Abstract
Cys-231 of Torpedo californica acetylcholinesterase (EC 3.1.1.7) was selectively labeled with the mercury derivative of a stable nitroxyl radical. In 1.5 M guanidinium chloride, this conjugate exists in a molten globule state (MG), whereas in 5 M denaturant, it is in an unfolded state (U). The transition between the two states is reversible. In the MG, the label is highly immobilized, whereas in the U, it is almost freely rotating. The clearly distinct electron paramagnetic resonance (EPR) spectra of the two states permits the study of this transition. Upon elevating the guanidinium chloride concentration, a decrease in the EPR signal of the MG occurs concomitantly with an increase in the U signal, the total intensity of the EPR spectra remaining constant. This behavior is characteristic of a two-state transition. The thermodynamic characteristics of this transition (delta G0 and m), whether estimated directly from the EPR data or from both CD and fluorescence data analyzed by assuming a two-state scheme, are in good agreement.
Full text
PDF
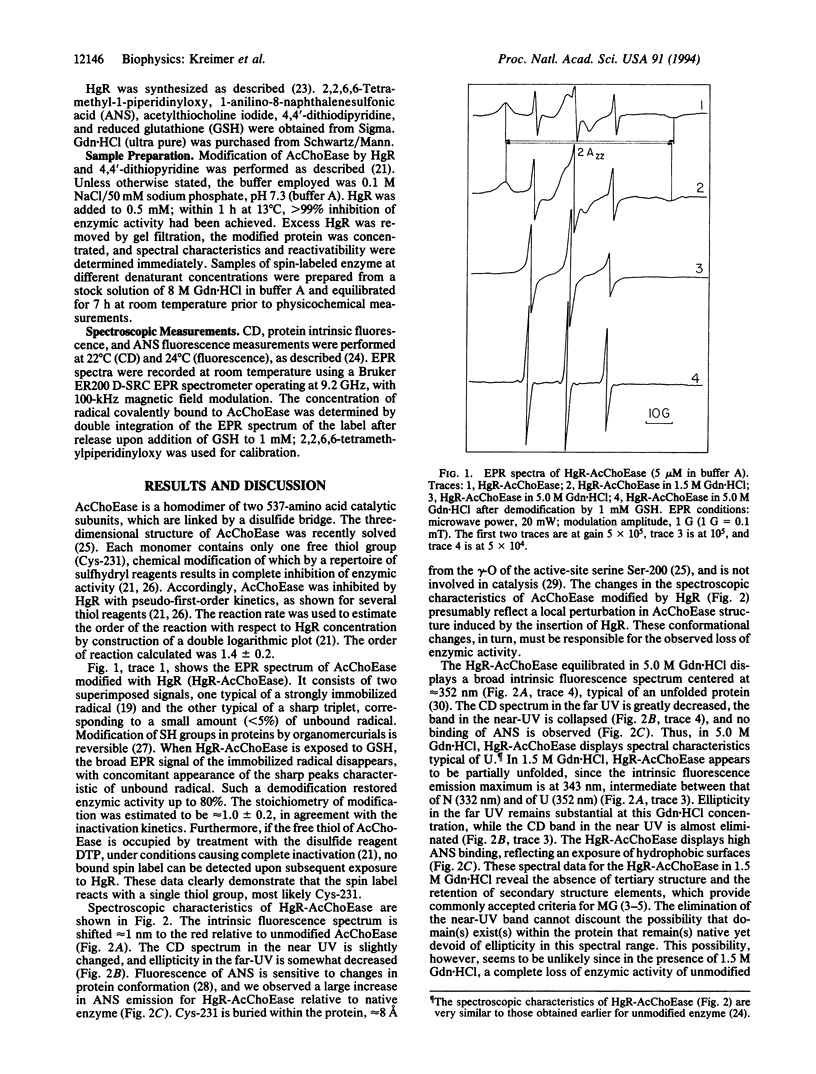

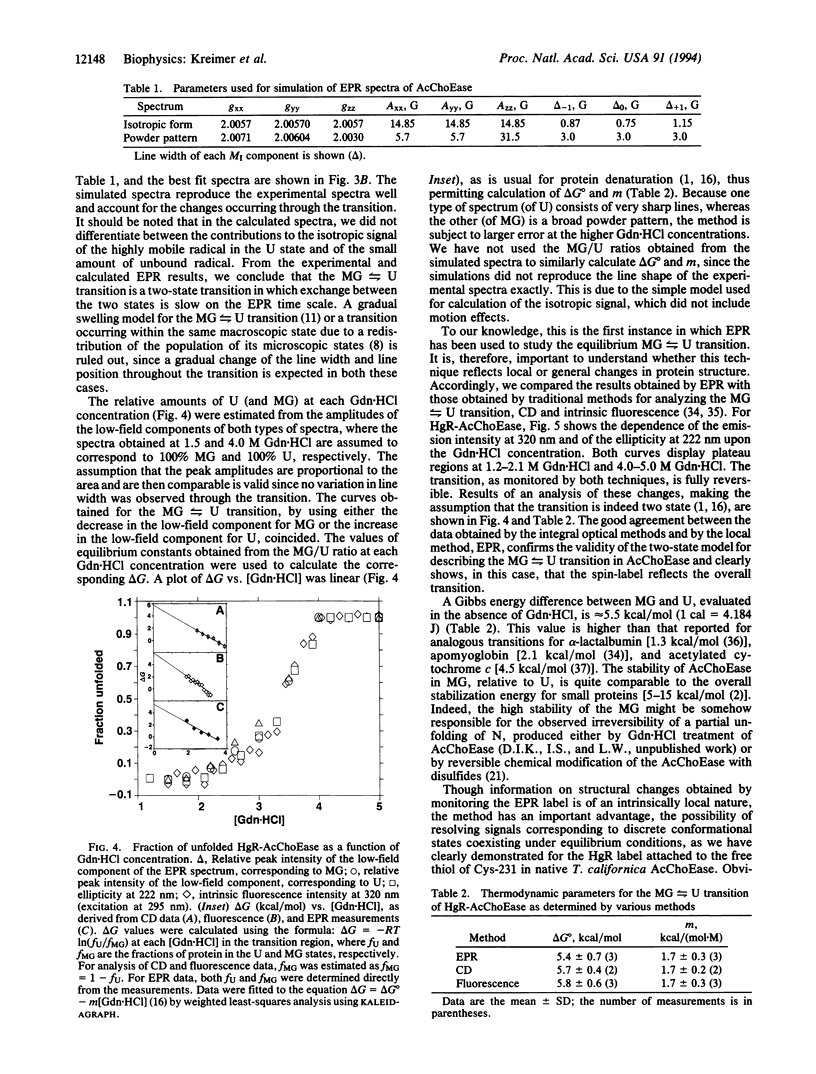
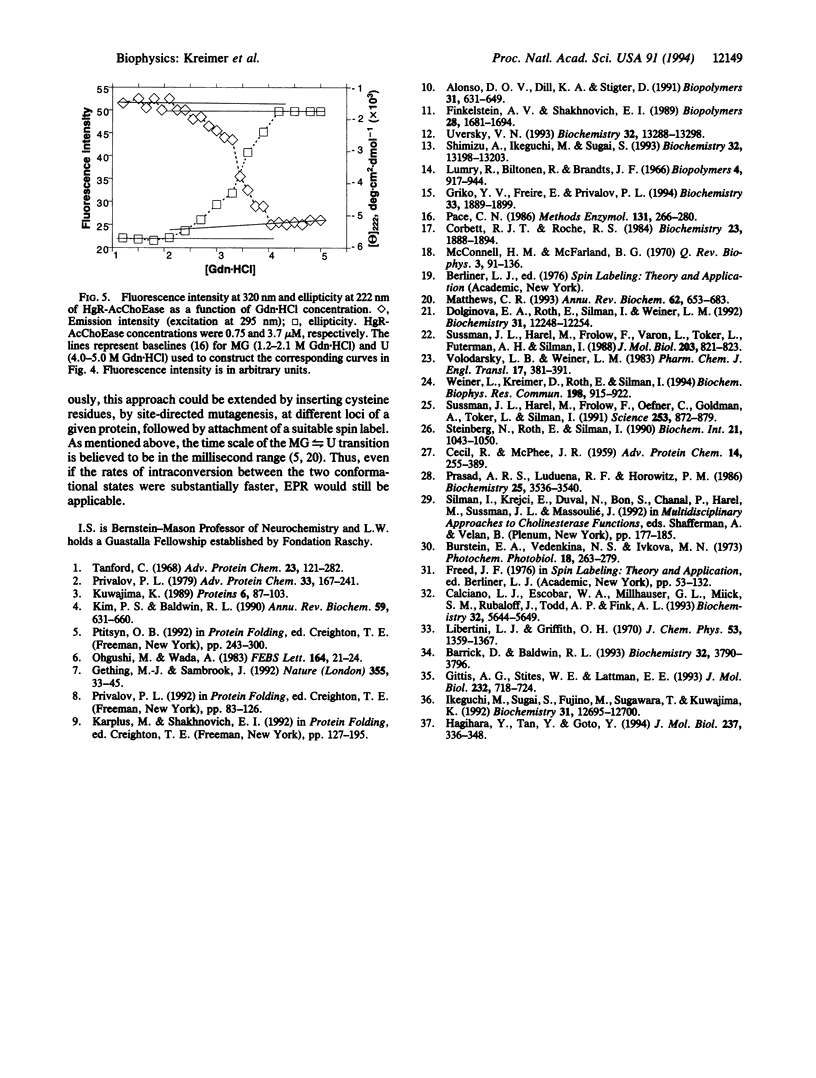
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrick D., Baldwin R. L. Three-state analysis of sperm whale apomyoglobin folding. Biochemistry. 1993 Apr 13;32(14):3790–3796. doi: 10.1021/bi00065a035. [DOI] [PubMed] [Google Scholar]
- Burstein E. A., Vedenkina N. S., Ivkova M. N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol. 1973 Oct;18(4):263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- CECIL R., McPHEE J. R. The sulfur chemistry of proteins. Adv Protein Chem. 1959;14:255–389. doi: 10.1016/s0065-3233(08)60613-0. [DOI] [PubMed] [Google Scholar]
- Calciano L. J., Escobar W. A., Millhauser G. L., Miick S. M., Rubaloff J., Todd A. P., Fink A. L. Side-chain mobility of the beta-lactamase A state probed by electron spin resonance spectroscopy. Biochemistry. 1993 Jun 1;32(21):5644–5649. doi: 10.1021/bi00072a021. [DOI] [PubMed] [Google Scholar]
