxic Metals in Caprine Blood – Level 1 (not certified for Mn). Various matrix effects in ICP-MS produced inaccurate results for SRM 1643e and discrepant results for SRM 955c. In the absence of a certified value for Mn in SRM 955c, we assigned a “consensus” value by combining data from the New York State Department of Health (NYS), the Centers for Disease Control and Prevention (CDC) and the Centre de toxicologie du Québec (CTQ). With this interlaboratory approach, we established an “all-lab” consensus value of 16.3 ± 0.8 μg L−1 based on data from DRC-ICP-MS with simple dilution sample preparation and blood-based calibration. We also assigned an “all-method” consensus value of 16.3 ± 0.9 μg L−1 based on GFAAS and SF-ICP-MS data from the NYS lab and the DRC-ICP-MS all-lab consensus value. Although the expanded uncertainty (U) calculated for the consensus values may not fully account for all sources of uncertainty, it does show the relative variation that might be expected from one study to the next for the determination of Mn in blood.
Introduction
Manganese is an essential element that serves multiple purposes in the body including bone mineralization, reproductive function, and carbohydrate/lipid metabolism.1 It is known that Mn overexposure leads to neurotoxicity. Effects of occupational exposure to Mn include decreased cognition and motor performance2 and these effects can progress to a disease known as manganism. The clinical symptoms of manganism include psychiatric disturbances, characteristic “cock walk,” and propensity to fall backward.3 Environmental Mn exposures are associated with outcomes that could indicate neurotoxicity. In children, Mn exposure from drinking water was associated with lower working memory, perceptual reasoning,4 and full scale IQ,5 and with increased hyperactivity and opposition;6 while exposure from a Mn mineral processing plant was associated with lower total, verbal, and performance IQ.7 Recent studies suggest a synergistic effect between Mn and Pb. In children with higher blood Mn concentrations, greater decreases in full scale IQ, verbal IQ, mental development and psychomotor development were associated with increasing blood Pb concentrations.8, 9
A good biomarker for Mn exposure has still yet to be established and validated. Exposure studies have commonly used whole blood, urine, or serum, but other biomarkers have been explored including hair,7 nails,10 red blood cells,11 serum prolactin,12 and magnetic resonance imaging (MRI).11 There are problems associated with each of these biomarkers. For example, hair Mn concentrations depend on pigmentation,13 serum can be affected by hemolysis, and urine is a minor route of excretion such that concentrations are typically very low. Whole blood Mn does not consistently reflect Mn in tissues and it is difficult to determine exposure on an individual basis; however, Mn in blood is effective in epidemiological studies for differentiating between exposed and unexposed groups.14 Additionally, blood Mn has recently been added to the CDC’s National Health And Nutrition Examination Survey (NHANES) biomonitoring panel.
The most widely used instrumental techniques for determining Mn in blood are graphite furnace atomic absorption spectrometry (GFAAS), quadrupole-based (Q-) inductively coupled plasma mass spectrometry (ICP-MS), and sector field (SF-) ICP-MS. The comparability of these techniques was explored for the determination of Mn in whole blood and urine in a previous study.15 That work showed in a quadrupole mass spectrometer operated without a DRC, polyatomic interferences can affect the 55Mn signal in urine and blood, and that an abundance sensitivity issue is caused by the large 56Fe signal overlapping the 55Mn signal in blood. The abundance sensitivity affects Q-ICP-MS results because of low resolution of the instrument, but 55Mn can be resolved from 56Fe in SF-ICP-MS at a resolution of m/Δm = 4000.
As part of our previous work, 34 proficiency testing (PT)/external quality assessment scheme (EQAS) samples for blood Mn were analyzed by different techniques. In each technique, samples were prepared by simple volumetric dilution and analyzed using (a) an aqueous calibration curve with GFAAS, and (b) a blood-based calibration curve with both DRC- and SF-ICP-MS. The PT/EQAS data showed that GFAAS had no apparent bias, while the DRC-ICP-MS and SF-ICP-MS methods exhibited a small, negative bias. However, PT/EQAS assigned values are not “certified” (unlike certified reference materials – CRMs), and are not generally traceable to SI units, so definitive statements on accuracy are not recommended. These previous findings led us to the current study wherein we investigate potential sources of negative bias in ICP-MS techniques with a series of sample preparations including: (a) simple dilution, (b) microwave-assisted digestion, (c) volumetric, and (d) gravimetric; and various calibration protocols including: (a) aqueous, (b) blood-based, and (c) the method of standard additions. These investigations were completed using the National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1643e Trace Elements in Water, SRM 1598a Inorganic Constituents in Animal Serum, and SRM 955c Toxic Metals in Caprine Blood.
