Abstract
Hepatocellular carcinoma (HCC) is primarily a malignancy of the liver, advancing from a damaged, cirrhotic liver to HCC. Globally, HCC is the sixth most prevalent cancer and the third-most prevalent reason for neoplastic disease-related deaths. A diverse array of infiltrating immunocytes regulates the development and progression of HCC, as is the case in many other cancers. An understanding of the various immune components during HCC becomes necessary so that novel therapeutic strategies can be designed to combat the disease. A dysregulated immune system (including changes in the number and/or function of immune cells, cytokine levels, and the expression of inhibitory receptors or their ligands) plays a key role in the development of HCC. Alterations in either the innate or adaptive arm of the immune system and cross-talk between them make the immune system tolerant to tumors, leading to disease progression. In this review, we have discussed the status and roles of various immune effector cells (e.g., dendritic cells, natural killer cells, macrophages, and T cells), their cytokine profile, and the chemokine-receptor axis in promoting or impeding HCC.
Keywords: Hepatocellular carcinoma, Immune cells, Immune-dysregulation, Adaptive immunity, Innate immunity
Core tip: Hepatocellular carcinoma (HCC) is a heterogeneous disease caused by multiple factors, and has its immunopathogenesis complicated by the paradoxical role of various immune cells. This review provides a comprehensive insight into the immunological mechanisms that control hepatocarcinogenesis. A better and fuller understanding of the precise function of each cellular subset may open new avenues for the treatment of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a cancer that originates in the liver, and is thus different from metastatic liver cancer that hails from other organs and culminates in the liver. Worldwide, HCC is the sixth most prevalent cancer, as well as being the third most common cause of mortality and poor-prognosis malignancy due to recurrence after surgery and metastasis[1]. It accounts for approximately 70%-80% of all primary liver cancer cases[2]. HCC is most prevalent in Asian nations like China and Japan, where it has a high mortality rate within weeks or months after detection. The disease is generally diagnosed at a late stage, which significantly brings down the survival rate to less than 14% within a span of five years[3]. The available treatment options are not 100% successful and the estimated recurrence rates are around 50% over a span of 3 years post-surgery and with a survival rate of only 30%-40% at five years post-surgery[4].
The major risk factors for chronic liver disease and subsequent HCC include prior infection with viruses like hepatitis B and hepatitis C[5]. Studies in mouse models have indicated the major role of local intra-hepatic chronic inflammation in promoting hepatocarcinogenesis in animals with non-alcoholic steatohepatitis (NASH)[6]. Accumulating data in humans also indicate an increasing role for NASH as a risk factor for HCC development[7]. In addition, other emerging risk factors are: obesity (especially visceral adiposity leading to non-alcoholic fatty liver disease), alcohol consumption, tobacco use, consumption of foodstuffs contaminated with aflatoxin B1, diabetes, overuse of oral contraceptive pills, and iron overload[4].
Factors promoting tumor antigen tolerance, such as decreased recognition of malignant cells, suppression of immunity, and chronic inflammation (either mediated by virus[8] or immune dysregulation), all lead to carcinogenesis[9]. Recent studies have provided evidence that a dysregulated immune system, including changes in the number or function of immune cells, cytokine levels, and expression of inhibitory receptors or their ligands significantly contribute to the development of HCC[10,11]. Alterations in the function or expression of immune components shift the immune response towards tumor tolerance, resulting in its progression. Tumor-related immune cells, such as cytotoxic T cells, CD4+ T cells, regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, and the cross-talk between these have all been reported to be involved in the development of HCC (Figure 1). In this review, we have discussed the immunology of HCC in terms of the status of various immune effector cells.
Figure 1.
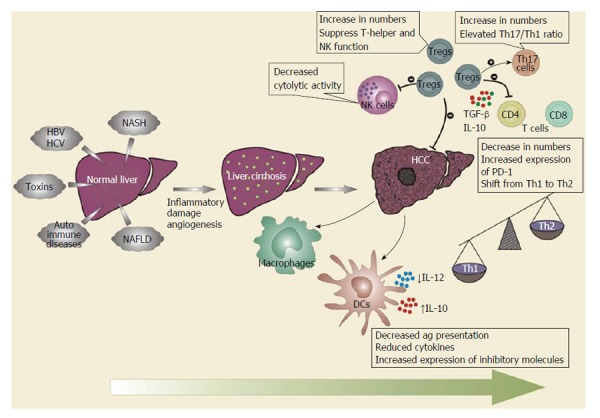
Role of immune cells in hepatocellular carcinoma. As the disease progresses from cirrhosis of the liver to hepatocellular carcinoma (HCC), the functions of various immune cells become dysregulated. Dendritic cells (DCs) lose their antigen presentation capabilities with the reduced secretion of Th1 cytokines. Macrophages differentiate into an “alternatively-activated phenotype” that generates a Th2-type immune response that promotes regulatory T cell (Tregs) recruitment and development. Natural killer (NK) cells have reduced cytolytic activities. T cells, both CD4+ and CD8+, decrease in numbers with attenuated function and increased expression of inhibitory receptors during HCC. Th17 cells increase in number and correlate with angiogenesis and poor-prognosis. Tregs exert negative effects on T cells, DCs, and NK cells, and may promote the differentiation of Th17 cells via immunosuppressive cytokines. There is shift in overall cytokine milieu from a Th1 to Th2 profile. HBV: Hepatitis B virus; HCV: Hepatitis C virus; IL-12: Interleukin 12; TGF: Transforming growth factor.
