Abstract
A special group of mitochondrial outer membrane (MOM) proteins spans the membrane several times via multiple helical segments. Such multispan proteins are synthesized on cytosolic ribosomes before their targeting to mitochondria and insertion into the MOM. Previous work recognized the import receptor Tom70 and the mitochondrial import (MIM) complex, both residents of the MOM, as required for optimal biogenesis of these proteins. However, their involvement is not sufficient to explain either the entire import pathway or its regulation. To identify additional factors that are involved in the biogenesis of MOM multispan proteins, we performed complementary high-throughput visual and growth screens in Saccharomyces cerevisiae. Cardiolipin (CL) synthase (Crd1) appeared as a candidate in both screens. Our results indeed demonstrate lower steady-state levels of the multispan proteins Ugo1, Scm4, and Om14 in mitochondria from crd1Δ cells. Importantly, MOM single-span proteins were not affected by this mutation. Furthermore, organelles lacking Crd1 had a lower in vitro capacity to import newly synthesized Ugo1 and Scm4 molecules. Crd1, which is located in the mitochondrial inner membrane, condenses phosphatidylglycerol together with CDP-diacylglycerol to obtain de novo synthesized CL molecules. Hence, our findings suggest that CL is an important component in the biogenesis of MOM multispan proteins.
INTRODUCTION
All mitochondrial outer membrane (MOM) proteins are nuclearly encoded and synthesized on cytosolic ribosomes. Therefore, they have to bear proper signals that ensure both their correct import into the organelle and their ability to acquire different topologies in the lipid bilayer. None of the known MOM proteins contain a canonical cleavable N-terminal presequence; rather, they carry internal noncleavable targeting and sorting signals that are difficult to identify (1). Multispan proteins comprise a distinct class of such proteins embedded into the lipid bilayer via multiple α-helical transmembrane segments (TMS) that are interconnected by loops. Some of them, like Fzo1 in yeast (Mfn1/2 in mammals), cross the membrane twice, exposing N- and C-terminal domains toward the cytosol. Additional multispan MOM proteins with three or more TMSs are, for example, Ugo1, Scm4, and Om14 in Saccharomyces cerevisiae and the human peripheral benzodiazepine receptor (PBR). Members of this group fulfil various functions such as serving as a mitochondrial receptor for cytosolic ribosomes (Om14) or mediating mitochondrial fusion and dynamics (Fzo1 and Ugo1).
Studies on the import pathway of multispan proteins suggested that import receptors appear to play a role in the membrane integration of these proteins. For example, Fzo1 was reported to require a protease-sensitive import receptor(s) for its integration into the MOM (2). Furthermore, import of PBR and Mfn2 into mammalian organelles involves interactions with Tom70 and an unknown intermembrane space (IMS) component but is independent of other components of the translocase of the outer membrane (TOM) (3). Recently, we and others reported on a unique import pathway in yeast for MOM multispan proteins (4, 5). This pathway involves the import receptor Tom70 but not its partner, Tom20, in the initial recognition of the multispan precursor proteins. Other TOM subunits and components residing in the mitochondrial IMS were not required for this process. On the other hand, the MOM protein Mim1 was found to play a crucial role in the membrane integration of these proteins (4, 5). In a subsequent study, the novel protein Mim2 was demonstrated to interact with Mim1 and to form with the latter the functional MIM insertase complex (6).
Despite the recent progress, the full mechanism by which multispan proteins are recognized and inserted into the MOM remains poorly defined. To shed new light on this process, we performed two high-throughput screens covering all yeast mutants' backgrounds using the model multispan protein Om14. The first screen was based on the ability to visually monitor the correct intracellular distribution of the green fluorescent protein-Om14 (GFP-Om14) fusion protein on all yeast mutant backgrounds. The second screen included the ability of strains expressing the chimeric protein Ura3-Om14-Degron to grow on medium lacking uracil as a probe for the correct membrane topology of the protein. One mutated gene that was identified by both screens as an effector of integration was the cardiolipin (CL) synthase Crd1.
CL is considered to be the signature lipid of mitochondria as it is rarely found in any other cellular membrane whereas in mitochondria it compromises a large fraction, about 10% to 15%, of total phospholipids (7–9). De novo synthesis of CL occurs within mitochondria, and the CL synthase, Crd1, is essential for the last reaction in this multistep pathway (10, 11). Although CL was traditionally believed to exist only in the mitochondrial inner membrane, several studies demonstrated that it is also a component of the MOM as well as of contact sites between the two membranes (7, 9, 12). CL is essential for the function of many proteins residing in mitochondrial membranes. It interacts with proteins in the mitochondrial inner membrane such as the ADP/ATP carrier and respiratory chain complexes facilitating formation of respiratory supercomplexes that are essential for energy production by respiration (13–16). However, the importance of CL extends beyond respiration, as it also modulates the function of the general translocase of the MOM, the TOM complex (12). Deficiency in CL therefore leads to many alterations in cellular functions such as mitochondrial dynamics, mitochondrial protein import, apoptosis, cell cycle, aging, mitophagy, cell wall biogenesis, and lysosome function (17–21). The importance of this phospholipid is further reflected by the fact that alterations in CL underlie diseases such as Barth syndrome (22).
The results of our current study suggest yet another function for this special phospholipid and substantiate the importance of CL for the proper biogenesis of MOM multispan proteins.
MATERIALS AND METHODS
Construction of Om14 variants and yeast strains.
Unless stated otherwise, the yeast strains used here were based on the BY4741 background. Strains included in this study are listed in Table 1. For cloning of URA3-hemagglutinin (HA)-OM14-SL17 into the pYX142 plasmid, URA3 without a stop codon was amplified from pYX142-URA3-SL17 and cloned into the vector pGEM4 using EcoRI and BamHI restriction sites. Subsequently, OM14 without a stop codon was cloned into the same vector via BamHI and SalI restriction sites. Additionally, an HA tag was added at the N terminus during amplification from pGEM4-OM14 with an appropriately designed forward primer. SL17, in contrast, was amplified from pYX142-URA3-SL17 and cloned into pYX142 with flanking SalI and XhoI restriction sites. Finally, URA3 and HA-OM14 were subcloned sequentially from pGEM4-URA3-HA-OM14-“nostop” into pYX142 containing SL17. The URA3-HA-OM14-SL17 query strain (YJS02) was created by homologous recombination using an insertion cassette amplified from the pYX142-URA3-HA-OM14-SL17 plasmid with homology arms for the URA3 locus.