NIST has certified the Mn content of SRMs 1643e and 1598a, but not SRM 955c. SRM 955c is a frozen caprine (goat) blood material that was originally produced as SRM 955c Lead in Caprine Blood.16 It was renamed SRM 955c Toxic Metals in Caprine Blood, and has been expanded to include certified values for inorganic As, Cd, Hg, ethyl-Hg, and methyl-Hg in some or all of its four levels (Murphy et al., in prep).17 In the absence of a certified value for Mn in SRM 955c, we sought to interpret the NYS data obtained from the various sample preparation methods and calibration protocols, and compare them to results for Mn obtained from the CDC and the CTQ, Institut national de santé publique du Québec (INSPQ). Here, we describe an “all-lab” consensus value for Mn in Level 1 of SRM 955c based on DRC-ICP-MS data from NYS, CDC, and CTQ. Another consensus value was calculated based on the “all-method” mean value. We anticipate that NIST will eventually provide a certified value for Mn and full statement of uncertainty at a later time.
Experimental
As part of our experimental approach, we exchanged Mn data on SRM 955c with two other laboratories that also specialize in measuring blood Mn for biomonitoring purposes. Our three laboratories are also responsible for coordinating our own EQAS for trace elements in blood: NYS PT Program for Trace Elements in Whole Blood; CTQ Québec Multielement External Quality Assessment Scheme; and the CDC Lead and Multielement Proficiency Program. The analytical method used by each laboratory is described below and a summary is provided in Table 1.
Table 1.
Summary of instrument platforms, sample preparation methods, and calibration protocols used by each laboratory.
| Laboratory | Instrument Platform | Sample Preparation | Calibration |
|---|---|---|---|
| NYS | 4100ZL | Variable | Variable |
| NYS | DRCII | Variable | Variable |
| NYS | Element2 | Variable | Variable |
| CDC | DRCII | Diluted | Blood-based |
| CTQ | DRCII/NexION | Diluted | Blood-based |
A. NYS
Instrumentation
Instrumental details are given in more detail elsewhere.15 Briefly, a PerkinElmer (PE) Model 4100ZL atomic absorption spectrometer (Shelton, CT, USA) equipped with longitudinal Zeeman background correction was used for the GFAAS work. For DRC-ICP-MS, a PE Elan DRC II ICP-MS was operated in the DRC mode with NH3 as the reaction gas (1.5 mL min−1 flow rate, 0.7 RPq). SF-ICP-MS measurements were conducted using a Thermo Fisher Scientific Element2 (Bremen, DE) operated in medium resolution mode (R = m/Δm = 4000). Both ICP-MS instruments were equipped with a parallel path Burgener Mira Mist® (Mississauga, ON, CA) nebulizer for diluted blood samples, and a glass concentric Meinhard® (Golden, CO, USA) nebulizer for digested blood samples. A PE quartz cyclonic spray chamber was used with the DRC-ICP-MS, while a Thermo quartz double pass spray chamber was used with the SF-ICP-MS.
Reagents and Solutions
Doubly de-ionized (DI) water (>18 MΩ cm) was produced from a NANOpure DiamondTM unit (Barnstead, Waltham, MA, USA) and used for all analytical work. Concentrated HNO3 was distilled in-house from reagent grade acid via a DuoPUR sub-boiling acid still (Milestone, Shelton, CT, USA). Standard solutions for digested blood calibration were prepared gravimetrically from NIST SRM 3132 Manganese Standard Solution, which is certified to contain 10.00 mg g−1 Mn with an expanded uncertainty, U(xNIST), equal to ± 0.02 mg g−1. The SRM 3132 standard was diluted to 1000 mg kg−1, then diluted to an intermediate concentration of 10 mg kg−1, and then further diluted as working standards of 0, 5, 10, 20, 30, 50 μg kg−1 in 0.5% (v/v) HNO3. Standard solutions for diluted blood calibration were prepared volumetrically from a SPEX CertiPrep, Inc. (Metuchen, NJ, USA) Mn standard. This solution is traceable to SRM 3132 and has a certified value of 1000 mg L−1 Mn with an expanded uncertainty (U) equal to ± 3 mg L−1. The SPEX 1000 mg L−1 standard was diluted to an intermediate concentration of 10 mg L−1 and then diluted further to obtain working standards of 0, 5, 10, 20, 30, 50 μg L−1 in 0.5% (v/v) HNO3.The modifier for GFAAS was 0.015% (w/v) Mg(NO3)2 · 6H2O (99.999% metal basis, Sigma-Aldrich Inc., St. Louis, MO, USA) and it also contained 0.1% (v/v) Triton® X-100 (Sigma Ultra Grade, Sigma Aldrich Inc.) and 0.2% (v/v) HNO3.
Two CRMs were used to investigate the accuracy of the blood Mn methods in this study. NIST SRM 1643e Trace Elements in Water has a certified Mn concentration of 38.97 μg L−1 with U(xNIST) equal to ± 0.45 μg L−1. NIST SRM 1598a Inorganic Constituents in Animal Serum is certified for Mn at 1.78 μg L−1 with U(xNIST) equal to 0.33 μg L−1. NIST SRM 955c Toxic Metals in Caprine Blood does not currently have a certified value for Mn, but Level 1 was analyzed for study purposes.