INNATE IMMUNE SYSTEM
Dendritic cells
Efficient recognition, processing, and presentation of tumor antigens by dendritic cells (DCs) are prerequisites for an effective immune response against tumors. Failed HCC-associated antigen presentation by DCs might not only be due to a decreased expression of human leukocyte antigen (HLA) class-I molecules[11], as maturation defects like reduced endocytosis, allostimulation, and interleukin 12 (IL-12) secretion can lead to a weak T cell immune response[12]. Even in the presence of strong maturation stimuli like high levels of inflammatory cytokines, DCs remain refractory to these stimulatory signals. Studies have previously shown that there is a numerical and functional defect in the peripheral DCs in HCC patients with hepatitis B and C virus infections, although it is unclear whether this defect is a cause or an effect[13,14]. On the other hand, there have been reports that have shown the frequency of activated CD83+ DCs in the peripheral circulation of HCC patients was comparable to patients with liver cirrhosis and normal healthy controls[15]. However, when compared to peripheral blood, activated DCs were present at a much lower frequency in the liver tissues of the other study groups. Additionally, the activated DCs in HCC patients were not able to infiltrate the cancer nodules, resulting in impaired recruitment of tumor-specific lymphocytes to tumor areas.
Recently, a new regulatory subset of DCs called CD14+ cytotoxic T-lymphocyte-associated protein (CTLA)-4+ DCs, which expresses inhibitory molecule-like CTLA-4 and programmed death receptor (PD)-1, were observed in the peripheral blood lymphocytes and tumor masses of HCC patients[16]. High levels of anti-inflammatory cytokine, IL-10, and indoleamine 2, 3-dioxygenase secreted by these cells’ post-stimulation suppressed the CD4+ T-cell immune response, thereby assisting tumor progression and immune escape.
Macrophages and myeloid-derived suppressor cells
Tumor-associated macrophages (TAMs) represent the main inflammatory cells associated with cancer-related inflammation[17]. While in infiltrating tumors, TAMs differentiate towards an M2 phenotype characterized by the expression of immunomodulatory cytokines [e.g., IL-10 and transforming growth factor (TGF)-β] and poor antigen presentation capacity. TAMs also express chemokines like CCL17, CCL22, and CCL24, along with arginase and low levels of proinflammatory cytokines and reactive oxygen species[18]. In HCC, the cytokines IL-6 and TGF-β (in particular) favor tumor growth, tumor necrosis factor (TNF)-α and IL-6 are involved in invasion and metastasis, and TGF-β, in concert with IL-10, has been shown to promote the suppression of anti-tumor immune response[19]. This alternative phenotype of macrophages further participates in the activation of a T helper type 2 (Th2) immune response, thereby promoting the recruitment and development of Tregs. Chronic inflammation was reported primarily to be coupled with a higher prevailing level of macrophage colony stimulating factor and a higher infiltration of macrophages, which were reportedly associated with HCC progression and intrahepatic metastasis, thereby signifying the role of TAMs in the recurrence and metastasis of HCC[20,21].
Another heterogeneous population of cells called MDSCs, which are a subset of inflammatory monocytes, has been identified that comprises immature myeloid progenitors not already committed to any cell lineage[20]. They can exert inhibitory functions and regulate T cell responses through the up-regulated expression of several factors, such as free radicals, arginase activity, and production of TGF-β, thereby encouraging the induction of Treg cells[22]. Like typical monocytes, these cells express CD14 but have a lower or no expression of HLA-DR. An increased frequency of these cells has been reported in the peripheral circulation and tumor environment of HCC patients[23].
Similarly, neutrophils are a common inflammatory infiltrate in tumors that could also provide a prediction of poor survival in HCC patients, since their numbers correlated positively with the stage of cancer. Kuang et al[24] demonstrated that peritumoral stromal cells were fortified with neutrophil populations under the influence of Th17 cells through chemokines, like CXCL8, produced by epithelial cells. These neutrophils produce proteases like matrix metalloproteinase-9 in HCC tissues, promoting angiogenesis. Thus, neutrophils provide a connection between immune cells and angiogenesis, as well as promoting tumor growth.
NK cells
An exaggerated cytolytic population of NK cells serves as an immune invigilator in the liver microenvironment[25]. NK cells are cytotoxic and regulate the activity of other immune cells through the cytokines they release[26]. Under normal physiological conditions, NK cells mediate their functions in the liver via the production of “cytolytic granules” containing perforin, granzymes, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and interferon (IFN)-γ[27]. However, their functions are not completely imparted in the case of many cancers, including HCC. For instance, in HCC patients, a significant decrease in the CD56dim NK subsets in the peripheral blood has been reported as compared to healthy subjects[28]. A significantly lower proportion of these NK cell subsets exhibited reduced levels of IFN-γ, and cytotoxic potential also being reported in tumor regions compared with non-tumor regions in HCC patients[29]. Multiple mechanisms have been put forward to explicate the decreased functioning of NK cells and their association with cancer and cirrhosis of the liver, including fibrotic damage to NK cells[30], phagocytic uptake of NK cells by activated hepatic stellate cells[31], and the up-regulation of inhibitory or down-regulation of activating receptors, respectively[32].
ADAPTIVE IMMUNE SYSTEM
T lymphocytes
T lymphocytes, both CD4+ T helper cells and CD8+ cytotoxic T cells, are mostly considered to be significant players in inhibiting, impeding, and killing tumor cells. Their existence in cancer areas has been observed and correlated with a favorable prognosis in many cancers[33]. The IFN-γ produced during the Th1 immune response play a crucial role in the evasion and amelioration of HCC. In addition to helper T cells, the role of cytotoxic CD8+ T-cells during HCC disease has been evaluated in many studies, where they were attributed a significant role in the killing of tumor cells.
