Abstract
IMPORTANCE
Severe obesity is increasingly common in the adolescent population but, as of yet, very little information exists regarding cardiovascular disease (CVD) risks in this group.
OBJECTIVE
To assess the baseline prevalence and predictors of CVD risks among severely obese adolescents undergoing weight-loss surgery.
DESIGN, SETTING, AND PARTICIPANTS
A prospective cohort study was conducted from February 28, 2007, to December 30, 2011, at the following 5 adolescent weight-loss surgery centers in the United States: Nationwide Children’s Hospital in Columbus, Ohio; Cincinnati Children’s Hospital Medical Center in Cincinnati, Ohio; Texas Children’s Hospital in Houston; University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania; and Children’s Hospital of Alabama in Birmingham. Consecutive patients aged 19 years or younger were offered enrollment in a long-term outcome study; the final analysis cohort consisted of 242 participants.
MAIN OUTCOMES AND MEASURES
This report examined the preoperative prevalence of CVD risk factors (ie, fasting hyperinsulinemia, elevated high-sensitivity C-reactive protein levels, impaired fasting glucose levels, dyslipidemia, elevated blood pressure, and diabetes mellitus) and associations between risk factors and body mass index (calculated as weight in kilograms divided by height in meters squared), age, sex, and race/ethnicity. Preoperative data were collected within 30 days preceding bariatric surgery.
RESULTS
The mean (SD) age was 17 (1.6) years and median body mass index was 50.5. Cardiovascular disease risk factor prevalence was fasting hyperinsulinemia (74%), elevated high-sensitivity C-reactive protein levels (75%), dyslipidemia (50%), elevated blood pressure (49%), impaired fasting glucose levels (26%), and diabetes mellitus (14%). The risk of impaired fasting glucose levels, elevated blood pressure, and elevated high-sensitivity C-reactive protein levels increased by 15%, 10%, and 6%, respectively, per 5-unit increase in body mass index (P < .01). Dyslipidemia (adjusted relative risk = 1.60 [95% CI, 1.26–2.03]; P < .01) and elevated blood pressure (adjusted relative risk = 1.48 [95% CI, 1.16–1.89]; P < .01) were more likely in adolescent boys compared with adolescent girls. White individuals were at greater risk of having elevated triglyceride levels (adjusted relative risk = 1.76 [95% CI, 1.14–2.72]; P = .01) but were less likely to have impaired fasting glucose levels (adjusted relative risk = 0.58 [95% CI, 0.38–0.89]; P = .01).
CONCLUSIONS AND RELEVANCE
Numerous CVD risk factors are apparent in adolescents undergoing weight-loss surgery. Increasing body mass index and male sex increase the relative risk of specific CVD risk factors. These data suggest that even among severely obese adolescents, recognition and treatment of CVD risk factors is important to help limit further progression of disease.
Childhood obesity has reached epidemic proportions and has established itself as a major threat to the health and welfare of millions of children and adolescents worldwide. Data from the United States estimate that approximately 17% of the pediatric and adolescent populations are considered obese (ie, body mass index [BMI; calculated as weight in kilograms divided by height in meters squared] ≥ 95th percentile) while corresponding reports demonstrate that 2% to 7% of affected youth are further categorized having the most severe form of obesity (ie, BMI ≥ 120% of the 95th percentile).1–4 There is evidence for an association between the rising prevalence of childhood obesity and a corresponding increase in numerous obesity-related comorbid illnesses including type 2 diabetes mellitus, hypertension (HTN), dyslipidemia, nonalcoholic fatty liver disease, and cardiovascular disease (CVD).5,6
A strong link between severe obesity and the development of CVD in adults is well established and previous data highlight the relationship between increasing excess body weight and declining cardiovascular health in the pediatric population.7 However, there is a relative paucity of data examining the specific CVD risk factors in severely obese adolescents.2,8 In addition, it is currently unknown whether a graded increase in the prevalence of cardiovascular risks continues throughout the full spectrum of adolescent severe obesity (eg, BMI values, 40–70) or whether such risks plateau at some threshold of BMI in adolescents. To address these knowledge gaps, we analyzed data collected from a cohort of 242 severely obese adolescents within 30 days preceding a scheduled weight-loss surgery (WLS) at 5 adolescent centers in the United States. We hypothesized that even in a severely obese young cohort, higher BMI levels would be associated with greater likelihood of having CVD risk factors and that the probability of having specific CVD risk factors would also be influenced by age, sex, and race/ethnicity.