TABLE 1.
Yeast strains used in this study
| Strain | Mating type | Genotype | Source or reference |
|---|---|---|---|
| BY4741 | MATa | S288C his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| YMS116 | MATa | S288C his3Δ1 leu2Δ0 met15Δ0 ura3Δ0::KANR | ATCC |
| YMS135 | MATα | S288C his3Δ1 leu2Δ0 lys2+ met15Δ0 ura3Δ0 can1Δ::STE2pr-spHIS5 lyp1Δ | 25 |
| YMS721 | MATα | S288C his3Δ1 leu2Δ0 met15Δ0ura3Δ0 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU2 | 23 |
| YMS1169 | MATα | S288C his3Δ1::TEF2pr-Cherry::URA3 leu2Δ0 lys2+ lys+ met15Δ0 ura3Δ0 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU2 | 23 |
| YMS1641 | MATα | YMS1169 om14Δ::NatR::ADHpr-GFP-OM14 | Dalia Elinger |
| YJS01 | MATα | YMS721 ugo1Δ::NatR::TEF2pr-GFP-UGO1 | This study |
| YJS02 | MATα | S288C his3Δ1 leu2Δ0 lys2+ met15Δ0 ura3Δ0::TPIpr-URA3-HA-OM14-SL17::LEU2 can1Δ::STE2pr-spHIS5 lyp1Δ | This study |
| ugo1Δ strain | MATa | BY4741 ugo1Δ::KANR | 24 |
| crd1Δ strain | MATa | BY4741 crd1Δ::KANR | 24 |
| fmp32Δ strain | MATa | BY4741 fmp32Δ::KANR | 24 |
| fmp33Δ strain | MATa | BY4741 fmp33Δ::KANR | 24 |
| hit1Δ strain | MATa | BY4741 hit1Δ::KANR | 24 |
| jlp2Δ strain | MATa | BY4741 jlp2Δ::KANR | 24 |
| ngr1Δ strain | MATa | BY4741 ngr1Δ::KANR | 24 |
| yfl034wΔ strain | MATa | BY4741 yfl034w2Δ::KANR | 24 |
| ypl067cΔ strain | MATa | BY4741 ypl067cΔ::KANR | 24 |
| SGA crd1Δ GFP-OM14 strain | MATa | S288C crd1Δ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU2 | This study |
| SGA fmp32Δ GFP-OM14 strain | MATa | S288C fmp32Δ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
| SGA fmp33Δ GFP-OM14 strain | MATa | S288C fmp33Δ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
| SGA hit1Δ GFP-OM14 strain | MATa | S288C hit1Δ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
| SGA jlp2Δ GFP-OM14 strain | MATa | S288C jlp2Δ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
| SGA ngr1Δ GFP-OM14 strain | MATa | S288C ngr1Δ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
| SGA yfl034wΔ GFP-OM14 strain | MATa | S288C yfl034wΔ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
| SGA ypl067cΔ GFP-OM14 strain | MATa | S288C ypl067cΔ::KANR om14Δ::NatR::ADHpr GFP-OM14 his3Δ1::TEF2pr-Cherry::URA3 can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU | This study |
To create the query strain harboring GFP-Om14, an insertion cassette for homologous recombination was amplified from the vector pYM-N9 natNT2-ADHpr-yEGFP (yeast-enhanced green fluorescent protein) with homology arms for the 5′ untranslated region (UTR) of OM14 and the beginning of its coding sequence. The insertion cassette itself consisted of a nourseothricin resistance (Natr) gene upstream of an ADHpr-yEGFP construct. The cassette was introduced into the genome of strain YMS1169, resulting in the generation of the GFP-Om14 query strain (YMS1641). Hence, the endogenous OM14 gene in this strain was replaced by GFP-OM14 under the control of the constitutive ADH promoter. The GFP-Ugo1 query strain (YJS01) was generated in a similar way. The insertion cassette for homologous recombination was amplified from the vector pFA6a-NatMX4-TEF2pr-EGFP-ADH1term with homology arms for the 5′ UTR of UGO1 and the onset of its coding sequence. The insertion cassette, comprising a Natr gene upstream of a TEF2pr-eGFP construct, was integrated into the genome of strain YMS721. Consequently, the generated query strain revealed overexpression of GFP-Ugo1 under the control of the TEF2 promoter instead of endogenously expressed Ugo1.
Synthetic genetic array.
The synthetic genetic array (SGA) procedure was used to systematically insert GFP-OM14 and URA3-HA-OM14-SL17 into entire yeast libraries (25, 26).
Fluorescence microscopy.
High-throughput screens were performed with a system previously described (26, 27). The microscopy for follow-up analysis was performed using an Olympus IX71 microscope controlled by Delta Vision SoftWoRx 3.5.1 software with either 60× or 100× oil lenses. Images were captured by a Photometrics Coolsnap HQ camera with excitation at 490/20 nm and emission at 528/38 nm (GFP) or excitation at 555/28 nm and emission at 617/73 nm (mCherry). Images were transferred to Adobe Photoshop CS2 or ImageJ for slight contrast and brightness adjustments.
Biochemical procedures.
Mitochondria were isolated from yeast cells by differential centrifugation as previously described (28). Subcellular fractionation was performed according to published procedures (29). In the carbonate extraction reaction, mitochondria were dissolved in 0.1 M Na2CO3. After 30 min on ice, the samples were centrifuged (100,000 × g, 30 min, 2°C) and pellets as well as supernatant were analyzed. Protein samples were analyzed by SDS-PAGE and blotting to nitrocellulose membranes followed by incubation with antibodies and visualization by the enhanced-chemoluminescence (ECL) method. The intensity of the observed bands was quantified using AIDA software. Unless stated otherwise, the data from each presented experiment represent results of at least three independent repetitions.
In vitro protein import.
Import experiments were performed with radiolabeled precursor proteins and isolated mitochondria in an import buffer containing 250 mM sucrose, 2.5 mg/ml bovine serum albumin (BSA), 80 mM KCl, 5 mM MgCl2, 10 mM MOPS (morpholinepropanesulfonic acid)-KOH, 2 mM NADH, and 2 mM ATP [pH 7.2]. Radiolabeled precursor proteins were synthesized in rabbit reticulocyte lysate in the presence of [35S]methionine. Protease treatment of mitochondria was performed by adding trypsin or proteinase K (50 μg/ml) for 30 min on ice. The protease was then inhibited by adding for 10 min on ice either soybean trypsin inhibitor (1.5 mg/ml) or phenylmethanesulfonyl fluoride (PMSF) (4 mM), respectively.