In one study, it was reported that there was a significant decrease in CD4+ T-cells in patients with liver cirrhosis and HCC, indicating their importance in facilitating malignancy among cirrhotic patients[34]. They likewise noticed a decreased ratio of helper T cells/suppressive T cells in the peripheral blood of patients with liver cirrhosis and HCC. Upon assessment of the genetic profile, a gene signature consisting of 17 immune related genes that changes the tumor microenvironment from a Th1 to a Th2 type milieu has been identified[20]. This foreshadows the development of venous metastasis in HCC, as well as impaired disease outcome, thereby indicating that progression of liver diseases is linked with a dysregulated cellular immune response.
Several mechanisms to deduce the immunosuppressive nature of T cells have been explained by many authors. Previous studies have shown increased levels of the soluble IL-2 receptor alpha chain, CD25 in the serum of cancer patients[35,36]. These studies have also shown a positive correlation between sCD25 and disease severity, serving as a surrogate indicator of survival and response to therapy[35]. The serum of HCC patients was evaluated, and revealed elevated levels of sCD25 as compared to normal, healthy individuals and patients with cirrhotic livers[36]. The authors observed an improvement in T cell responses after sCD25 depletion, suggesting that sCD25 is indeed involved in suppressing effector T cell functions.
Many human cancer cells express the ligand for inhibitory receptor PD-1. An up-regulated expression of its ligand, PD-L1, on intra-tumoral Kupffer cells and a concurrent increase in PD-1 expression on CD8+ T-cells is detrimental in cancer[37]. Moreover, MDSCs were also found to have up-regulated expression of PD-L1, leading to functional exhaustion of effector cells through ligand-receptor interaction[37]. These data provide clues that strategies to block the PD-L1/PD-1 axis in HCC can increase tumor-specific immunity[38].
Another important effector subset of T helper cells are follicular T-helper cells (Tfh). These are important to B cells during germinal center reactions in secondary lymphoid tissues and function to support B-cell activation, affinity maturation, and isotype switching, leading to the generation of memory B cells and long-lived plasma cells[39]. Although only a few studies have focused on humoral immunity in HCC and its regulatory mechanisms, impairment of CD4+ Tfh cells has been indicated to influence the development of HBV-associated HCC[40]. A decreased proportion of CXCR5+CD4+Tfh cells was found to be associated with HCC disease progression. Furthermore, these cell types were found to have an attenuated function with reduced secretion of IL-21, along with the inability to promote B cell maturation, and hence were suggested to be associated with low survival rates in HCC[41].
Regulatory T lymphocytes
Aside from anti-tumor cells that get functionally impaired during various cancers, there is another class of cells, termed Tregs, that express CD25 on their surface, along with the intracellular transcription factor forkhead box P3 (Foxp3), that have been reported to play a very important role in carcinogenesis[42,43]. Under normal physiological conditions, natural Tregs (nTregs) limit autoimmune reactions by suppressing self-reactive immune cells, and are also engaged in sustaining immunological self-tolerance and homeostasis.
It has been demonstrated in many studies that the number of a class of Tregs called induced Tregs (iTregs) increase in the peripheral blood and tumor infiltrating lymphocytes of patients with HCC[44]. Depletion of Tregs led to the manifestation of anti-tumor immune responses in this study in around 38% of HCC cases[45]. While the original investigations only demonstrated an increase in the frequencies of Tregs in patients with HCC[46], subsequent research was focused on the possible correlation of Tregs with disease progression and the clinical outcome of disease in patients[47]. It has been reported that the number of Tregs correlated with disease severity, as patients with advanced stages of HCC demonstrated a higher percentage of intra-hepatic CD8+Foxp3 regulatory T cells than patients in initial stages, suggesting that CD8+Foxp3+ regulatory T cells represent another immune-escape mechanism. Moreover, there was reduced infiltration of CD8+ T-cells in tumors consequent to the abundant accumulation of Tregs in these areas as compared to non-tumor regions[48]. It has further been reported in another study that FoxP3+ Tregs were highly amassed as activated cells expressing CD69 and HLA-DR (terminally differentiated subpopulation) in tumors where they could suppress T-cell proliferative capabilities and IFN-γ secretion by T cells[48]. Hence, it is suggested that the increased number of tumor-infiltrating Tregs fosters tumor progression and serves as a poor prognostic marker in HCC patients.
Furthermore, Tregs through their membrane-bound TGF-β, could also dampen NK cell responses by down-regulating NK group 2 member D expression and by participating in HCC progression[49]. Tumor-iTreg seem to differentially regulate NK cell activity in the tumor microenvironment, as well as being endowed with abilities to modulate T-cell proliferative abilities and the functions of DCs via anti-inflammatory cytokines like IL-10 and TGF-β. In contrast with the nTregs, tumor iTreg cells interfere with NK cells activated with IL-2, while IL-2 independent activation of NK cells was augmented in the presence of iTregs[50].
Th17 cells
Ever since it became known that tumor cells of HCC, TAMs, and MDSCs are all capable of producing adequate quantities of IL-6 and TGF-β, it has been speculated that differentiation of Th17 cells in such an environment would be favored, especially in established tumor tissues. Coupled with extreme inflammatory conditions in growing tumors, an increased frequency of Th17 cells was more eminent in HCC tissues than non-tumor tissues, which positively correlated with microvessel density, a marker of tumor angiogenesis in tissues associated with poor endurance in patients with HCC[51]. Despite the positive correlation of Th17 cells with reduced survival in HCC cases, the role of these cells in HCC still remains incompletely defined. Some studies have recently suggested that IL-17 plays a dual role in tumor immunology; it can either promote anti-tumor cytotoxic T cell responses or foster angiogenesis of surrounding endothelial cells and fibroblasts facilitating tumor growth[52]. In HCC patients, increased levels of Th17 and Th1 cells were observed in tumor regions as compared to non-tumor regions, with the frequency of these cells being associated with overall disease-free survival[53]. Thus, an elevated Th17 to Th1 ratio may promote tumor progression and serve as a prognostic marker at the same time.