Sample Preparation and Instrument Calibration
For digested blood samples (hereafter referred to as “Dig”), all steps of sample preparation were completed gravimetrically. Approximately 0.8 g of blood was digested overnight in 5.0 mL of HNO3. The following day, 1.0 mL of 30% (v/v) H2O2 (‘Baker Analyzed’ A.C.S. Reagent Grade, J.T. Baker, Phillipsburg, NJ, USA) was added and the samples digested at room temperature for two hours prior to microwave assisted digestion at atmospheric-pressure with a Microwave Assisted Reaction System 5 (MARS5) (CEM Corporation, Matthews, NC, USA). In the MARS5, samples were ramped to 83°C over one hour and then held at 83°C for two hours. Microwave vessels were 15-mL polypropylene conical tubes (Becton Dickinson, Franklin Lakes, NJ, USA) with holes drilled into the caps for venting during digestion. The digestates were diluted after digestion to approximately 17 g with DI water. Before analysis by ICP-MS, 2.5 mL of digestate was diluted 1+1 with a diluent containing 0.5% (v/v) HNO3, 0.005% (v/v) Triton® X-100, and 5 μg L−1 71Ga (GFS Chemicals® Inc., Columbus, OH, USA). This yielded a solution with ~15% (v/v) HNO3 for analysis by ICP-MS. For analysis by GFAAS, digestates were analyzed directly, without further dilution since the concentration of Mn was very low. This meant that the sample deposited on the platform contained ~30% (v/v) HNO3.
In addition to analyzing digested samples, undigested blood was simply diluted volumetrically prior to analysis (hereafter referred to as “Dil”). For ICP-MS measurements, 200 μL of blood were diluted 1+49 with the same diluent used for digested samples. For GFAAS, 100 μL of blood were diluted 1+9 with a modifier solution containing Mg(NO3)2.
Each of the three instrumental techniques used in this study, GFAAS, DRC-ICP-MS, and SF-ICP-MS, were calibrated with either aqueous or blood-based external calibration standards. For each technique, the aqueous calibration standards contained a HNO3 concentration equivalent to that of the sample being analyzed. For blood-based calibration standards, human blood containing ~11 μg L−1 Mn was added to each standard. It should be noted that all mammalian species have some endogenous Mn in their blood, so it is not possible to find a true base blood that is “free” of Mn. The method of standard additions was also investigated as a check on matrix effects occurring within the caprine blood sample. For each SRM 955c sample analyzed, the standard additions curve was composed of a sample blank and then three additional sample aliquots spiked with 10, 20, and 30 μg L−1 of Mn. 71Ga was used as the internal standard for all ICP-MS calibrations and samples.
B. CDC
Analysis of SRM 955c was carried out using a PE Elan DRCII ICP-MS operated in the DRC mode with O2 as the reaction gas (1.2 mL min−1 flow rate, 0.6 RPq). Samples were nebulized with an Elemental Scientific MicroFlow PolyPro-ST nebulizer (Omaha, NE, USA) into a quartz cyclonic spray chamber.
Blood samples (50 μL) were diluted with 50 μL of deionized water (>18 MΩ cm) 1+49 in diluent containing 1% (v/v) CH3CH2OH, 0.25% (v/v) tetramethylammonium hydroxide (TMAH), 0.05% (v/v) Triton X-100®, and 0.01% (v/v) ammonium pyrrolidine dithiocarbamate (APDC). For the external calibration, 50 μL of human blood and 50 μL of standards containing 0, 1.5, 4.5, 10.5, 15, and 30 μg L−1 Mn were diluted 1+49. 103Rh was used as the internal standard for calibration standards and blood samples.
C. CTQ
SRM 955c was analyzed using both a PE Elan DRCII and a PE NexION 300S operated in the standard mode, i.e., without a reaction gas. Both systems were equipped with a cyclonic spray chamber, with an Elemental Scientific MicroFlow PFA-ST nebulizer for the DRCII and a quartz concentric Meinhard® nebulizer for the NexION. The resolution setting for 55Mn was customized in order to minimize overlap from 56Fe.
Blood samples (250 μL) were diluted 1+39 in diluent containing 0.5% (v/v) NH4OH and 0.1% (v/v) octylphenol ethoxylate. External calibration curves were prepared by diluting human blood (250 μL) 1+39 in diluent and spiking with differing volumes of 1 mg L−1 Mn standard solution in order to emulate 0, 8, 40, 160, and 400 μg L−1 in the standards. The internal standard for calibration standards and blood samples was 195Pt on the NexION and 89Y on the DRCII.
Results and Discussion
A. NYS
NIST SRM 1643e Trace Elements in Water
In order to assess measurement accuracy for each method, SRM 1643e was analyzed (Fig. 1). The average number of days of analysis was 7 and the average total number of measurements was 13. Each data point in Fig. 1 represents the grand mean of each daily mean. The error bars represent the U calculated according to:
| (1) |
where, k is the coverage factor (calculated as the two-tailed Student’s t statistic for a 95% confidence interval for n-1 degrees of freedom), sR is the standard deviation for results of each method, n is the number of days of analysis for each method (except for GFAAS+Dig+Aqueous calibration, in which n is four measurements in a single day), and U(xNIST) is the expanded uncertainty reported by NIST.18
Fig. 1.