Methods
Study Design and Patients
The study methods for the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS), an ancillary study to the Longitudinal Assessment of Bariatric Surgery (LABS) Study (NCT00465829), have been previously described in detail.1,9 Consecutive severely obese adolescents (<19 years) scheduled for bariatric surgery were offered enrollment into the study at 5 Teen-LABS centers between February 28, 2007, and December 30, 2011. Although there was no attempt to standardize or align clinical care algorithms in this observational study, clinical decision making, including the indications for adolescent WLS and routine patient evaluation and management during the preoperative period, generally followed previously reported adolescent-specific guidelines.10–12 Severely obese individuals who were not responsive to previous attempts at nonsurgical weight loss and with multiple comorbidities, such as diabetes mellitus, HTN, dyslipidemia, and nonalcoholic steatohepatitis, were considered appropriate to undergo surgical weight loss based on previously published best-practice guidelines.11 Written informed consent for study participation was obtained from participants who were between 18 and 19 years. Parental/caregiver assent was obtained for those adolescents younger than 18 years. The study protocol, assent/consent forms, and data and safety monitoring plans were approved by institutional review boards at each of the 5 institutions (Nationwide Children’s Hospital in Columbus, Ohio; Cincinnati Children’s Hospital Medical Centerin Cincinnati, Ohio; Texas Children’s Hospital in Houston; University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania; and Children’s Hospital of Alabama in Birmingham) and an independent data and safety monitoring board prior to initiation of the study.
Collection of Data
Data collection methods for this study1,9 were adapted from the LABS-2 Study.9,13,14 All data were collected within 30 days of the planned bariatric operation at an in-person study visit with trained study personnel. Study staff followed standard definitions to determine the presence or absence of associated comorbid conditions using participant interviews, physical examinations, review of medical records, and centralized laboratory testing. The current study focused on specific clinical and anthropomorphic elements used to define CVD risks in this cohort at study enrollment.
CVD Risk Factor Definitions
An individual was scored as having dyslipidemia if she or he had abnormally high low-density lipoprotein (LDL) cholesterol or triglyceride (TG) levels, an abnormally low high-density lipoprotein (HDL) cholesterol level, or was taking medication(s) for dyslipidemia. Low-density lipoprotein cholesterol and TG levels were considered abnormally high if either was 130 mg/dL or more (to convert triglyceride levels to millimoles per liter, multiply by 0.0113 and to convert LDL cholesterol to millimoles per liter, multiply by 0.0259), consistent with the Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report.15 Abnormally low levels of HDL cholesterol were defined as levels of 30 mg/dL or less (to convert HDL cholesterol to millimoles per liter, multiply by 0.0259) and represent what is considered a conservative cut point that encompasses approximately 5% of children in a prior population-based study.16 An abnormal TG:HDL cholesterol level ratio was defined as 3.0 or higher for nonblack individuals and 2.5 or higher for black individuals.17 Elevated blood pressure was defined as systolic blood pressure or diastolic blood pressure in the 95th percentile or higher for age/sex/height for patients younger than 18 years or a systolic blood pressure of 140 mm Hg or higher or diastolic blood pressure of 90 mm Hg or higher for those aged 18 years or older. Blood pressure measurements were obtained in identical manner at all clinical research facilities using a Welch Allyn Spot Vital Signs monitor (4200B). Blood pressure readings comprised the average systolic and diastolic measurement (minimum of 2 separate measurements). Additional methodological details are available in an online supplement from our published description of the Teen-LABS Study cohort.1 Irrespective of age, participants were considered to have an elevated blood pressure if taking antihypertensive medication(s). Prehypertension (pre-HTN) was defined as a systolic or diastolic blood pressure in the 90th percentile or higher and less than the 95th percentile for age/sex/height or a systolic blood pressure greater than or equal to 120 mm Hg but less than 140 mm Hg or diastolic blood pressure greater than or equal to 80 mm Hg but less than 90 mm Hg for patients younger than 18 years. Prehypertension was defined for individuals aged 18 years or older as a systolic blood pressure of 120 mm Hg or higher but less than 140 mm Hg or a diastolic blood pressure of 80 mm Hg or higher but less than 90 mm Hg. Impaired fasting glucose (IFG) was defined as a serum fasting glucose level of 100 mg/dL or more (to convert to millimoles per liter, multiply by 0.0555). Diabetes mellitus was considered present if previously diagnosed by a