More recent studies have shown that an imbalanced proportion of Th17 cells and Tregs are also associated with cancer progression, but not much is known about the implication of this disproportion in cases of HCC[54]. The density of liver-infiltrated FoxP3+ Tregs increased gradually from chronic hepatitis B infection to patients with atypical hyperplasia, then to HCC, while the density of Th17 cells and CD8+ T cells in these cases trended towards a decrease as the disease progressed to HCC. In less differentiated HCC cases, the population of tumor-resident Tregs was lower, while the percentages of Th17 cells and CD8+ T-cells were significantly greater. These findings indicate that Th17 cells and Tregs cooperate in the liver niche, thereby promoting cancer advancement.
NKT cells
NKT cells are a subset of T lymphocytes that have overlapping properties with both T cells and NK cells, expressing both the αβ T-cell receptor and many receptors of NK cells, and are a potent source of cytokines like IL-4, IFN-γ, and TNF-α. Depending on the diversity and extent of cytokines produced, their effects could be either beneficial or deleterious to the host. These cells recognize the non-polymorphic molecule CD1d, to which self and foreign lipid antigens are presented. These typical NKT cells, known as invariant NKT cells, act like a double-edged sword in cancer cases by promoting anti-tumor response via the activation of effector cells, while at the same time boosting the suppressor cell compartment and inducing tolerance[55].
Although NKT cells constitute a major population in the liver, their role in hepatocarcinogenesis remains incompletely understood. The frequency of NKT cells was increased in tumors, especially in HCC patients, with a gradual increment from blood to liver to tumor. A subset of these cells characterized by CD4 expression has been shown to accumulate in the tumor environment and is able to generate Th2 cytokines that inhibit the tumor-specific CD8+ T-cell response[56], while the other subset, CD4-NKT cells, has anti-tumor effects and constitutes a key role in dampening the inflammatory response mediated by β catenin-driven hepatocarcinogenesis[57].
ROLE OF CYTOKINES AND CHEMOKINES
Dysregulated cytokine milieu
Hepatocytes express receptors for several cytokines, thus making them susceptible to their action. Consequently, cytokines are involved not only in the optimum functioning of the liver, development, and regeneration, but may also aid in the pathogenesis of liver cirrhosis, fibrosis, and HCC. The cytokine milieu in livers with metastatic HCC is skewed towards a Th2 profile, with an increase in levels of anti-inflammatory cytokines and a concomitant reduction in pro-inflammatory cytokines. This also highlights the importance of Th1-type immune response in inhibiting tumor relapse[58].
Th1 and Th2 cytokines
In Th1 cytokine levels, IL-2 is shown to have a direct correlation with prognosis in HCC patients, as the increased levels of IL-2 were associated with an increase in the number of CD8+ T-cells[59]. Similarly, other Th1 cytokines like IFN-γ, IL-8, IL-15, and IL-18 have been indicated to correlate with invasiveness and metastasis during HCC[60]. Alterations in these cytokines may help to control or ameliorate carcinogenesis, as they are capable of changing the functional status of cells like NK cells and cytotoxic T lymphocytes[61].
The levels of Th2 cytokines, IL-4, and IL-5 were found to be high in the tumor microenvironment of metastatic HCC in patients with hepatitis B virus (HBV)-positive metastatic HCC, showing a shift from a Th1 to Th2 profile[21]. The causative factor associated with the switching of the cytokine balance is unknown, but factors produced by the tumor or microenvironment may play a role in tumorigenesis by polarizing cytokine production towards a Th2 phenotype.
Another cytokine released by Th22 cells[62] is IL-22, which has been found to be significantly elevated in HCC patients, suggesting an involvement in T-cell-mediated immunity in HCC. A direct relationship between the levels of IL-22 and IL-17 in HCC patients indicates their interplay in the pathogenesis of HCC[63].
Pro-inflammatory and anti-inflammatory cytokines
TNF-α is an important mediator of inflammatory and autoimmune diseases and is strongly involved in the pathogenesis of HCC by promoting invasion, angiogenesis, and metastasis[64]. In many cancers, including HCC, the serum levels of TNF-α has been reported to be very high, which correlated with disease and nutrition status in these patients[65,66]. Although, in solid tumors, the levels of TNF-α were higher in normal tissues than in tumor cells, the serum levels were found to be lower in patients with HCC. Because of this discrepancy, the precise impact of cytokines associated with liver cancer development remains unclear[67]. TNF-α is also known to stimulate the expression of the negative co-stimulatory molecule B7 homolog 1 or PD-L1 on macrophage surfaces, thus suppressing the CD8+ T-cell anti-tumor immune response[38]. The principal downstream mediator of pro-tumoral TNF-α activity is nuclear factor κB (NF-κB), whose target genes are involved in cell proliferation and survival[68]. TNF-α is also notably induced by NF-κB in a positive feedback loop.