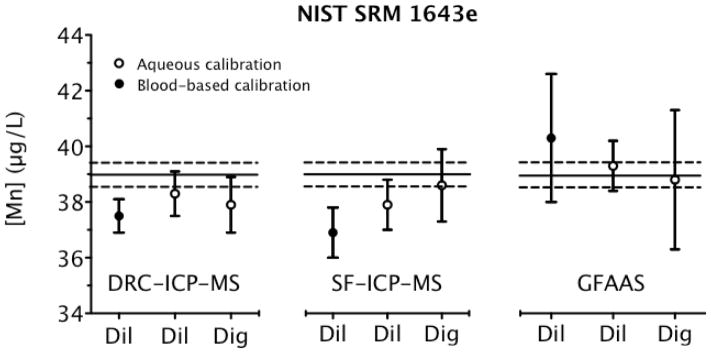
Analysis of NIST SRM 1643e Trace Elements in Water for Mn determined via three instrumental techniques, comparing dilution with digestion, and aqueous and blood-based calibration protocols. Each data point is the mean ±U (error bars). The solid black line is the certified value for Mn and the dashed lines represent U(xNIST) limits from the NIST certificate.
In Fig. 1, results from each method are compared to the certified value (solid line) and U(xNIST) (dashed line) for SRM 1643e. Each mean value derived from an aqueous calibration curve is in reasonable agreement with the certified value ±U(xNIST). Thus, both the acid digestion and dilution sample preparations are validated for water with an aqueous calibration. Also, the density correction is validated since the units of the digested samples prepared gravimetrically were μg kg−1 and were then converted into μg L−1 using a density value (1.0250 g mL−1) inferred from data provided on the certificate for SRM 1643e.
In contrast to the aqueous calibration, the blood-based calibration for both ICP-MS instruments exhibits an unacceptable low bias for SRM 1643e. Results from a blood-based calibration with GFAAS show a positive bias, but the uncertainty encompasses the certified value. The difference in calibration slope between the blood-based and aqueous calibration standards explains this discrepancy. For GFAAS, the blood-based calibration slope is on average 3% less than the aqueous slope, thus yielding higher values than those calculated with an aqueous calibration line. On the contrary, a blood-based calibration with ICP-MS yields a slope that is 3% greater than the aqueous calibration, and, on average, results from a blood-based calibration will be lower than those from an aqueous calibration. In additional investigations, it is clear that a calibration line based on digested blood gives a 3% lower slope than an aqueous calibration (data not shown). This suggests that the SRM 1643e results should be about 3% higher with a calibration including digested blood compared to an aqueous calibration.
Matrix effects are well known in ICP-MS and the use of an appropriate internal standard is one way to correct for such effects.19 Here, we used 71Ga as the internal standard for 55Mn. Differences between slopes for the various calibration protocols used with ICP-MS as described above were based on the ratio of the analyte to internal standard counts. Evidently, 71Ga does not completely correct for biological matrix effects, as there is a residual discrepancy in the slope of −7 to −1% for a digested blood matrix, and −2 to +6% for diluted blood matrix relative to an aqueous calibration curve for DRC-ICP-MS. Without an internal standard, these discrepancies in the slope intensify from −18 to +10% for digested blood and from −24 to +15% for diluted blood compared to aqueous calibration curves. The average 3% discrepancy between aqueous and blood-based calibration is clinically insignificant, but it is much more important when assigning a certified value to a reference material.
While there are no previous reports in the literature regarding blood matrix effects for Mn with ICP-MS, there are two reports for 52Cr in blood, which is close in mass to 55Mn. First, Sarmiento-Gonzalez et al.20 reported a difference of ~ +15% between digested blood-based calibration slopes compared to aqueous calibration without an internal standard and ~ −1% with 71Ga internal standard for SF-ICP-MS (Element). Additionally, when using Q-ICP-MS (Agilent 7500c), the difference was found to be ~ +13% without versus ~ −1% with 71Ga internal standard.20 Second, Bonnefoy et al.21 found a −26 to −14% difference in the uncorrected slopes of a blood-based calibration compared to an aqueous calibration on a PE Sciex 6100 with NH3 as the DRC gas. These previous reports for 52Cr are consistent with our observations reported here for 55Mn: that significant matrix effects from blood can complicate measurements performed by ICP-MS, and while the internal standard can help correct to some extent, residual effects persist. In this study, we investigated several alternatives to 71Ga as the internal standard including 45Sc, 69Ga, 74Ge, 103Rh, and 193Ir, but none of them adequately corrected for blood matrix effects (data not shown). Therefore, 71Ga was selected as the internal standard for 55Mn determination in a blood matrix.