medical professional, taking medications for the treatment of diabetes mellitus (unless taking metformin with a concomitant diagnosis of polycystic ovary syndrome), a serum hemoglobin A1C level of 6.5% or higher (to convert to proportion of total hemoglobin, multiply by 0.01), fasting serum glucose level of 126 mg/dL or more, or 2-hour glucose value on an oral glucose tolerance test of 200 mg/dL or more within 2 weeks prior to study enrollment.18 Fasting insulin, serum hemoglobin A1c, and high-sensitivity C-reactive protein (hs-CRP) levels were considered abnormal if greater than 17 μIU/mL (to convert insulin to picomoles per liter, multiply by 6.945), 6.5% or higher, and 0.3 mg/dL or more (to convert CRP to nanomoles per liter, multiply by 9.524), respectively. All laboratory values reported were measured centrally at the Northwest Lipid Metabolism and Diabetes Research Laboratory in Seattle, Washington. For some analyses, a composite score (CVD risk factor [RF] total) was also created, which took into account multiple CVD risk factors with equal weighting (elevated blood pressure, dyslipidemia, diabetes mellitus, and abnormal hs-CRP). The homeostasis model assessment insulin resistance (HOMA-IR) was also calculated for each participant.19,20 The HOMA-IR values were calculated as (glucose [mg/dL] × insulin [mg/dL])/405. Values of HOMA-IR 4 or higher were considered abnormally elevated because this corresponded to the 85th percentile for lean adolescents based on our prior unpublished data and correlated with cross-sectional studies in various populations where HOMA-IR values 4 or higher represented a moderate degree of insulin resistance.21,22
Statistical Methods
The prevalence of CVD risk factors was assessed using percentages and frequencies. In addition to analyzing BMI as a continuous variable, the following BMI groups were created for categorical analysis: group 1 (BMI < 50); group 2 (BMI, ≥50–<60); and group 3 (BMI ≥ 60). These BMI categories were chosen to ensure that each BMI group had sufficient participants to optimize subsequent statistical analysis. The relationship between risk factors and BMI categories was assessed using Fisher exact test and the Cochran-Armitage test for trend. Analysis for the composite score (CVD RF total), which consisted of 4 primary CVD risk factors (elevated blood pressure, dyslipidemia, diabetes mellitus, and abnormal hs-CRP), required extensive computation time and memory; therefore, Monte Carlo estimation of Fisher exact test and Mantel-Haenszel exact χ2 test were performed.
Regression analyses were performed while simultaneously accounting for the independent variables of BMI, age, sex, and race/ethnicity to evaluate the association between the CVD risk factors (or the CVD total score) and each of these independent variables. Separate regression analyses were performed using BMI as either a continuous or categorical variable. In each regression analysis, age was modeled continuously while sex (boys/girls) and race/ethnicity (white/nonwhite individuals) were modeled categorically. The relative risk (RR) was estimated for each of the predictor variables. The analyses used a generalized estimating equation approach with a Poisson distribution to obtain robust confidence intervals for the RR estimates.23 No participants were excluded from the previously described statistical analyses based on the presence of specific comorbid conditions. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Characteristics of the Study Participants
The 242 participants in the Teen-LABS cohort are predominantly female (76%), white (72%), and non-Hispanic (93%), with a mean age of 17 years (range, 13–19 years). The median BMI at baseline was 50.5 (range, 34–88). As shown in Table 1, the following CVD risk factors were commonly observed in this cohort: fasting hyperinsulinemia (74%), elevated hs-CRP (75%), dyslipidemia (50%), elevated blood pressure (49%), and diabetes mellitus (14%). Also noted by others, the most common lipid abnormalities contributing to dyslipidemia in this cohort were elevated triglyceride levels (40%) and depressed HDL cholesterol levels; 16% for HDL cholesterol levels less than 30 mg/dL, 42% for HDL cholesterol levels less than 35 mg/dL, and 63% for HDL cholesterol levels less than 40 mg/dL. Although the proportion of participants meeting the definition of abnormally low HDL levels varied considerably by the cut point used (eg, <30 mg/dL, <35 mg/dL, or <40 mg/dL) as shown earlier, HDL cholesterol levels less than 30 mg/dL (defined as a conservative cut point in the Methods section) was used to define abnormally low HDL cholesterol levels in our further analyses. Using this conservative HDL cholesterol cut point, 16% of our study cohort had abnormally low HDL cholesterol level values. Nearly three-quarters of the cohort (71%) were insulin resistant, as determined by elevated HOMA-IR. Most participants (61%) had at least 2 of the 4 CVD risk factors used to define CVD RF total. The percentages of individuals with at least 1, 2, 3, or 4 CVD risk factors were 95%, 61%, 24%, and 5%, respectively. Only 5% of participants had no measured CVD risk factors.