Higher production of IL-1β may help increase the production of other cytokines, such as IL-2, IL-6, and TNF-α, and trigger the complex immunological processes to eliminate the virus in cases of hepatitis-induced HCC. Interestingly, besides its major role as a pro-inflammatory cytokine, IL-1β has been implicated as an important factor for tumor growth. Several independent lines of evidence have also suggested that genetic polymorphisms within the IL-1β gene are associated with gastric cancer and HCC induced by HCV infection[69,70]. Moreover, supplementing cytokines like TNF-α, IL-1β, or IL-18 has been shown to induce growth of CD8+ T-cells and induce TRAIL in many HCC cell lines, thereby contributing to tumor evasion[71].
The most studied anti-inflammatory cytokine in HCC is IL-10, which has been shown to be increased in HCC tumors vs non-tumorous tissue adjacent to the tumor and tissues of healthy cohorts, respectively[72]. These studies suggest that an increase in IL-10 in conjunction with other Th2 cytokines correlates with progression. Another multifunctional inflammatory cytokine, IL-6, which is produced mostly by resident macrophages, was found to be linked with poor prognosis in HCC patients[73]. IL-6 exerts its oncogenic activity by triggering downstream signal transducer and activator of transcription 3 and extracellular-signal-regulated kinase pathways, which in turn control target genes involved in both cell proliferation and survival. It has been found that IL-6 levels and receptor expression were raised in a number of cancers, including HCC, where it may contribute to tumor progression[74]. Recently, in a study carried out to investigate the use of novel serum biomarkers for predicting the recurrence and survival of patients with HBV-related HCC, low serum IL-6 level, low platelet count, and low serum albumin level were found to be independent prognostic factors for disease-free survival in these patients[75]. IL-37, a recently recognized anti-inflammatory cytokine has been shown to suppress cells of the innate immune system[76]. The study indicates that in HCC specimens, the expression of IL-37 was found to be decreased in tumor tissues and its expression level was negatively related to tumor burden and survival improvement.
Hence, it could be concluded that cytokines regulate the microenvironment of immune cells with allied and opposing roles, involving different signaling pathways to affect the course of HCC disease.
Chemokine ligand-chemokine receptor axis
Chemokines are known to direct lymphocyte recruitment into liver tumors expressing the corresponding chemokine receptors[77]. The CXCL12-CXCR4 axis is regarded to be critical as a factor regulating tumor growth and progression during HCC. Previous studies have depicted higher expression of CXCL12 and CXCR4 in HCC specimens than the surrounding tissues[78]. It has been demonstrated in different studies that CXCR4 and CXCL12 may play a significant part in HCC metastasis and invasiveness of the tumor[79,80]. A significant correlation was observed between CXCR4 expression, tumor progression, metastasis, and a decreased survival rate[80]. However, the lack of a loss of function mutation of the tumor suppressor gene p53 gene on CXCR4 expression in HCC indicated yet another unidentified mechanism[81].
However, an ambiguity as to whether CXCR4-CXCL12 actually promotes tumor growth as a down-modulation of CXCR4-CXCL12 expression in HCC, both in vitro and in vivo has been reported, where CXCL12/CXCR4 also lacked an association with death and HCC recurrence[82]. Therefore, although it appears that the CXCL12-CXCR4 axis is indispensable in HCC, its precise role still remains paradoxical in this disease. The possible involvement of the CCL20-CCR6 axis in HCC has been suggested because of the significantly up-regulated expression of both CCL20 and its chemokine receptor CCR6 that has been observed in HCC tissues with different rates of tumor progression[83]. Although the role of fractalkine (CX3CL1) and its receptor CX3CR1 in HCC indicated a role in the regulation of immune response, the relationship of between the fractalkine-CX3CR1 axis and HCC is as yet unclear. According to recent studies, the fractalkine-CX3CR1 axis is critical in the diagnosis of HCC, as it can regulate both the immune response and the cell cycle of HCC[84].
Furthermore, the expression levels of some chemokine receptors like CCR5, CCR6, and CXCR3 on the surface of peripheral lymphocytes of HCC patients was reduced, while the expression of these receptors on tumor-infiltrating cells was higher, suggesting a role of these chemokine receptors in controlling the trafficking of effector T cells to the tumor regions in response to the corresponding chemokines[85]. In addition to this, the expression levels of CXCR3 have been reported to be particularly high in tumor infiltrating cells, as compared to non-tumor infiltrating cells, implying that lymphocytes preferentially migrate to the tumor tissue rather than the surrounding non-tumor regions. This increased expression was negatively correlated with tumor burden and the stage of cancer. The literature citing the role of various immune components in HCC is summarized in Table 1.
Table 1.
Summary of the status of various immune components in hepatocellular carcinoma
| Immune component | Status in HCC | Ref. |
| Dendritic cells | Decreased antigen presentation, decreased numbers, impaired functions | [12,13] |
| Macrophages | Poor antigen presentation, activated Th2 immune responses, promoted Tregs | [17,18,20] |
| Myeloid-derived suppressor cells | Exerted suppressive functions through free radicals, arginase activity, and TGF-β | [21,22] |
| Neutrophils | Promoted angiogenesis through metalloproteinase-9 | [24] |
| NK cells | Decreased numbers, low cytolytic activity | [26,28] |
| T lymphocytes | Decreased frequency, fewer Th1 cytokines, increased expression of inhibitory receptors | [36,37] |
| Tregs | Increased frequency, suppressed T-cell proliferation and IFN-γ secretion, inhibited NK cell responses | [42,48,86] |
| Th17 cells | Increased numbers, incompletely defined role, correlated with disease progression | [51,52] |
| NKT cells | Dual roles, increased frequency, promoted Th2 cytokines | [55,56] |
| Th1 cytokines | Decreased in tumor microenvironment, induced CD8+ T-cells | [59,61,87] |
| Th2 cytokines | Increased levels, correlation with tumor progression | [21] |
| Proinflammatory cytokines | Involved in pathogenesis of HCC | [65,69] |
| Anti-inflammatory cytokines | Increased in HCC, correlated with progression | [72,73,76] |
| Chemokine-receptor axis | Tumor progression and metastasis | [78,83,84] |
HCC: Hepatocellular carcinoma; NK: Natural killer cells; Tregs: Regulatory T cells; Th17: T helper type 17; NKT: Natural killer T; TGF: Transforming growth factor; IFN: Interferon.