NIST SRM 1598a Inorganic Constituents in Animal Serum
Serum is the liquid component of whole blood that remains after the red blood cells clot, yet it is somewhat similar to whole blood inasmuch as it contains dissolved ions and proteins. SRM 1598a is one of just a few biological CRMs for which a certified value for Mn exists. SRM 1598a was analyzed for Mn content to assess method bias using a matrix that was more representative of blood than water. Fig. 2 shows the results of the SRM 1598a analyses using different instruments, sample preparation methods, and calibration protocols. The total number of measurements performed was seven on average, while the average number of days of analysis was four. Each data point in Fig. 2 shows the grand mean calculated from each daily mean value. The error bars represent U calculated according to eqn (1) (n is the number of days analyzed, except for GFAAS+Dig+Aqueous calibration, where n is four measurements collected in a single day). The density value (1.0274 g mL−1) obtained from the SRM 1598a certificate was used to convert the mass fraction (μg kg−1) for Mn into concentration units (μg L−1). All methods yielded results that were in good agreement with the certified value and U(xNIST).
Fig. 2.
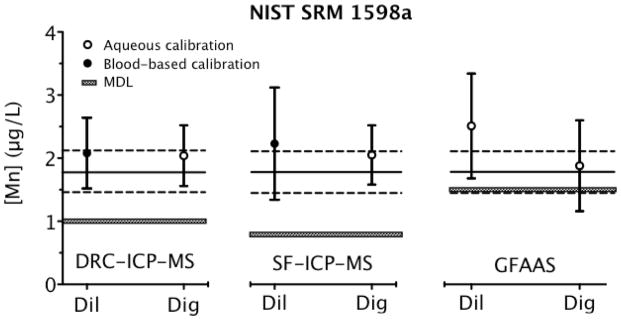
Analysis of NIST SRM 1598a Inorganic Constituents in Animal Serum for Mn determined via three instrumental techniques, comparing dilution with digestion, and aqueous and blood-based calibration protocols. Each data point is the mean ±U (error bars). The solid black line is the certified value for Mn and the dashed lines represent U(xNIST) limits from the NIST certificate. The method detection limits (MDL) are also shown as gray bars for each technique.
The certified value assigned to Mn in SRM 1598a is accompanied by a relative U(xNIST) (19%) that is much larger than the corresponding value cited for Mn relative U(xNIST) (1%) in SRM 1643e. This is most likely due to the value in SRM 1598a being closer to the method detection limits (MDL) of the certification methods. Estimates for the relative U in our Mn values for these SRMs are larger than those cited on the NIST certificates. For example, our average U in the reported value for Mn in SRM 1598a was 31% relative, while for SRM 1643e it was 3% relative. It should be noted that our results for Mn in SRM 1598a are very close to our MDLs (0.8–1.5 μg L−1), making quantification less precise. Discrepancies due to sample preparation method, calibration protocol, or instrumental technique used were not observed with SRM 1598a, probably on account of the low Mn content.
NIST SRM 955c Toxic Metals in Caprine Blood
There is currently no certified value for Mn in SRM 955c. Therefore, a key goal of this study was to characterize the Mn content of SRM 955c for interlaboratory comparison purposes. We analyzed Level 1 of this material using different sample preparation methods, calibration protocols and instrumental techniques to investigate how these variables might affect Mn results. The average number of days of analysis was 6 and the average total number of measurements was 17. Each data point in Fig. 3 represents the grand mean of each daily mean. The error bars represent U calculated as shown:
| (2) |
Fig. 3.
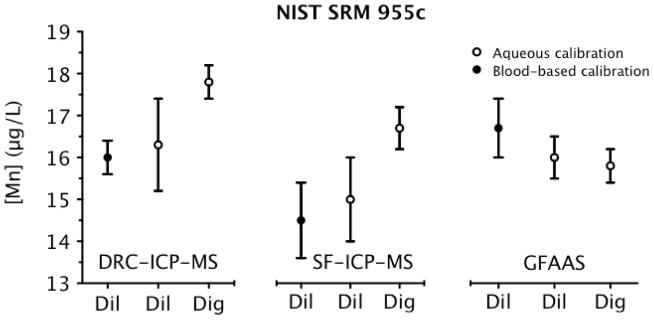
Analysis of NIST SRM 955c Toxic Metals in Caprine Blood – Level 1 for Mn determined via three instrumental techniques, comparing dilution with digestion, and aqueous and blood-based calibration protocols. Each data point is the mean ±U (error bars). NIST SRM 955c currently has no certified value for Mn.
Again, n is the number of days of analysis, except for GFAAS+Dig+Aqueous calibration in which n is 16 measurements in a single day. Eqn (2) is similar to eqn (1), which was used for calculating U for Mn in SRMs 1643e and 1598a. However, since there is no certified value (yet) for Mn in 955c, there is no CRM uncertainty term included in eqn (2).