Table 1.
Demographic, Anthropometric, and Comorbid Disease Characteristics and Categorical Analysis Based on BMI Stratification for 242 Participantsa
| Variable | Sample Size | No. (%) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Overall Prevalence | Group 1 | Group 2 | Group 3 | ||||
| 30 < BMI < 50 (n = 115) |
50 ≤ BMI < 60 (n = 77) |
BMI ≥ 60 (n = 50) |
Fisher | Trend Test | |||
| Dyslipidemia | 238 | 120 (50.4) | 59 (52.2) | 41 (53.2) | 20 (41.7) | .39 | .30 |
| Elevated blood pressure | 239 | 117 (49.0) | 44 (38.6) | 43 (56.6) | 30 (61.2) | .01 | <.01 |
| Prehypertension | 239 | 63 (26.4) | 34 (29.8) | 17 (22.4) | 12 (24.5) | .51 | .36 |
| Impaired fasting glucose | 238 | 62 (26.1) | 20 (17.7) | 24 (31.2) | 18 (37.5) | .01 | <.01 |
| Diabetes mellitus | 242 | 33 (13.6) | 13 (11.3) | 12 (15.6) | 8 (16.0) | .55 | .35 |
| LDL cholesterol level, ≥130 mg/dL | 238 | 20 (8.4) | 7 (6.2) | 9 (11.7) | 4 (8.3) | .38 | .46 |
| HDL cholesterol level, <30 mg/dL | 238 | 38 (16.0) | 20 (17.7) | 12 (15.6) | 6 (12.5) | .76 | .41 |
| TG level, ≥130 mg/dL | 238 | 96 (40.3) | 47 (41.6) | 31 (40.3) | 18 (37.5) | .90 | .64 |
| Abnormal TG:HDL cholesterol level ratio | 238 | 124 (52.1) | 59 (52.2) | 42 (54.5) | 23 (47.9) | .77 | .72 |
| HOMA, ≥4.0 | 232 | 165 (71.1) | 79 (71.2) | 52 (71.2) | 34 (70.8) | >.99 | .97 |
| Insulin, >17.0 μIU/mL | 232 | 172 (74.1) | 82 (73.9) | 54 (74.0) | 36 (75.0) | >.99 | .89 |
| HbA1c, ≥6.5% | 231 | 14 (6.1) | 4 (3.7) | 8 (10.5) | 2 (4.2) | .17 | .58 |
| hs-CRP, ≥0.3 mg/dL | 238 | 179 (75.2) | 76 (67.3) | 66 (85.7) | 37 (77.1) | .01 | .06 |
| CVD risk factor total | 242 | .17b | .05c | ||||
| None | 13 (5.4) | 10 (8.7) | 1 (1.3) | 2 (4.0) | |||
| Only 1 | 81 (33.5) | 44 (38.3) | 20 (26.0) | 17 (34.0) | |||
| Only 2 | 89 (36.8) | 40 (34.8) | 31 (40.3) | 18 (36.0) | |||
| Only 3 | 46 (19.0) | 16 (13.9) | 20 (26.0) | 10 (20.0) | |||
| Only 4 | 13 (5.4) | 5 (4.4) | 5 (6.5) | 3 (6.0) | |||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; TG, triglyceride.