Blue native PAGE.
Mitochondria were lysed in 50 μl digitonin buffer (1% digitonin, 20 mM Tris-HCl, 0.1 mM EDTA, 50 mM NaCl, 10% glycerol, 1 mM PMSF, pH 7.4). After incubation for 30 min at 4°C and a clarifying spin procedure (30,000 × g, 15 min, 2°C), 5 μl sample buffer (5% [wt/vol] Coomassie brilliant blue G-250, 100 mM Bis-Tris, 500 mM 6-aminocaproic acid, pH 7.0) was added, and the mixture was analyzed by electrophoresis in a blue native gel containing a 6%-to-13% gradient of acrylamide (30). Gels were blotted onto polyvinylidene fluoride membranes, and proteins were further analyzed by immunodecoration or autoradiography.
RESULTS
GFP-Om14 serves as a model protein for correct membrane insertion of mitochondrial outer membrane multispan proteins.
To identify novel effectors of the biogenesis of MOM multispan proteins, we set out to look for a suitable MOM multispan model protein for a high-content screen. We hypothesized that MOM multispan polypeptides that are not properly integrated into the MOM are likely to be degraded or mistargeted to other organelles. For example, we anticipated that a green fluorescent protein (GFP)-tagged model protein on the background of mutations in required genes would display altered localization of the fusion proteins (altered GFP signal pattern) and/or a reduction in its detected levels (altered GFP intensity). Accordingly, our aim was to monitor the localization of a model protein and its levels on the background of all deletion yeast strains (of nonessential genes) or of strains harboring downregulation alleles (of essential genes). Such screens would allow us to spot those proteins that support the biogenesis of these multispan proteins.
To that end, we added a GFP tag at the N terminus of Om14 (GFP-Om14) in a query strain suitable for automated mating approaches like the use of a Synthetic Genetic Array (SGA) (see strain YMS1641 data in Table 1 and references 25 and 26). To validate the correct mitochondrial targeting of GFP-Om14, we used fluorescence microscopy and observed that the GFP signal indeed colocalizes with the mitochondrial marker protein Aco2-Cherry (Fig. 1A). To substantiate this finding, we also monitored the location of the protein by subcellular fractionation. Similar to native Om14 (as observed in the wild-type [WT] strain), GFP-Om14 was highly enriched in purified mitochondria and cofractionated with the Tom70 mitochondrial marker protein (Fig. 1B). Next, we were interested to test whether the tagged protein is properly embedded within the MOM or only associates with the organelle. For this, isolated mitochondria harboring the protein were subjected to carbonate extraction. GFP-Om14, like native Om14, was found mainly in the pellet fraction that also contained other MOM proteins such as Tom20 and porin. Of note, as was previously reported (31), a small portion of GFP-Om14 and of native Om14 is found in the supernatant fraction that harbors peripheral and soluble proteins such as aconitase (Fig. 1C, lanes 3 and 7).
FIG 1.
Establishment of an in vivo visual assay to monitor the biogenesis of Om14. (A) The GFP-Om14 query strain expressing mitochondrial Aco2-Cherry was subjected to fluorescence microscopy. DIC, differential inference contrast. (B) Subcellular fractionation of the GFP-Om14 query strain. Equal amounts of whole-cell lysate (WCL) and of fractions corresponding to cytosol (cyt), ER, and mitochondria (mito) were analyzed by SDS-PAGE and immunodecoration. The indicated antibodies against marker proteins for mitochondria (Om14 and Tom70), cytosol/nucleus (Bmh1), and ER (Erv2) were used. (C) Carbonate extraction (CE) of mitochondria isolated from either the wild-type strain or the GFP-Om14 query strain was performed, and samples were separated to membrane-embedded proteins in the pellet fraction (P) and soluble proteins in the supernatant (SN). Where indicated (lanes 4 and 8), additional mitochondrial samples were treated with 20 μg/ml proteinase K (PK). All samples were analyzed by SDS-PAGE and immunodecoration. Porin, MOM protein resistant to PK; Tom20, MOM protein sensitive to PK; aconitase, matrix protein resistant to PK. (D) Fluorescence microscopy of representative deletion strains expressing GFP-Om14. (E) Cellular functions of proteins identified as hits by the visual screen. Functions have been assigned according to the Saccharomyces Genome Database.
To verify that membrane integration occurred in the correct and functional topology, we used a protease protection assay. Since the N terminus of Om14 is known to be located in the cytosol (31), the fusion protein GFP-Om14 should expose the GFP moiety on the mitochondrial surface. To verify this assumption, we added proteinase K to isolated mitochondria harboring the fusion protein. As expected, this treatment resulted in a cleavage of both the native and the GFP-tagged proteins and disappearance of the signal (Fig. 1C, lanes 4 and 8). The matrix protein aconitase was barely affected under these conditions, demonstrating that the organelles were intact. Taken together, these findings indicate that GFP-Om14 is targeted to mitochondria and can be integrated into the MOM with a native-like conformation.
OM14 was recently demonstrated to serve as a mitochondrial receptor for cytosolic ribosomes (32). However, since om14Δ cells display neither a mitochondrial morphology phenotype nor a growth phenotype (31, 32), the functionality of the fusion protein was difficult to test. However, the normal behavior of GFP-Om14 in all available assays prompted us to continue using it as a probe for MOM protein integration in a high-throughput visual screen.
A high-content screen identifies factors involved in the biogenesis of multispan MOM proteins.