It is evident from Fig. 3 that similar discrepancies exist with ICP-MS consistent with those observed for SRM 1643e (Fig. 1) between use of a blood-based versus aqueous calibration protocol. That is, Mn values based on using diluted blood in combination with a blood-based calibration curve were lower relative to an aqueous calibration protocol when determined by ICP-MS. The fact that lower values were observed by ICP-MS regardless of the technology used, i.e., both SF- and Q-ICP-MS instrumentation were consistently low, suggests the cause is not due to a spectral or polyatomic interference. In contrast, Mn results by ICP-MS based on the analysis of digested blood and using an aqueous calibration line were higher compared to those based on analyzing diluted blood and using a blood-based calibration line. The discrepancy in this case was 11% for DRC-ICP-MS and 13% for SF-ICP-MS. In the case of DRC-ICP-MS, 6% of the discrepancy can be attributed to the observation that results from a diluted blood-based calibration protocol yields a 3% negative bias on average, while results from a digested blood-based line yields a 3% positive bias on average relative to an aqueous calibration protocol. In the case of SF-ICP-MS, as much as 8% can be attributed to calibration protocol differences since the effects are slightly worse with SF-ICP-MS compared to DRC-ICP-MS. This would leave a balance of ~5% of the observed discrepancy that is unexplained, but which could be due to uncertainties in the gravimetric versus volumetric sample preparation procedures. Additional uncertainty may be attributed to use of the certified density value (1.05182 g mL−1) from the SRM 955c certificate to convert mass fractions (μg kg−1) for Mn into concentration units (μg L−1). Another potential source of uncertainty that might explain the 5% discrepancy is between-vial heterogeneity for SRM 955c – Level 1. Since some degree of material heterogeneity was found to be statistically significant in Level 1 for Pb, Cd, and total Hg,22 it is entirely possible that heterogeneity is also a factor for Mn, and may explain the discrepancies observed between the various methods.
In order to investigate further the observed discrepancies between the various sample preparation and calibration protocols for ICP-MS techniques, the method of standard additions was explored for both diluted and digested SRM 955c samples with analysis by DRC-ICP-MS (Table 2). When analyzing diluted blood, an aqueous calibration curve yields higher values compared to either a blood-based calibration or the method of standard additions. It is interesting to note that calibration with blood-based and standard additions both produce similar results, and both are ~3% lower than the corresponding value based on aqueous calibration. This is entirely consistent with the observations reported above for SRM 1643e (water). For digested blood, the SRM 955c value from aqueous calibration is 2% higher than that based on standard additions. This is contrary to what would be expected. As stated in the SRM 1643e discussion, additional investigations show that the result from a digested blood matrix-matched curve should average out to be ~3% higher than the result from an aqueous calibration. Regardless, the data from SRM 955c standard additions suggest that calibration protocol alone does not fully account for the discrepancies mentioned in the preceding paragraph that were observed between analyzing diluted blood with a blood-based calibration compared to digested blood analyzed with an aqueous calibration.
Table 2.
Comparison of NYS calibration protocols for Mn determination in NIST SRM 955c –Level 1. Mn concentrations are in μg L−1 (U) for four measurements within a single day using DRC-ICP-MS.
| Calibration Mode | Diluted Blood | Digested Blood |
|---|---|---|
| Aqueous | 16.8 (0.2) | 17.1 (0.3) |
| Blood-based | 16.4 (0.2) | |
| Standard additions | 16.2 (1.2) | 16.8 (0.2) |
In GFAAS, the same trend is seen with SRM 955c as with SRM 1643e, that Mn values determined by a blood-based calibration are higher than those determined by an aqueous calibration. However, both digested and diluted blood samples analyzed with an aqueous calibration line yield the same result. This suggests that with GFAAS, digested and diluted blood samples behave in a similar manner. Given digested blood was only analyzed on one day, and given the uncertainty associated with the density conversion from μg kg−1 to μg L−1, one cannot be sure that differences are absent.
Analysis of SRM 955c for Mn using varying sample preparation methods, calibration protocols, and instrumental techniques has yielded a spread of values ranging from 14.5 μg L−1 with SF-ICP-MS+Dil+Blood-based calibration to 17.8 μg L−1 with DRC-ICP-MS+Dig+Aqueous calibration. This spread of values would be acceptable in the context of clinical or occupational exposure assessments. However, from a biomonitoring perspective, reduced uncertainty and metrological traceability are preferred so that different populations can be compared across studies. In order to place our found values based on different methods in the context of data produced by other labs, we compared our results to data on Mn in SRM 955c – Level 1 provided by CDC and CTQ.
B. Clinical laboratory comparison of data on Mn in SRM 955c – Level 1
Interestingly, the Mn data provided by both CDC and CTQ show a spread of values for SRM 955c – Level 1. The CDC provided data obtained from three different PE DRCII instruments with two different analysts and their results ranged from 14.9 to 17.4 μg L−1, with a mean value of 16.6 μg L−1. The CTQ provided data from two ICP-MS instruments (a DRCII and a NexION) with two different analysts and their Mn results ranged from 14.9 to 17.5 μg L−1, with a mean value of 15.9 μg L−1 for the DRCII and 17.1 μg L−1 for the NexION. A summary of the Mn data obtained on SRM 955c – Level 1 from CDC, CTQ, and NYS based on DRC-ICP-MS instrumentation, with simple dilution sample preparation and a blood-based calibration protocol is provided in Table 3a. The U for mean value from each lab was calculated using eqn (2). The CTQ value is combined data from both the DRCII and NexION instruments, operated in standard mode but with a “customized” resolution designed to reduce the degree of Fe overlap at m/z 55. We used the combined data from the three clinical laboratories to compute an all-lab consensus value of 16.3 ± 0.8 μg L−1, based on the un-weighted mean of each lab value, and U was calculated according to eqn (2) with two degrees of freedom. For comparison purposes, the mean value from NYS based on standard additions by DRC-ICP-MS is also provided in Table 3a showing that blood-based and standard additions calibration protocols yield similar results.