SI conversion factors: To convert CRP to nanomoles per liter, multiply by 9.524; glucose to millimoles per liter, multiply by 0.0555; HbA1c to proportion of total hemoglobin, multiply by 0.01; HDL and LDL cholesterol to millimoles per liter, multiply by 0.0259; insulin to picomoles per liter, multiply by 6.945; and TG to millimoles per liter, multiply by 0.0113.
Prevalence of multiple CVD risk factor variables determined at baseline (preweight loss surgery) and associated categorical analysis determined by BMI range stratification in a cohort of 242 severely obese individuals. Group 1 (BMI < 50), group 2 (BMI, ≥50–<60), and group 3 (BMI ≥ 60). Cardiovascular disease risk factor total represents a composite score of 4 equally weighted independent CVD risk factors (ie, elevated blood pressure, dyslipidemia, diabetes mellitus, and abnormal hs-CRP). A P value ≤.05 was considered statistically significant.
Monte Carlo estimate of Fisher exact P value of the exact P value.
Monte Carlo estimate of exact P value for Mantel-Haenszel χ2 test.
Predictors of CVD Risk Factors
To test the hypothesis that BMI was related to the presence of specific CVD risk factors in this cohort, we first examined the relationship between individual CVD risk factors and discrete categories of BMI. Specifically, we segregated the study cohort into the following 3 BMI groups: group 1 (BMI < 50), group 2 (BMI, ≥50–<60), and group 3 (BMI ≥ 60). As shown in Table 1 and in the Figure, the prevalence of IFG differed markedly between those in the lowest BMI category compared with those in the highest 2 categories (18%, 31%, and 38% for groups 1, 2, and 3, respectively; P < .01). Similar to the observation with IFG, we also noted an increase in the prevalence of elevated blood pressure between the lowest BMI category and both of the higher categories (39%, 57%, and 61% for BMI groups 1, 2, and 3, respectively; P < .01). The prevalence of other CVD risk factors did not appear to significantly increase or decrease across progressively higher BMI categories.
Figure. Prevalence of Elevated Blood Pressure and Impaired Fasting Glucose Levels Based on Body Mass Index (BMI) Range.
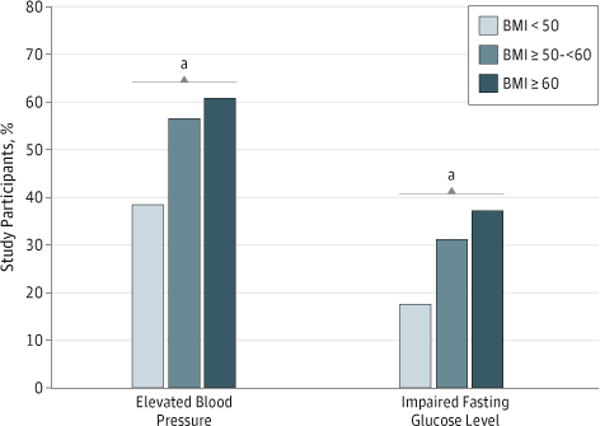
Body mass index is calculated as weight in kilograms divided by height in meters squared.
aA significant increasing linear trend across the 3 BMI groups. Both elevated blood pressure and the impaired fasting glucose level observed P < .01 when performing this test for trend.
To further explore this relationship between participant characteristics (BMI, age, sex, and race/ethnicity) and specific CVD risk factors, regression modeling was used. Relative risk and 95% CI estimates were obtained for each baseline characteristic (predictor variable). The predictor variables that approached significance (P < .10) for predicting RR in adjusted analyses are shown in the far left column of Table 2. For these variables, adjusted RR (ARR) estimates were obtained for a 5-unit increase in BMI, a 1-year increase in age, boys compared with girls, and white individuals compared with nonwhite individuals (Table 2). When BMI was examined as a continuous variable, we confirmed a strong association with risk of IFG in these adjusted analyses. While controlling for age, race/ethnicity, and sex, we also found that for every 5-unit increase in BMI, a 15% increase in the risk of IFG resulted (P < .01; Table 2). Increasing BMI was also associated with an increased risk of elevated blood pressure (ARR = 1.10; P < .01) and elevated hs-CRP (ARR = 1.06; P < .01) in this severely obese cohort.
Table 2.