To integrate the GFP-Om14 into a library of deletions in all nonessential genes (24) and a library of hypomorphic alleles of all essential genes (23), we used SGA to attain nearly 6,000 haploid strains, with each expressing GFP-OM14 on the background of one mutation. The strains were then visualized using a previously described high-content microscopy setup (27). Normal mitochondrial staining was observed in the vast majority (97.8%) of the inspected mutated strains (see, for example, Fig. 1D, gapdhΔ). In contrast, in some strains (1.5%), we observed strong cytosolic mislocalization of GFP-Om14 (see, for example, Fig. 1D, jlp2Δ), whereas the mitochondrial staining by GFP seemed to be altered for other strains (0.7%) (see, for example, Fig. 1D, ngr1Δ). In the latter cases, mitochondria were usually aggregated, probably due to the elevated levels of GFP-Om14 in the MOM. However, in contrast to mutants that affect targeting of MOM single-span proteins (33, 34), none of the inspected strains displayed mistargeting to other organelles. Manually annotating the resulting images for changes in localization or automatically extracting intensity data, we could find 146 strain backgrounds with altered intensity and/or altered localization of the GFP construct (see Table S1 in the supplemental material). Analyzing the functions of these hits, it appears that the two largest groups were of genes involved in DNA and RNA homeostasis, transcription, and translation (Fig. 1E). In addition, genes that are involved in protein turnover, the cytoskeleton, and ion homeostasis were also represented.
Of note, factors with known function in the biogenesis of MOM multispan proteins (such as Tom70, Tom71, Mim1, and Mim2) did not appear as hits in this screen (see Table S1 in the supplemental material). We assume that the single deletion of either Tom70 or Tom71 did not result in a clear phenotype, since the two receptor proteins have redundant functions and in the absence of one of them the paralogue protein can take over its function. In addition, both can be partially replaced by the other import receptor, Tom20. The Mim1 gene was initially annotated as an essential gene and therefore is not included in the deletion collection but rather in the Decreased Abundance by mRNA Perturbation (DAmP) library, where the levels of depletion are not clear. Finally, the Mim2 gene open reading frame (ORF) was first annotated as a dubious ORF and hence is not included in any library.
We assumed that many of the effects of the genes belonging to the groups mentioned above (such as general effects on transcription and/or translation of the GFP-Om14 marker) might be indirect. Hence, based on their potential relevance for mitochondrial protein biogenesis and the extent of the fluorescence alterations, these initial 146 hits were narrowed down to 9 strains with which we were interested in continuing to work (Table 2).
TABLE 2.
The most promising hits of the visual screen performed with GFP-Om14
| Systematic name | Standard name | Size (kDa) | Description (according to the Saccharomyces Genome Database) | Hit in GFP-Ugo1 screen |
|---|---|---|---|---|
| YFL046W | Fmp32 | 24 | Putative protein of unknown function | No |
| YJL161W | Fmp33 | 20.2 | Putative protein of unknown function | No |
| YMR132C | Jlp2 | 24.6 | Protein of unknown function that contains sequence closely resembling a J domain | No |
| YDL142C | Crd1 | 32 | Cardiolipin synthase produces cardiolipin | Yes |
| YFL034W | 119 | Putative integral membrane protein that interacts with a component of the ribosomal stalk | No | |
| YJR055W | Hit1 | 18 | Protein of unknown function that is required for growth at high temp | No |
| YGR078C | Gim2 | 23 | Part of the heteromeric cochaperone GimC/prefoldin complex that promotes efficient protein folding | Yes |
| YBR212W | Ngr1 | 75 | RNA binding protein that negatively regulates growth rate | No |
| YPL067C | 23 | Putative protein of unknown function | No |
To further characterize this limited group of hits, we created another query strain in which a GFP tag was added at the N terminus of another MOM multispan protein, Ugo1 (GFP-Ugo1; strain YJS01 in Table 1). This fusion protein was correctly localized to mitochondria and inserted into the outer membrane but was not functional, as it could not support regular mitochondrial morphology (see Fig. S1 in the supplemental material). Crossing this query strain with the nine deletion strains revealed an altered biogenesis of GFP-Ugo1 in only two of those strains, the crd1Δ and gim1Δ strains (Fig. 2 and Table 2). As Crd1 is a mitochondrial protein, while a clear relevance of Gim1 to mitochondrial function is not obvious, CRD1 was the most promising hit from the visual assays.
FIG 2.
GFP-Om14 and GFP-Ugo1 have an altered appearance in crd1Δ and gim2Δ deletion strains. Strains with BDH2 (as a control), CRD1, or GIM2 deleted, expressing either GFP-Om14 (upper panels) or GFP-Ugo1 (lower panels), were observed by fluorescence microscopy. Selected regions from each image were enlarged and are shown separately.
Ura3-Om14-degron can be used to probe membrane integration of Om14 in vivo.
The visual screen was aimed to uncover proteins that are essential for targeting Om14 to the mitochondria. However, fluorescence staining of mitochondria could not monitor whether the model protein obtained its correct topology at the MOM. Thus, as a complementary method to identify factors that are required for the correct membrane integration of multispan outer membrane proteins, we also employed a growth screen. As a probe, we used a fusion protein that is based on Om14. This hybrid protein contains the Ura3 enzyme, a cytosolic enzyme that catalyzes one of the steps in UTP synthesis, fused to the cytosol-facing N terminus of Om14. In addition, our hybrid protein contained the 50-amino-acid “degron” SL17 sequence, which targets proteins for degradation by the ubiquitin-proteasome system (35), attached to the C terminus of the protein that should face the IMS (Fig. 3A). We have previously used a similar method to successfully monitor the membrane insertion of the MOM single-span protein Mim1 (34). When the Om14 fusion protein is integrated into the MOM with its native topology (31), Ura3 is exposed to the cytosol (where it is functional), whereas SL17 is hidden in the IMS and is not exposed to the ubiquitin-proteasome system (Fig. 3A). Thus, the native topology is expected to abolish the uracil auxotrophy of the yeast strains in the collection of the mutants. However, if Om14 membrane integration is compromised, then the degron region is exposed to the cytosol where it causes degradation of the entire hybrid protein. Accordingly, the cells become auxotrophic for uracil.
FIG 3.
Growth assay to monitor the biogenesis of Om14. (A) Schematic representation of the various fusion proteins used. The expected potential of these proteins to support growth on synthetic medium without uracil is indicated at the bottom. (B) Yeast cells expressing the indicated constructs were analyzed at 30°C by drop-dilution assay on synthetic medium lacking either leucine only (SD-Leu) or leucine and uracil (SD-Leu-Ura). (C) Subcellular fractionation of the Ura3-HA-Om14-SL17 query strain. Equal amounts of whole-cell lysate (WCL) and of fractions corresponding to cytosol (cyt), ER, and mitochondria (mito) were analyzed as described in the Fig. 1B legend. (D) Mitochondria isolated from either the WT strain or the Ura3-HA-Om14-SL17 query strain were treated and analyzed as described in the Fig. 1C legend. (E) crd1Δ and cmk2Δ (as a control) cells expressing Ura3-HA-Om14-SL17 were grown on galactose-containing liquid medium in the presence (SGal comp.) or absence (SGal-Ura) of uracil. The optical density at 600 nm (OD600) of the cultures was measured and is depicted as a function of incubation time.