Table 3.
Consensus values for Mn in NIST SRM 955c – Level 1 as determined by (a) all labs: CDC, CTQ, and NYS, and (b) all-methods.
| (a) All labs
| |||
|---|---|---|---|
| Lab | Mean [Mn] (μg L−1) | U (μg L−1) | n (days) |
| CDC DRCII | 16.6 | 0.3 | 13 |
| CTQ DRCII/NexION | 16.4 | 0.7 | 8 |
| NYS DRCII | 16.0 | 0.4 | 5 |
|
| |||
| NYS DRCII (Standard additions) | 16.2 | 1.2 | 1 |
|
| |||
| All-lab consensus value: | 16.3 | 0.8 | |
| (b) All methods
| |||
|---|---|---|---|
| Method | Mean [Mn] (μg L−1) | U (μg L−1) | n (days) |
| NYS SF-ICP-MS (Dig+Aqueous calibration) | 16.7 | 0.5 | 4 |
| NYS GFAAS (Dig+Aqueous calibration) | 16.0 | 0.5 | 16 |
|
| |||
| All lab DRC-ICP-MS | 16.3 | 0.8 | 24 |
| All-method consensus value: | 16.3 | 0.9 | |
In addition to an all-lab consensus value, we also calculated an all-method consensus value with data from three instrumental techniques (Table 3b). The all-method consensus value includes the all-lab consensus value based on DRC-ICP-MS and data from GFAAS and SF-ICP-MS provided by NYS. With regard to the latter technique and from our previous work, analysis by SF-ICP-MS combined with a dilution sample preparation and blood-based calibration consistently yields a negative bias when compared to other instrumental techniques and assigned values of archived PT/EQAS samples.15 Thus, here we chose to represent SF-ICP-MS by including data based on the analysis of digested blood with an aqueous calibration protocol. For GFAAS, however, we used the dilution sample preparation with an aqueous calibration, since this approach has shown excellent performance in PT/EQAS for both archived and current samples.15 With this approach, an all-method consensus mean value was also computed along with U (eqn (2), two degrees of freedom) and found to be 16.3 ± 0.9 μg L−1.
Fig. 4 depicts the range of values that contribute to the all-method consensus mean, which is shown as a solid horizontal line. The limits of U, equivalent to 5.5% relative, are shown as dashed lines in Fig. 4. Each method value and lab-specific value is shown along with U (error bars). From the all-method consensus value, we have 95% confidence that the true value lies somewhere between 15.4 and 17.2 μg L−1. Considering each of the NYS investigational methods in the context of the all-method consensus value, U of each would have some overlap with the 95% confidence interval of this value, with the exception of the SF-ICP-MS+Dil+Blood-based calibration, and the DRC-ICP-MS+Dig+Aqueous calibration experiments.
Fig. 4.
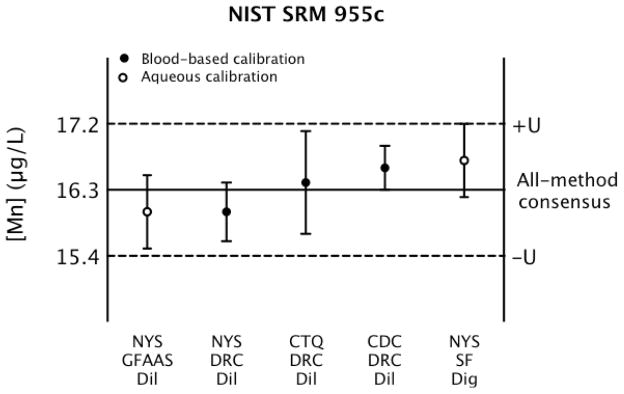
All-method consensus value (solid line) and associated U (dashed lines) for NIST SRM 955c – Level 1 showing mean values and U (error bars) calculated for method-specific results as reported by NYS, CDC, and CTQ.
SRM 955c – Level 1 has a clinically relevant Mn concentration, as the “reference concentration” for Mn in human blood according to the US Agency for Toxic Substances and Disease Registry is 4–15 μg L−1.23 The U for our consensus values could be underestimated, as a comprehensive determination of all sources of uncertainty was not completed. However, the consensus values and associated U give us some idea of what variability can be expected between laboratories using similar methods (all-lab) and of the variability between methods (all-method) at this Mn concentration.