Predictors of CVD Risk Based on BMI, Age, Sex, and Race/Ethnicitya
| Variable | Predictor Variable | ARR (95% CI) |
|---|---|---|
| Abnormal LDL cholesterol level, >130 mg/dL | Age (1-y increase) | 1.32 (0.97–1.78) |
| Abnormal TG level, >130 mg/dL | Sex (adolescent boys vs girls) | 1.55 (1.14–2.11) |
| Race/ethnicity (white vs nonwhite individual) | 1.76 (1.14–2.72) | |
| Abnormal HDL cholesterol level, <30 mg/dL | Age (1-y increase) | 0.86 (0.72–1.03) |
| Abnormal TG:HDL cholesterol level ratio | Sex (adolescent boys vs girls) | 1.42 (1.12–1.81) |
| Race/ethnicity (white vs nonwhite individuals) | 1.31 (0.96–1.79) | |
| Dyslipidemia | Sex (adolescent boys vs girls) | 1.60 (1.26–2.03) |
| Elevated blood pressure | BMI (5-unit increase) | 1.10 (1.04–1.16) |
| Sex (adolescent boys vs girls) | 1.48 (1.16–1.89) | |
| Prehypertensiona | ||
| Impaired fasting glucose | BMI (5-unit increase) | 1.15 (1.05–1.26) |
| Age (1-y increase) | 0.88 (0.78–0.99) | |
| Race/ethnicity (white vs nonwhite individuals) | 0.58 (0.38–0.89) | |
| Diabetes mellitusb | ||
| Abnormal HOMA-IR | Race/ethnicity (white vs nonwhite individuals) | 1.26 (1.01–1.57) |
| Abnormal insulin | Race/ethnicity (white vs nonwhite individuals) | 1.28 (1.04–1.58) |
| Abnormal HbA1cb | ||
| Abnormal hs-CRP | BMI (5-unit increase) | 1.06 (1.02–1.10) |
| Age (1-y increase) | 1.06 (1.01–1.12) |
Abbreviations: ARR, adjusted relative risk; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; HDL, high-density lipoprotein; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment insulin resistance; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; TG, triglyceride.
SI conversion factors: To convert CRP to nanomoles per liter, multiply by 9.524; glucose to millimoles per liter, multiply by 0.0555; HbA1cto proportion of total hemoglobin, multiply by 0.01; HDL and LDL cholesterol to millimoles per liter, multiply by 0.0259; insulin to picomoles per liter, multiply by 6.945; and TG to millimoles per liter, multiply by 0.0113.
Regression analysis displayed as adjusted relative risk and 95% CI estimates. Results for predictor variables with P values greater than .10 are not displayed. Adjusted data are shown for individual CVD risk factors using the predictor variables of BMI, age, sex, and race/ethnicity. A P value < .05 was considered statistically significant.
No predictors were observed for this specific outcome variable.
Adolescent boys were at a markedly higher risk compared with adolescent girls for abnormal TG level (ARR = 1.55; P = .01), TG:HDL cholesterol levels ratio (ARR = 1.42; P < .01), and dyslipidemia (ARR = 1.60; P < .01). Boys were also 48% more likely to have elevated blood pressure when compared with girls (ARR = 1.48; P < .01). White participants were at greater risk for elevated TG levels (ARR = 1.76; P = .01), abnormal insulin levels (ARR = 1.28; P = .02), and elevated HOMA-IR (ARR = 1.26; P = .04) compared with nonwhite individuals. However, the risk for IFG was lower in white individuals compared with nonwhite individuals (ARR = 0.58; P = .01). Older participants were at modestly increased risk for elevated hs-CRP levels (ARR = 1.06; P = .01) but were at lower risk of IFG when compared with younger participants (ARR = 0.88; P = .04).
Discussion
To our knowledge, this was the first study to examine the prevalence and predictors of CVD risk factors in a large cohort of severely obese teenagers prior to WLS. We found that most participants demonstrated evidence of insulin resistance and inflammation while approximately half met criteria for dyslipidemia and elevated blood pressure. Most participants had evidence of at least 2 CVD risk factors. Despite the fact that all participants were already severely obese, we were nonetheless able to detect a graded risk for elevated blood pressure and IFG across categories of BMI in this cohort.