- Corbett R. J., Roche R. S. Use of high-speed size-exclusion chromatography for the study of protein folding and stability. Biochemistry. 1984 Apr 10;23(8):1888–1894. doi: 10.1021/bi00303a047. [DOI] [PubMed] [Google Scholar]
- Dolginova E. A., Roth E., Silman I., Weiner L. M. Chemical modification of Torpedo acetylcholinesterase by disulfides: appearance of a "molten globule" state. Biochemistry. 1992 Dec 8;31(48):12248–12254. doi: 10.1021/bi00163a039. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. V., Shakhnovich E. I. Theory of cooperative transitions in protein molecules. II. Phase diagram for a protein molecule in solution. Biopolymers. 1989 Oct;28(10):1681–1694. doi: 10.1002/bip.360281004. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gittis A. G., Stites W. E., Lattman E. E. The phase transition between a compact denatured state and a random coil state in staphylococcal nuclease is first-order. J Mol Biol. 1993 Aug 5;232(3):718–724. doi: 10.1006/jmbi.1993.1425. [DOI] [PubMed] [Google Scholar]
- Griko Y. V., Freire E., Privalov P. L. Energetics of the alpha-lactalbumin states: a calorimetric and statistical thermodynamic study. Biochemistry. 1994 Feb 22;33(7):1889–1899. doi: 10.1021/bi00173a036. [DOI] [PubMed] [Google Scholar]
- Hagihara Y., Tan Y., Goto Y. Comparison of the conformational stability of the molten globule and native states of horse cytochrome c. Effects of acetylation, heat, urea and guanidine-hydrochloride. J Mol Biol. 1994 Apr 1;237(3):336–348. doi: 10.1006/jmbi.1994.1234. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M., Sugai S., Fujino M., Sugawara T., Kuwajima K. Contribution of the 6-120 disulfide bond of alpha-lactalbumin to the stabilities of its native and molten globule states. Biochemistry. 1992 Dec 22;31(50):12695–12700. doi: 10.1021/bi00165a021. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 1989;6(2):87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- Lumry R., Biltonen R. Validity of the "two-state" hypothesis for conformational transitions of proteins. Biopolymers. 1966 Sep;4(8):917–944. doi: 10.1002/bip.1966.360040808. [DOI] [PubMed] [Google Scholar]