To perform such a screen, we first constructed the Ura3-HA-Om14-SL17 fusion protein and cloned it into a yeast expression vector. Next, to validate its compatibility with the growth screen, wild-type cells were transformed with an expression vector encoding Ura3, Ura3-SL17, or Ura3-HA-Om14-SL17. Of note, the yeast expression plasmid used in this study contains LEU2 as a selection marker, and, as expected, all transformed cells grew equally well on synthetic dextrose (SD)-Leu plates (Fig. 3B). In contrast, as hypothesized, Ura3 and Ura3-HA-Om14-SL17, but not Ura3-SL17, which is constantly degraded, also supported growth in the absence of uracil (SD-Leu-Ura plates, Fig. 3B). These findings demonstrate that Ura3 is functional even when localized to the mitochondrial surface by Om14. Next, this construct was introduced into the URA3 locus of a query strain, resulting in construction of the Ura3-HA-Om14-SL17 query strain (YJS02, Table 1). This strain also contains the native endogenous Om14 protein. To test the suitability of the generated query strain for the desired growth screen, both the mitochondrial targeting and membrane insertion of the hybrid protein were analyzed in more detail. The Ura3-HA-Om14-SL17 query strain was subjected to subcellular fractionation, and, as expected, the hybrid protein was detected solely in the mitochondrial fraction (Fig. 3C). Next, we verified by carbonate extraction and protease treatment that the fusion protein behaves like native Om14 and is indeed embedded correctly within the outer membrane (Fig. 3D).
A growth screen uncovers Crd1 as an important factor for correct membrane topology of Om14.
To perform the growth screen, we used SGA to integrate Ura3-HA-Om14-SL17 on the background of the same mutant arrays as used for the visual screens. We performed the growth screen by comparing the colony sizes of the resulting haploids on synthetic galactose-containing medium (SGal) to the colony sizes seen on the same medium lacking uracil (SGal-Ura). Galactose was used to allow robust growth while avoiding the repression of mitochondrial biogenesis that is caused by glucose. This screen uncovered many mutants in whose absence strains grew more poorly on SGal-Ura (see Table S2 in the supplemental material). However, among those potential candidates, only the crd1Δ strain also produced a hit in the visual screen. We further verified on the single-gene level that the crd1Δ strain expressing the Ura3-HA-Om14-SL17 hybrid protein grew slower when uracil was omitted from a galactose-containing liquid medium (Fig. 3E). In a control experiment, the deletion of an unrelated gene, CMK2, did not show this phenotype (Fig. 3E). We anticipated that an important protein for biogenesis of MOM multispan proteins should affect both the association of the protein with the membrane and its correct membrane topology. As both (visual and growth) screens identified Crd1 as a protein that is required for optimal biogenesis of the Om14 variants, we decided to focus on this protein in subsequent biochemical assays.
Mitochondria from crd1Δ cells display reduced insertion efficiency of multispan proteins.
To confirm the outcome of the high-throughput screens, we isolated crude mitochondria from either control or crd1Δ cells overexpressing the GFP-Om14 fusion protein and found that the fusion protein was indeed detected at reduced levels (Fig. 4A). When we next analyzed pure organelles, we observed that the steady-state levels of native Om14 were not affected whereas those of two other MOM multispan proteins, Ugo1 and Scm4, were reduced to about half of normal (Fig. 4B). These observations suggest that Crd1 functionality is especially required when the system is challenged with elevated amounts of multispan proteins. Of note, neither single-span proteins such as Mim1, Tom70, and Tom20 nor the Yah1 matrix protein was affected in the mutant cells (Fig. 4A and B).
FIG 4.
The absence of cardiolipin affects the steady-state levels of multispan proteins. (A) Steady-state levels of GFP-Om14 are decreased in crd1Δ mitochondria. (Left panel) Crude mitochondria from wild-type (WT) and crd1Δ yeast strains were isolated and analyzed by SDS-PAGE and immunodecoration with the indicated antibodies. (Right panel) The bands resulting from three independent experiments were quantified and normalized to the level of Tom20, and the protein levels in the WT strain were set to 100%. Error bars represent standard deviations (n = 3). rel., relative. (B) Steady-state levels of Ugo1 and Scm4 are decreased in crd1Δ organelles. Mitochondria were isolated from either WT or crd1Δ cells, and the indicated amounts were analyzed as described for panel A. The resulting bands were quantified and normalized to the level of Yah1, and the protein levels in the WT strain were set to 100% (right panel). Error bars represent standard deviations (n = 6 [3 biological experiments with 2 technical repeats each]). (C) Mitochondria isolated as described for panel B were subjected to blue native PAGE and immunodecoration with antibodies against Mim1 or Tom40. The MIM and the TOM complexes are indicated.
It is well-established that Mim1 and Tom70 are required for the biogenesis of multispan proteins (4, 5). Therefore, we asked whether the observed effect on GFP-Om14, Scm4, and Ugo1 is secondary to reduced amounts of Tom70 and/or Mim1. However, both proteins were detected in the mutated organelles at levels that were not significantly altered compared to their amounts in control organelles (Fig. 4A and B). As a further control, we analyzed both the TOM and MIM complexes by blue native PAGE. Although, as previously reported (12), the TOM complex from the mutated cells migrated at an apparently lower molecular mass, the two complexes displayed similar levels in mutated and control mitochondria (Fig. 4C). Hence, it appears that the absence of Crd1 can directly influence the biogenesis of multispan proteins.
To determine whether the reduced amounts of Ugo1, Scm4, and GFP-OM14 were really a result of reduced targeting and not of reduced production or stability, we next used established in vitro insertion assays based on purified mitochondria and cell-free synthesized radiolabeled Ugo1 or Scm4 molecules (4, 5). Supporting the aforementioned observations, we found that mitochondria isolated from crd1Δ cells had a reduced capacity to import Ugo1 and Scm4 compared to organelles isolated from control cells (Fig. 5A and B). In contrast, the import of the model matrix protein, pSu9-dihydrofolate reductase (pSu9-DHFR), was not hampered by the deletion of Crd1 (Fig. 5C). Taking the results together, it appears that the absence of Crd1 does not affect the general import capacity of mitochondria but instead specifically reduces the ability of the organelle to integrate multispan proteins.