GAPS IN EXISTING KNOWLEDGE
Insights into the immune signaling pathways are being provided by recent studies analyzing the role of immune effector cells. However, a complete understanding of many immune components, such as NKT cells, gamma delta T cells, and the role of many cytokines and chemokines, has not yet been achieved. It is generally believed that T lymphocytes play a protective role in inhibiting tumor growth and development, while TAMs, MDSC, Tregs, Th17 cells, and their associated cytokines (IL-6, TNF-α, IL-1β, IL-23, and TGF-β) may play important roles in promoting the growth and survival of cancer. However, defining their roles as pro-tumor or anti-tumor still requires caution. It is also unclear as to how TAMs and TGF-β regulate the generation and function of Tregs in the development and establishment of the solid tumor microenvironment. Of further importance is understanding whether TGF-β production preferentially induces Tregs or promotes the development of Th17 cells within the tumor microenvironment. Further research into better understanding the balance between all immune components at all stages of carcinogenesis is essential for the development of effective cancer therapies that target or utilize immunological mechanisms.
Recent observation of many solid tumors suggests the use of checkpoint inhibitors that decide a balance between co-stimulatory and inhibitory signals in inducing a strong anti-tumor response that needs to be evaluated in HCC. Tumor vaccines and therapeutic agents for targeting various checkpoints represent some novel strategies for inducing immune resistance. These combinatorial approaches induce tumor regression in patients that would not have responded to either treatment alone. Strategies to deliver genetically modified T cells into the tumor microenvironment, such as via a hepatic artery, are underway and being evaluated in clinical trials that have already proven successful in the treatment of other cancers[88]. Novel epitopes specific for tumor-associated antigens should be designed using high throughput “omics” technology with the aim to induce anti-tumor CD4+ and CD8+ T-cell responses. In this context, high resolution mass spectrometry has been used for directly sequencing peptides presented by HLA molecules from tumor cells so as to identify naturally processed class I and II tumor-associated peptides[89]. Combining key components of the tumor microenvironment, as compared to chemotherapy alone, would improve the clinical outcome. Finally, therapeutic agents capable of reversing the immunosuppressive nature of HCC tumors via administration alone or in combination with other modalities will be critical in optimizing clinical outcomes for HCC patients.
CONCLUSION
Since HCC accounts for 90% of all liver cancers and is usually multifocal at the time of diagnosis, treatment is difficult and affronted with a higher recurrence rate in these patients. The incidence of the disease is accelerating at a regular rate and will likely increase further over the coming years. Hence, in this context, there is an imperative demand for newer and better therapeutic strategies to combat this predicament. This requires a fuller discernment of the function of various components of our immune system and how they interplay in creating immune responses against tumors. Immune suppression is predominantly mediated by cytokine secreted in the local milieu by Tregs that down-regulate the effector and cytotoxic activities of CD8+ T-cells and NK cells. The antigen presenting functions of DCs are also affected due to the expression of several inhibitory receptors that further suppress the functions of helper T cell. TAMs and MDSCs contribute to the ongoing inflammation and participate in the activation of a Th2 immune response that favors Treg recruitment and development, thus promoting angiogenesis. These cell types can help in the differentiation of Th17 cells that also infiltrate the tumor microenvironment, and correlate with poor survival in HCC patients; however, their roles still remain incompletely defined. Similarly, despite being the predominant population in the liver, the role of NKT cells in hepatocarcinogenesis remains to be completely elucidated. Soluble factors, including cytokines and chemokines, play a crucial role in immunosurveillance and immunoregulation. The cytokine milieu in livers with metastatic HCC is skewed towards a Th2 profile, with a concomitant decrease in pro-inflammatory cytokines. The roles of many cytokines like IL-22 have recently been deciphered in HCC, which adds to the current knowledge about the milieu of liver tumors. The chemokine ligand-chemokine receptor axis plays a role in regulating the differential recruitment of effector T cells to the tumor and the interconnections between different axes, as not just a single axis should be surmised. Future studies are warranted to understand the complexity of interactions between these immune cells to potentiate the immune system and for the designing of newer immune-therapeutics against HCC.