Cardiovascular disease has been identified as the leading cause of death among adults in the United States as a consequence of several synergistic processes, including the development of HTN, atherosclerotic disease, and metabolic dysregulation (ie, insulin resistance, metabolic syndrome, and type 2 diabetes mellitus), which lead to an increased risk of cardiac ischemic events, heart failure, and stroke.24 Although such overt manifestations of CVD are rarely present in the pediatric population, associated clinical and biological risk factors have been identified during childhood and adolescence25 and these CVD risk factors are known to track into adulthood.26 Taken together, the rising prevalence of severe childhood obesity coupled with the increased propensity of obese adolescents becoming obese adults has led to concerns about the cumulative impact of obesity-related CVD in this population.6
An increasing number of clinical studies, including our study from the Teen-LABS Consortium, have shown that obese adolescents and, in particular, severely obese teenagers are at increased risk for numerous obesity-related comorbidities.1,2,27–29 However, there are limited data available examining the prevalence and predictors of CVD risk in this severely affected group undergoing surgery for weight loss. In a prior analysis of severely obese adolescents (mean age, 17.8 years; mean BMI, 50) with diabetes mellitus undergoing WLS (n = 11), we showed a high prevalence of elevated blood pressure (46%), pre-HTN (9%), and IFG (40%). In addition, dyslipidemia and hyperinsulinemia were quite prevalent in this cohort.2 Finally, we and others have found that adolescents undergoing WLS had evidence of a pathologic impact of severe obesity on cardiac structure and physiology (ie, diastolic dysfunction and elevated cardiac workload).27,30,31
Cardiovascular disease risk data from the National Health and Nutrition Examination Survey suggest that some CVD risk factors are increased in youth with increasing BMI.24 Youth with normal BMI (<85th percentile for age) have a relatively low prevalence of pre-HTN (10%), borderline/high LDL cholesterol levels (≥110 mg/dL; 18%), low HDL cholesterol levels (<35 mg/dL; 3%), and IFG/diabetes mellitus (13%). On the other hand, obese youth (BMI ≥ 95th percentile) have higher prevalence of pre-HTN (25%), borderline/high LDL cholesterol levels (32%), low HDL cholesterol levels (<35 mg/dL; 16%), and IFG/diabetes mellitus (20%). In the Teen-LABS cohort, we found that the prevalence of borderline/high LDL cholesterol levels and IFG were similar at 27% and 26%, respectively. However, in contrast to the obese National Health and Nutrition Examination Survey cohort, considerably more Teen-LABS participants met criteria for elevated blood pressure/pre-HTN (75%) and abnormally low HDL cholesterol levels (HDL < 35 mg/DL; 42%).
In this analysis, we showed a dose-response relationship between BMI and some CVD risk factors (eg, elevated blood pressure and IFG) but not others (eg, LDL cholesterol levels). Large epidemiologic studies of healthy children demonstrated that obese youth have higher LDL cholesterol and TG and lower HDL cholesterol levels compared with their lean counterparts.32 It is not surprising that further increases in the prevalence of high LDL cholesterol levels are not seen in our severely obese adolescents because high LDL cholesterol levels are more likely to be related to familial hypercholesterolemia, a genetic disorder less influenced by an obesogenic lifestyle. The fact that BMI in our cohort was not associated with an increased RR for an elevated TG:HDL cholesterol level ratio may indicate that the threshold for this effect is seen at a lower BMI because this abnormality is found in a high proportion of youth with mild obesity and insulin resistance.33 To our knowledge, our study is the first to demonstrate a continued graded effect of extreme increases in BMI on blood pressure and impaired glucose metabolism, suggesting that reversal of severe obesity may be important in preventing future CVD in these patients.
The study had several important limitations. First, the population examined in this study was likely not to be representative of severely obese adolescents in the general population because they were a population that was referred because of clinical indications for WLS. Thus, the comorbidity burden may be greater in this group owing to referral bias. In addition, the overrepresentation of white female participants and underrepresentation of Hispanic individuals further limited the generalizability of the study findings. Lastly, elevated blood pressure recorded at a single time does not represent the definition of HTN, which generally requires 3 separate abnormal measurements at 2 to 3 separate visits. Despite these limitations, the strengths of the Teen-LABS Study were the large and multi-institutional nature of this cohort of severely obese adolescents, the uniformity of the prospective data collection using standardized methods and data definitions, and the use of a central laboratory for biochemical analyses.