FIG 5.

Mitochondria lacking Crd1 have a lower in vitro capacity to import multispan proteins. (A) Radiolabeled molecules of Ugo1-2HA were incubated with mitochondria isolated from either WT or crd1Δ cells for the indicated time periods. After import, mitochondria were treated with trypsin and subjected to SDS-PAGE and autoradiography. The trypsin-protected fragment is indicated (f). (Lower panel) Bands representing this fragment were quantified, and the intensity of the band upon import into control mitochondria for 20 min was set as 100%. Data represent averages of the results of three independent experiments. (B) Radiolabeled molecules of Scm4 were incubated with isolated mitochondria as described for panel A. After import, mitochondria were solubilized by the use of digitonin and subjected to blue native PAGE and autoradiography. The Scm4-containing band is indicated. (Lower panel) Bands were quantified, and the intensity of the band upon import into control mitochondria for 45 min was set as 100%. Data represent averages of the results of three independent experiments. (C) Radiolabeled molecules of pSu9-DHFR were incubated with isolated mitochondria as described for panel A. After import, mitochondria were treated with proteinase K (PK) and subjected to SDS-PAGE and autoradiography. The PK-protected mature form is indicated (m). (Lower panel) Bands representing the mature form were quantified, and the intensity of the band upon import into control mitochondria for 40 min was set as 100%. Data represent averages of the results of three independent experiments.
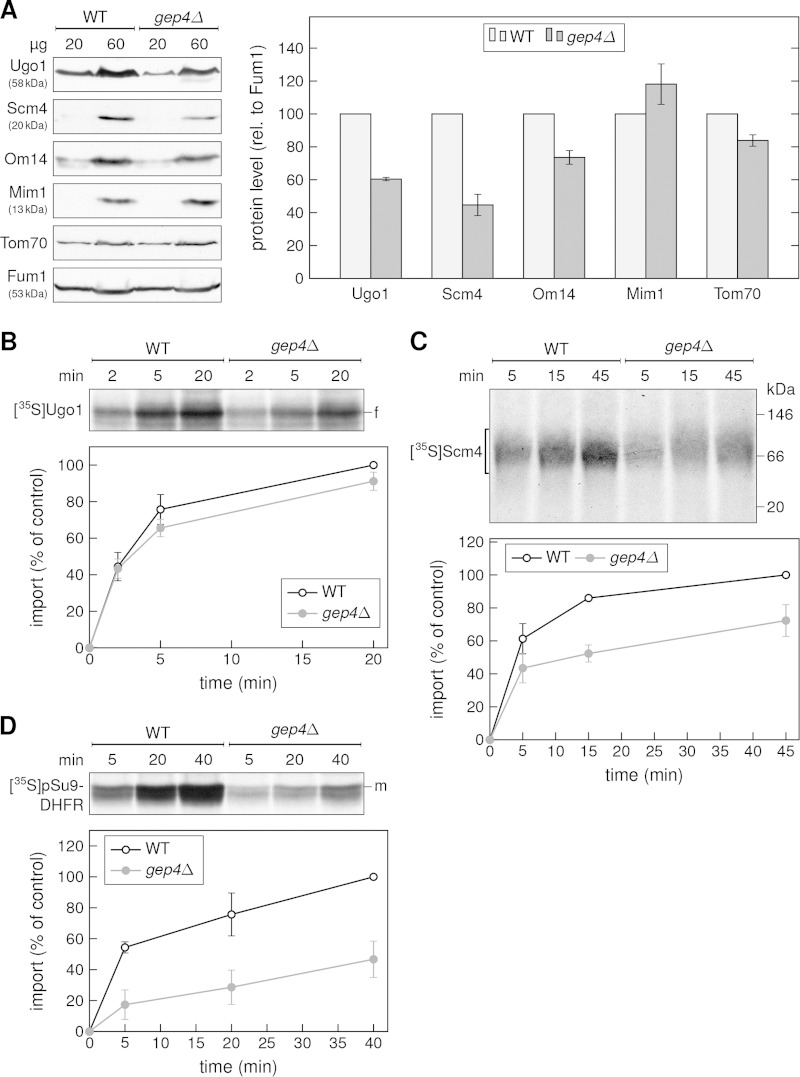
Crd1 catalyzes the last step in the biosynthesis pathway of CL. Hence, depletion of Crd1 could affect integration of multispan proteins due to either depletion of CL or accumulation of its precursor, phosphatidylglycerol (PG). To test this, we looked at the effect of mutating one enzyme, Gep4, upstream in the pathway that creates PG. First, we monitored the steady-state levels of proteins in mitochondria isolated from cells lacking Gep4. Our analysis demonstrates that all three MOM multispan proteins, Ugo1, Scm4, and Om14, are found in reduced levels in the altered organelles (Fig. 6A). Importantly, the levels of Tom70 and Mim1 were hardly affected in the mitochondria from gep4Δ cells. Next, we tested the capacity of such isolated mitochondria to import in vitro radiolabeled mitochondrial proteins. Whereas the absence of Gep4 resulted in a slight reduction in the import of Ugo1, a significant effect on the import of Scm4 was observed (Fig. 6B and C). Of note, the import of the matrix-destined protein pSu9-DHFR was highly reduced in the mutant mitochondria, suggesting a general import defect in this strain (Fig. 6D). Such a general biogenesis defect is in line with the severe growth phenotypes of cells lacking Gep4 and its requirement for the stability of respiratory chain supercomplexes (36). Collectively, these findings demonstrate that Crd1 and Gep4 are required for optimal biogenesis of MOM multispan proteins, strongly suggesting that the lack of CL itself is the cause for the compromised membrane integration.
FIG 6.
Deletion of Gep4 causes a reduction in the biogenesis of multispan proteins. (A) Mitochondria were isolated from either WT or gep4Δ cells, and the indicated amounts were analyzed as described in the Fig. 4B legend. The resulting bands were quantified and normalized to the level of fumarase (Fum1), and the protein levels in the WT strain were set to 100% (right panel). (B to D) The indicated radiolabeled proteins were incubated with isolated organelles and further analyzed as described in the Fig. 5 legend.