Footnotes
P- Reviewer: Calvisi DF, Chua MS, Geller DA, Simonovic SZ S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Liu SQ
Conflict-of-interest statement: The authors have no conflicts of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 1, 2015
First decision: April 23, 2015
Article in press: July 22, 2015
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 4.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 5.Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383–393. doi: 10.1111/j.1365-2893.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 6.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity‘s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 10.Aravalli RN. Role of innate immunity in the development of hepatocellular carcinoma. World J Gastroenterol. 2013;19:7500–7514. doi: 10.3748/wjg.v19.i43.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui M, Machida S, Itani-Yohda T, Akatsuka T. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J Gastroenterol Hepatol. 2002;17:897–907. doi: 10.1046/j.1440-1746.2002.02837.x. [DOI] [PubMed] [Google Scholar]
- 12.Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31:323–331. doi: 10.1016/s0168-8278(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakumu S, Ito S, Ishikawa T, Mita Y, Tagaya T, Fukuzawa Y, Yoshioka K. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2000;15:431–436. doi: 10.1046/j.1440-1746.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Akbar SM, Tanimoto K, Ninomiya T, Iuchi H, Michitaka K, Horiike N, Onji M. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 2000;148:49–57. doi: 10.1016/s0304-3835(99)00312-2. [DOI] [PubMed] [Google Scholar]
- 16.Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 17.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 18.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 19.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 21.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Subleski JJ, Wiltrout RH, Weiss JM. Application of tissue-specific NK and NKT cell activity for tumor immunotherapy. J Autoimmun. 2009;33:275–281. doi: 10.1016/j.jaut.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermijlen D, Luo D, Froelich CJ, Medema JP, Kummer JA, Willems E, Braet F, Wisse E. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol. 2002;72:668–676. [PubMed] [Google Scholar]
- 28.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology. 2008;48:963–977. doi: 10.1002/hep.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coulouarn C, Factor VM, Conner EA, Thorgeirsson SS. Genomic modeling of tumor onset and progression in a mouse model of aggressive human liver cancer. Carcinogenesis. 2011;32:1434–1440. doi: 10.1093/carcin/bgr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson ED, Enriquez HL, Fu YX, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207:1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Attallah AM, Tabll AA, El-Sadany M, Ibrahim TA, El-Dosoky I. Dysregulation of blood lymphocyte subsets and natural killer cells in schistosomal liver cirrhosis and hepatocellular carcinoma. Clin Exp Med. 2003;3:181–185. doi: 10.1007/s10238-003-0023-y. [DOI] [PubMed] [Google Scholar]
- 35.Murakami S. Soluble interleukin-2 receptor in cancer. Front Biosci. 2004;9:3085–3090. doi: 10.2741/1461. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera R, Ararat M, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR. Hepatocellular carcinoma immunopathogenesis: clinical evidence for global T cell defects and an immunomodulatory role for soluble CD25 (sCD25) Dig Dis Sci. 2010;55:484–495. doi: 10.1007/s10620-009-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 40.Wang XD, Wang L, Ji FJ, Zhu JM, Ayana DA, Fang XD. Decreased CD27 on B lymphocytes in patients with primary hepatocellular carcinoma. J Int Med Res. 2012;40:307–316. doi: 10.1177/147323001204000131. [DOI] [PubMed] [Google Scholar]
- 41.Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One. 2015;10:e0117458. doi: 10.1371/journal.pone.0117458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 45.Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–218. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 46.Thakur S, Singla A, Chawla Y, Rajwanshi A, Kalra N, Arora SK. Expansion of peripheral and intratumoral regulatory T-cells in hepatocellular carcinoma: a case-control study. Indian J Pathol Microbiol. 2011;54:448–453. doi: 10.4103/0377-4929.85073. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 48.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 49.Ghiringhelli F, Ménard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 50.Bergmann C, Wild CA, Narwan M, Lotfi R, Lang S, Brandau S. Human tumor-induced and naturally occurring Treg cells differentially affect NK cells activated by either IL-2 or target cells. Eur J Immunol. 2011;41:3564–3573. doi: 10.1002/eji.201141532. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 52.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 53.Yan J, Liu XL, Xiao G, Li NL, Deng YN, Han LZ, Yin LC, Ling LJ, Liu LX. Prevalence and clinical relevance of T-helper cells, Th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014;9:e96080. doi: 10.1371/journal.pone.0096080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z, Xu R, Du Z. Intrahepatic interleukin-17+ T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:851–859. doi: 10.1111/jgh.12418. [DOI] [PubMed] [Google Scholar]
- 55.Berzofsky JA, Terabe M. The contrasting roles of NKT cells in tumor immunity. Curr Mol Med. 2009;9:667–672. doi: 10.2174/156652409788970706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, Im JS, Alves PM, Martinet O, Halkic N, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009;182:5140–5151. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- 57.Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, Colnot S, Viguier M, Perret C, et al. Oncogenic β-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;122:586–599. doi: 10.1172/JCI43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo HG, Park ES, Cheon JH, Kim JH, Lee JS, Park BJ, Kim W, Park SC, Chung YJ, Kim BG, et al. Gene expression-based recurrence prediction of hepatitis B virus-related human hepatocellular carcinoma. Clin Cancer Res. 2008;14:2056–2064. doi: 10.1158/1078-0432.CCR-07-1473. [DOI] [PubMed] [Google Scholar]