- Matthews C. R. Pathways of protein folding. Annu Rev Biochem. 1993;62:653–683. doi: 10.1146/annurev.bi.62.070193.003253. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Neri P., Corti M., Lozzi L., Valensin P. E. Structure and antigenic activity of rubella E1 glycoprotein synthetic peptides. Biopolymers. 1991 May;31(6):631–635. doi: 10.1002/bip.360310607. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Wada A. 'Molten-globule state': a compact form of globular proteins with mobile side-chains. FEBS Lett. 1983 Nov 28;164(1):21–24. doi: 10.1016/0014-5793(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Pace C. N. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- Prasad A. R., Luduena R. F., Horowitz P. M. Detection of energy transfer between tryptophan residues in the tubulin molecule and bound bis(8-anilinonaphthalene-1-sulfonate), an inhibitor of microtubule assembly, that binds to a flexible region on tubulin. Biochemistry. 1986 Jun 17;25(12):3536–3540. doi: 10.1021/bi00360a010. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Ikeguchi M., Sugai S. Unfolding of the molten globule state of alpha-lactalbumin studied by 1H NMR. Biochemistry. 1993 Dec 7;32(48):13198–13203. doi: 10.1021/bi00211a031. [DOI] [PubMed] [Google Scholar]
- Steinberg N., Roth E., Silman I. Torpedo acetylcholinesterase is inactivated by thiol reagents. Biochem Int. 1990 Sep;21(6):1043–1050. [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Varon L., Toker L., Futerman A. H., Silman I. Purification and crystallization of a dimeric form of acetylcholinesterase from Torpedo californica subsequent to solubilization with phosphatidylinositol-specific phospholipase C. J Mol Biol. 1988 Oct 5;203(3):821–823. doi: 10.1016/0022-2836(88)90213-6. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Uversky V. N. Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry. 1993 Dec 7;32(48):13288–13298. doi: 10.1021/bi00211a042. [DOI] [PubMed] [Google Scholar]
- Weiner L., Kreimer D., Roth E., Silman I. Oxidative stress transforms acetylcholinesterase to a molten-globule-like state. Biochem Biophys Res Commun. 1994 Feb 15;198(3):915–922. doi: 10.1006/bbrc.1994.1130. [DOI] [PubMed] [Google Scholar]