DISCUSSION
In the current study, we performed visual and growth screens to identify proteins that are required for the biogenesis of multispan proteins residing in the MOM. We concentrated in these screens on the model protein Om14 and its variants. In this context, we confirmed that GFP-Om14 is targeted to mitochondria and integrated into the MOM with a native-like topology. Interestingly, our visual screen could not identify mutants that result in mistargeting of Om14 to the endoplasmic reticulum (ER). This is in contrast to previous studies with MOM single-span proteins such as Mim1 or Gem1 where deletions of DJP1 (34) or SPF1 (33), respectively, resulted in mislocalization of the mitochondrial protein to the ER. Hence, it seems that, whereas the single-span proteins can, in principle, under certain conditions, also get integrated into the ER membrane, the multispan proteins lack this capacity or else might get efficiently and rapidly degraded in the event that they do. A speculative explanation for this difference is the divergence in the integration pathways of single- or multispan proteins into the ER membrane. While the membrane insertion of single-span proteins is mediated by the guided entry of TA protein (GET) machinery and is probably more flexible regarding substrate specificity, the targeting pathways for proteins that must use the Sec translocon for their membrane integration might be more restrictive regarding their substrates.
The most prominent hit in our screens was Crd1, and subsequent biochemical assays verified its important role for optimal biogenesis of MOM multispan proteins. The performed screens provided, in addition to Crd1, various potential hits for proteins that might be involved in the biogenesis of multispan proteins. However, since Crd1 was the only gene that appeared as a hit in both screens, we decided in the current study to concentrate on the characterization of its contribution. Future studies can address the relevance of the other potential hits.
Crd1 catalyzes the final step in the de novo synthesis of the CL diphosphatidylglycerol. Due to its importance for various processes, deficiency of and/or variations in CL result in a wide variety of alterations in cellular functions such as the cell cycle, aging, mitophagy, cell wall biogenesis, mitochondrial dynamics, mitochondrial protein import, and apoptosis (21). Our report adds another important process in which CL is playing a central role, namely, in the membrane integration of MOM multispan proteins. We observed very specific alterations in the biogenesis of Ugo1, Scm4, and Om14 upon deletion of CRD1 but not of other MOM or matrix proteins. Our observations are also in line with a previous report of a study in which the absence of Crd1 caused a reduction in the steady-state levels of the two multispan proteins Ugo1 and Fzo1 (20). The requirement for CL in the biogenesis of MOM multispan proteins also supports, by providing functional relevance, the idea of the presence of CL in the MOM, as its occurrence in this membrane is rather controversial (12). The idea of the importance of CL for the biogenesis process is further supported by the similar effects of deletion of Gep4, the upstream enzyme in this pathway (36).
It is currently unclear how CL modulates all the aforementioned processes, specifically, the biogenesis of multispan protein. As it has a dimeric structure and harbors a net negative charge, one can speculate that its negative charges may contribute to this effect. However, CL forms only ca. 1% to 3% of the MOM phospholipids whereas phosphatidylinositol (PI), which is also negatively charged, is far more abundant (12% [9]). Thus, it is unlikely that the contribution of CL is limited to electrostatic interactions with membrane proteins, as those interactions probably could also be facilitated by other lipid molecules in the membrane. The reported observation that CL stabilizes the TOM complex (12) could have provided another explanation of the requirement for CL in this process. However, we disfavor this possibility, because we and others reported that it is not the core TOM complex that is involved in the biogenesis of multispan proteins but rather the import receptor Tom70 and the MIM complex (4, 5), both of which do not seem destabilized in this background. Therefore, we favor the idea of a direct contribution of CL to unique structures within the membrane that facilitate integration and stabilization of multispan proteins. Such structures might be CL-rich microdomains that provide a convenient insertion site for the newly synthesized multispan proteins. Regardless of the actual mechanism, the data from the current study indicate that a defined phospholipid composition of the MOM modulates the capacity of this membrane to integrate multispan proteins. As the importance of CL is emphasized by the fact that alterations in its composition underlie diseases such as Barth syndrome, our findings may help also to shed light on disease progression.
Supplementary Material
ACKNOWLEDGMENTS
We thank E. Kracker, L. Gal, and D. Elinger for excellent technical support and T. Becker and N. Pfanner for the kind gift of antibodies against Om14 and Scm4.
This work was supported by the Deutsche Forschungsgemeinschaft (RA 1048/5-1 and RA 1048/7-1 to D.R.), the PROMOS program of the DAAD (J.S.), and a Minerva grant and a European Research Council (ERC) starting grant (StG) (260395) to the laboratory of M.S.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00107-15.