Conclusions
Investigation into the effects of sample preparation methods, calibration protocols, and instrumental techniques revealed multiple sources of uncertainty that complicate the determination of Mn in whole blood. Discrepancies were seen due to matrix effects in ICP-MS that do not appear to be fully resolved by using an internal standard. Gravimetric and volumetric sample preparations yielded disparate results that may be further complicated by use of the blood density value to convert mass fraction into concentration units. In the absence of a definitive method of the highest metrological order, and without an existing blood-based CRM certified for Mn, establishing traceability to SI and reducing the relative uncertainty to less than 5% remains a daunting analytical challenge, and this may not be possible if the material is heterogeneous. However, despite this, we have established a consensus value for the Mn content in SRM 955c – Level 1 based on an all-method mean value of data provided by NYS, CDC and CTQ, and representing SF-ICP-MS, DRC-ICP-MS and GFAAS techniques. The U for this consensus value is ~5.5% relative, but we recognize there may be additional sources of uncertainty that are not accounted for. Certified values with a full statement of uncertainty for Mn in blood are desperately needed for biomonitoring purposes so that consistent results can be compared across labs, and across different populations. The present study gives us some insight into analytical variability in the determination of blood Mn. This is an important context for biomonitoring and epidemiological studies that seek to compare blood Mn of populations, as the difference between populations may be due to methodological variation rather than Mn exposures.
Acknowledgments
The authors are grateful to the staff of the NYS DOH Laboratory of Inorganic and Nuclear Chemistry’s Clinical Trace Elements section for advice and technical assistance, and we thank Dr. John Arnason for his assistance with SF-ICP-MS. We acknowledge NIST for providing SRM 1643e and 1598a materials for this study, and we are especially grateful to Karen Murphy and Will Guthrie of NIST for many helpful discussions. We also thank Dr. Ryszard Gajek of the California Department of Public Health for sharing his insights on SRM 955c. This work was supported in part by Cooperative Agreement Number U38 EH000464-02 from the US CDC. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
References
- 1.Shamberger RJ. In: Toxicity of Heavy Metals in the Environment. Oehme FW, editor. Vol. 2. Marcel Dekker, Inc; New York, NY: 1979. pp. 716–721. ch 29. [Google Scholar]
- 2.Meyer-Baron M, Knapp G, Schaper M, van Thriel C. Neurotoxicology. 2009;30:487–496. doi: 10.1016/j.neuro.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- 4.Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, Kline J, van Geen A, Mey J, Slavkovich V, Siddique AB, Islam T, Graziano JH. NeuroToxicology. 2011;32:450–457. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur M, Bouffard T, Limoges E, Bellinger DC, Mergler D. Env Health Persp. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Environ Health Persp. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, Rodriguez-Agudelo Y. Environ Health Perspect. 2010;118:1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henn BC, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M, Amarasiriwardena C, Hu H, Bellinger DC, Wright RO, Téllez-Rojo MM. Environ Health Perspect. 2012;120:126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, Bhang SY, Cho SC. NeuroToxicology. 2009;30:564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, Weisskopf MG. J Occup Environ Med. 2011;53:506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, Lu J, Fu X, Li W, Liu S, Long Q, Huang J, Pira E. NeuroToxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montes S, Riojas-Rodriguez H, Sabido-Pedraza E, Rios C. Environ Res. 2008;106:89–95. doi: 10.1016/j.envres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Cotzias GC, Papavasiliou PS, Miller ST. Nature. 1964;201:1228–1229. doi: 10.1038/2011228a0. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W, Fu SX, Dydak U, Cowan DM. NeuroToxicology. 2011;32:1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Praamsma ML, Arnason JG, Parsons PJ. J Anal At Spectrom. 2011;26:1224–1232. [Google Scholar]
- 16.Murphy KE, Guthrie WF, Vetter TW, Turk GC, Palmer CD, Lewis ME, Jr, Geraghty CM, Parsons PJ. J Anal At Spectrom. 2009;24:1170–1178. [Google Scholar]
- 17.Davis WC, Long SE. J Anal At Spectrom. 2011;26:431–435. [Google Scholar]
- 18.Sharpless KE, Duewer DL. J AOAC Int. 2008;91:1298–1302. [PubMed] [Google Scholar]
- 19.Vanhaecke F, Vanhoe H, Dams R, Vandecasteele C. Talanta. 1992;39:737–742. doi: 10.1016/0039-9140(92)80088-u. [DOI] [PubMed] [Google Scholar]
- 20.Sarmiento-González A, Marchante-Gayón JM, Tejerina-Lobo JM, Paz-Jiménez J, Sanz-Medel A. Anal Bioanal Chem. 2005;382:1001–1009. doi: 10.1007/s00216-005-3165-9. [DOI] [PubMed] [Google Scholar]
- 21.Bonnefoy C, Menudier A, Moesch C, Lachâtre G, Mermet JM. Anal Bioanal Chem. 2005;383:167–173. doi: 10.1007/s00216-005-3403-1. [DOI] [PubMed] [Google Scholar]
- 22.NIST Certificate of Analysis. SRM 955c Toxic Metals in Caprine Blood. 2010 Jul; http://www.nist.gov/srm.
- 23.ATSDR. Draft Toxicological Profile for Manganese. U.S. Department of Health and Human Services Public Health Service; Atlanta, GA: 2008. [Google Scholar]