- 59.Ikeguchi M, Hirooka Y. Interleukin-2 gene expression is a new biological prognostic marker in hepatocellular carcinomas. Onkologie. 2005;28:255–259. doi: 10.1159/000084695. [DOI] [PubMed] [Google Scholar]
- 60.Akiba J, Yano H, Ogasawara S, Higaki K, Kojiro M. Expression and function of interleukin-8 in human hepatocellular carcinoma. Int J Oncol. 2001;18:257–264. doi: 10.3892/ijo.18.2.257. [DOI] [PubMed] [Google Scholar]
- 61.Yamaji K, Nabeshima S, Murata M, Chong Y, Furusyo N, Ikematsu H, Hayashi J. Interferon-alpha/beta upregulate IL-15 expression in vitro and in vivo: analysis in human hepatocellular carcinoma cell lines and in chronic hepatitis C patients during interferon-alpha/beta treatment. Cancer Immunol Immunother. 2006;55:394–403. doi: 10.1007/s00262-005-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 63.Qin S, Ma S, Huang X, Lu D, Zhou Y, Jiang H. Th22 cells are associated with hepatocellular carcinoma development and progression. Chin J Cancer Res. 2014;26:135–141. doi: 10.3978/j.issn.1000-9604.2014.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 65.Wang YY, Lo GH, Lai KH, Cheng JS, Lin CK, Hsu PI. Increased serum concentrations of tumor necrosis factor-alpha are associated with disease progression and malnutrition in hepatocellular carcinoma. J Chin Med Assoc. 2003;66:593–598. [PubMed] [Google Scholar]
- 66.Morsi MI, Hussein AE, Mostafa M, El-Abd E, El-Moneim NA. Evaluation of tumour necrosis factor-alpha, soluble P-selectin, gamma-glutamyl transferase, glutathione S-transferase-pi and alpha-fetoprotein in patients with hepatocellular carcinoma before and during chemotherapy. Br J Biomed Sci. 2006;63:74–78. doi: 10.1080/09674845.2006.11732724. [DOI] [PubMed] [Google Scholar]
- 67.Bortolami M, Venturi C, Giacomelli L, Scalerta R, Bacchetti S, Marino F, Floreani A, Lise M, Naccarato R, Farinati F. Cytokine, infiltrating macrophage and T cell-mediated response to development of primary and secondary human liver cancer. Dig Liver Dis. 2002;34:794–801. doi: 10.1016/s1590-8658(02)80073-1. [DOI] [PubMed] [Google Scholar]
- 68.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka Y, Furuta T, Suzuki S, Orito E, Yeo AE, Hirashima N, Sugauchi F, Ueda R, Mizokami M. Impact of interleukin-1beta genetic polymorphisms on the development of hepatitis C virus-related hepatocellular carcinoma in Japan. J Infect Dis. 2003;187:1822–1825. doi: 10.1086/375248. [DOI] [PubMed] [Google Scholar]
- 70.Lee KA, Ki CS, Kim HJ, Sohn KM, Kim JW, Kang WK, Rhee JC, Song SY, Sohn TS. Novel interleukin 1beta polymorphism increased the risk of gastric cancer in a Korean population. J Gastroenterol. 2004;39:429–433. doi: 10.1007/s00535-003-1315-4. [DOI] [PubMed] [Google Scholar]
- 71.Shiraki K, Yamanaka T, Inoue H, Kawakita T, Enokimura N, Okano H, Sugimoto K, Murata K, Nakano T. Expression of TNF-related apoptosis-inducing ligand in human hepatocellular carcinoma. Int J Oncol. 2005;26:1273–1281. [PubMed] [Google Scholar]
- 72.Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, Grosse-Wilde H, Broelsch CE, Gerken G, Cicinnati VR. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10:7260–7269. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 73.Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- 74.Giannitrapani L, Cervello M, Soresi M, Notarbartolo M, La Rosa M, Virruso L, D’Alessandro N, Montalto G. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:46–52. doi: 10.1111/j.1749-6632.2002.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 75.Cho HJ, Kim SS, Ahn SJ, Park SY, Park JH, Kim JK, Wang HJ, Cheong JY, Cho SW. Low serum interleukin-6 levels as a predictive marker of recurrence in patients with hepatitis B virus related hepatocellular carcinoma who underwent curative treatment. Cytokine. 2015;73:245–252. doi: 10.1016/j.cyto.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 76.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoong KF, Afford SC, Jones R, Aujla P, Qin S, Price K, Hubscher SG, Adams DH. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100–111. doi: 10.1002/hep.510300147. [DOI] [PubMed] [Google Scholar]
- 78.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–533. [PubMed] [Google Scholar]
- 79.Sutton A, Friand V, Brulé-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poiré A, Saffar L, Kraemer M, Vassy J, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 80.Liu H, Pan Z, Li A, Fu S, Lei Y, Sun H, Wu M, Zhou W. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–378. doi: 10.1038/cmi.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schimanski CC, Bahre R, Gockel I, Müller A, Frerichs K, Hörner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nahon P, Sutton A, Rufat P, Simon C, Trinchet JC, Gattegno L, Beaugrand M, Charnaux N. Chemokine system polymorphisms, survival and hepatocellular carcinoma occurrence in patients with hepatitis C virus-related cirrhosis. World J Gastroenterol. 2008;14:713–719. doi: 10.3748/wjg.14.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rubie C, Frick VO, Wagner M, Weber C, Kruse B, Kempf K, König J, Rau B, Schilling M. Chemokine expression in hepatocellular carcinoma versus colorectal liver metastases. World J Gastroenterol. 2006;12:6627–6633. doi: 10.3748/wjg.v12.i41.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsubara T, Ono T, Yamanoi A, Tachibana M, Nagasue N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J Surg Oncol. 2007;95:241–249. doi: 10.1002/jso.20642. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. Am J Gastroenterol. 2004;99:1111–1121. doi: 10.1111/j.1572-0241.2004.30265.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chia CS, Ban K, Ithnin H, Singh H, Krishnan R, Mokhtar S, Malihan N, Seow HF. Expression of interleukin-18, interferon-gamma and interleukin-10 in hepatocellular carcinoma. Immunol Lett. 2002;84:163–172. doi: 10.1016/s0165-2478(02)00176-1. [DOI] [PubMed] [Google Scholar]
- 88.Essand M, Loskog AS. Genetically engineered T cells for the treatment of cancer. J Intern Med. 2013;273:166–181. doi: 10.1111/joim.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh-Jasuja H, Emmerich NP, Rammensee HG. The Tübingen approach: identification, selection, and validation of tumor-associated HLA peptides for cancer therapy. Cancer Immunol Immunother. 2004;53:187–195. doi: 10.1007/s00262-003-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