REFERENCES
- 1.Dukanovic J, Rapaport D. 2011. Multiple pathways in the integration of proteins into the mitochondrial outer membrane. Biochim Biophys Acta 1808:971–980. doi: 10.1016/j.bbamem.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Fritz S, Rapaport D, Klanner E, Neupert W, Westermann B. 2001. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J Cell Biol 152:683–692. doi: 10.1083/jcb.152.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otera H, Taira Y, Horie C, Suzuki Y, Suzuki H, Setoguchi K, Kato H, Oka T, Mihara K. 2007. A novel insertion pathway of mitochondrial outer membrane proteins with multiple transmembrane segments. J Cell Biol 179:1355–1363. doi: 10.1083/jcb.200702143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker T, Wenz LS, Kruger V, Lehmann W, Muller JM, Goroncy L, Zufall N, Lithgow T, Guiard B, Chacinska A, Wagner R, Meisinger C, Pfanner N. 2011. The mitochondrial import protein Mim1 promotes biogenesis of multispanning outer membrane proteins. J Cell Biol 194:387–395. doi: 10.1083/jcb.201102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papic D, Krumpe K, Dukanovic J, Dimmer KS, Rapaport D. 2011. Multispan mitochondrial outer membrane protein Ugo1 follows a unique Mim1-dependent import pathway. J Cell Biol 194:397–405. doi: 10.1083/jcb.201102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimmer KS, Papic D, Schumann B, Sperl D, Krumpe K, Walther DM, Rapaport D. 2012. A crucial role for Mim2 in the biogenesis of mitochondrial outer membrane proteins. J Cell Sci 125:3464–3473. doi: 10.1242/jcs.103804. [DOI] [PubMed] [Google Scholar]
- 7.Sperka-Gottlieb CD, Hermetter A, Paltauf F, Daum G. 1988. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 946:227–234. doi: 10.1016/0005-2736(88)90397-5. [DOI] [PubMed] [Google Scholar]
- 8.Gaigg B, Simbeni R, Hrastnik C, Paltauf F, Daum G. 1995. Characterization of a microsomal subfraction associated with mitochondria of the yeast, Saccharomyces cerevisiae. Involvement in synthesis and import of phospholipids into mitochondria. Biochim Biophys Acta 1234:214–220. [DOI] [PubMed] [Google Scholar]
- 9.de Kroon AIPM, Koorengevel MC, Goerdayal SS, Mulders PC, Janssen MJ, de Kruijff B. 1999. Isolation and characterization of highly purified mitochondrial outer membranes of the yeast Saccharomyces cerevisiae. Mol Membr Biol 16:205–211. doi: 10.1080/096876899294670. [DOI] [PubMed] [Google Scholar]
- 10.Osman C, Voelker DR, Langer T. 2011. Making heads or tails of phospholipids in mitochondria. J Cell Biol 192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura Y, Sesaki H, Endo T. 2014. Phospholipid transport via mitochondria. Traffic 15:933–945. doi: 10.1111/tra.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N. 2009. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, Rehling P. 2005. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth syndrome. Mol Biol Cell 16:5202–5214. doi: 10.1091/mbc.E05-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. 2008. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol 182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Böttinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, Pfanner N, Becker T. 2012. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J Mol Biol 423:677–686. doi: 10.1016/j.jmb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazán S, Mileykovskaya E, Mallampalli VK, Heacock P, Sparagna GC, Dowhan W. 2013. Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified Saccharomyces cerevisiae complexes III and IV. J Biol Chem 288:401–411. doi: 10.1074/jbc.M112.425876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsuka T, Nishijima M, Suzuki K, Akamatsu Y. 1993. Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J Biol Chem 268:22914–22919. [PubMed] [Google Scholar]
- 18.Chen S, Tarsio M, Kane PM, Greenberg ML. 2008. Cardiolipin mediates cross-talk between mitochondria and the vacuole. Mol Biol Cell 19:5047–5058. doi: 10.1091/mbc.E08-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. 2013. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi AS, Thompson MN, Fei N, Huttemann M, Greenberg ML. 2012. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J Biol Chem 287:17589–17597. doi: 10.1074/jbc.M111.330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren M, Phoon CK, Schlame M. 2014. Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res 55:1–16. doi: 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Schlame M, Kelley RI, Feigenbaum A, Towbin JA, Heerdt PM, Schieble T, Wanders RJ, DiMauro S, Blanck TJ. 2003. Phospholipid abnormalities in children with Barth syndrome. J Am Coll Cardiol 42:1994–1999. doi: 10.1016/j.jacc.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Breslow DK, Cameron DM, Collins SR, Schuldiner M, Stewart-Ornstein J, Newman HW, Braun S, Madhani HD, Krogan NJ, Weissman JS. 2008. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods 5:711–718. doi: 10.1038/nmeth.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Guldener U, Hegemann JH, Hempel S, Herman Z, Jaramillo DF, Kelly DE, Kelly SL, Kotter P, LaBonte D, Lamb DC, Lan N, Liang H, Liao H, Liu L, Luo C, Lussier M, Mao R, Menard P, Ooi SL, Revuelta JL, Roberts CJ, Rose M, Ross-Macdonald P, Scherens B, et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 25.Tong AH, Boone C. 2006. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 313:171–192. [DOI] [PubMed] [Google Scholar]
- 26.Cohen Y, Schuldiner M. 2011. Advanced methods for high-throughput microscopy screening of genetically modified yeast libraries. Methods Mol Biol 781:127–159. doi: 10.1007/978-1-61779-276-2_8. [DOI] [PubMed] [Google Scholar]
- 27.Breker M, Gymrek M, Schuldiner M. 2013. A novel single-cell screening platform reveals proteome plasticity during yeast stress responses. J Cell Biol 200:839–850. doi: 10.1083/jcb.201301120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daum G, Böhni PC, Schatz G. 1982. Import of proteins into mitochondria: cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem 257:13028–13033. [PubMed] [Google Scholar]
- 29.Walther DM, Papic D, Bos MP, Tommassen J, Rapaport D. 2009. Signals in bacterial β-barrel proteins are functional in eukaryotic cells for targeting to and assembly in mitochondria. Proc Natl Acad Sci U S A 106:2531–2536. doi: 10.1073/pnas.0807830106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schägger H, Cramer WA, von Jagow G. 1994. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem 217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- 31.Burri L, Vascotto K, Gentle IE, Chan NC, Beilharz T, Stapleton DI, Ramage L, Lithgow T. 2006. Integral membrane proteins in the mitochondrial outer membrane of Saccharomyces cerevisiae. FEBS J 273:1507–1515. doi: 10.1111/j.1742-4658.2006.05171.x. [DOI] [PubMed] [Google Scholar]
- 32.Lesnik C, Cohen Y, Atir-Lande A, Schuldiner M, Arava Y. 2014. OM14 is a mitochondrial receptor for cytosolic ribosomes that supports co-translational import into mitochondria. Nat Commun 5:5711. doi: 10.1038/ncomms6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krumpe K, Frumkin I, Herzig Y, Rimon N, Ozbalci C, Brugger B, Rapaport D, Schuldiner M. 2012. Ergosterol content specifies targeting of tail-anchored proteins to mitochondrial outer membranes. Mol Biol Cell 23:3927–3935. doi: 10.1091/mbc.E11-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papić D, Elbaz-Alon Y, Koerdt SN, Leopold K, Worm D, Jung M, Schuldiner M, Rapaport D. 2013. The role of Djp1 in import of the mitochondrial protein Mim1 demonstrates specificity between a cochaperone and its substrate protein. Mol Cell Biol 33:4083–4094. doi: 10.1128/MCB.00227-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilon T, Chomsky O, Kulka RG. 1998. Degradation signals for ubiquitin system proteolysis in Saccharomyces cerevisiae. EMBO J 17:2759–2766. doi: 10.1093/emboj/17.10.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osman C, Haag M, Wieland FT, Brugger B, Langer T. 2010. A mitochondrial phosphatase required for cardiolipin biosynthesis: the PGP phosphatase Gep4. EMBO J 29:1976–1987. doi: 10.1038/emboj.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.