Conclusions
In this first large-scale and uniform analysis, severely obese adolescents had a major burden of cardio metabolic risk factors. Participants with lower BMI had fewer CVD risk factors while white race/ethnicity and male sex were associated with a greater likelihood of having specific CVD risks. The clinical assessment and medical care of severely obese adolescents should focus on the identification of these risks and control or prevention of progression of them in this vulnerable population.
Acknowledgments
Funding/Support: Dr Courcoulas received grants from the National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases. The Teen-LABS consortium was funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases through grants U01DK072493, UM1DK072493, and UM1DK095710 (University of Cincinnati). The study was also supported by grants UL1 TR000077-04 (Cincinnati Children’s Hospital Medical Center), UL1RR025755 (Nationwide Children’s Hospital), M01-RR00188 (Texas Children’s Hospital/Baylor College of Medicine), UL1 RR024153, UL1TR000005 (University of Pittsburgh), and UL1 TR000165 (University of Alabama, Birmingham). We gratefully acknowledge the significant contributions made by the Teen-LABS Consortium and grant U01 DK066557 from our parent study, LABS Consortium.
Footnotes
Author Contributions: Dr Jenkins had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Michalsky, Inge, Simmons, Helmrath, Brandt, Harmon, Chen, Daniels, Urbina.
Acquisition, analysis, or interpretation of data: Michalsky, Inge, Simmons, Jenkins, Buncher, Helmrath, Harmon, Courcoulas, Chen, Horlick.
Drafting of the manuscript: Michalsky, Inge, Simmons, Harmon, Chen, Daniels, Urbina.
Critical revision of the manuscript for important intellectual content: Michalsky, Inge, Simmons, Jenkins, Buncher, Harmon, Courcoulas, Chen, Horlick, Daniels, Urbina.
Statistical analysis: Simmons, Jenkins, Buncher, Urbina.
Obtained funding: Inge, Helmrath, Harmon.
Administrative, technical, or material support: Courcoulas, Horlick.
Study supervision: Michalsky, Inge, Helmrath, Harmon, Daniels.
Conflict of Interest Disclosures: Dr Courcoulas received grants from Covidien, EndoGastric Solutions, Nutrisystem, and J&J Ethicon Scientific, and personal fees unrelated to the submitted work from J&J Ethicon Scientific. Dr Inge received grants from Ethicon Endosurgery unrelated to the submitted work. No other disclosures were reported.
Role of the Funder/Sponsor: The funders were directly involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The following individuals contributed to the planning or conduct of the study: Thomas Inge, MD, PhD, Meg Zeller, PhD, Michael Helmrath, MD, Avani Modi, PhD, Stavra Xanthakos, MD, MS, Larry Dolan, MD, Todd Jenkins, MPH, PhD, Rosie Miller, RN, CCRC, Rachel Akers, MPH, April Carr, BS, Lindsey Shaw, MS, Cynthia Spikes, CRC, Stephen Daniels, MD, PhD, Tawny Wilson Boyce, MS, MPH, Tara Schafer-Kalkhoff, MA, CCRP, at the Cincinnati Children’s Hospital Medical Center; Ralph Buncher, ScD, and Mark Simmons, MEng, at the University of Cincinnati; Mary Brandt, MD, Vadim Sherman, MD, Margaret Callie Lee, MPH, David Allen, BS, Gia Washington, PhD, and Karin Price, PhD, at the Texas Children’s Hospital, Baylor Medical Center; Carroll M. Harmon, MD, PhD, Ronald Clements, MD, Richard Stahl, MD, Molly Bray, PhD, Beverly Haynes, BSN, at the Children’s Hospital of Alabama, University of Alabama at Birmingham; Anita Courcoulas, MD, Ramesh Ramanathan, MD, Carol A. McCloskey, MD, George M. Eid, MD, Jessie Eagleton, MPH, William Gourash, MSN, CRNP, Sheila Pierson, BS, and Dana Rofey, PhD, MS, at the University of Pittsburgh Medical Center; Marc Michalsky, MD, Steven Teich, MD, Karen Carter, CCRC, Melinda Helton, RN, Bonny Bowen, RN, Robert David Murray, MD, and Amy Baughcum, PhD, at the Nationwide Children’s Hospital; and Mary Horlick, MD, Mary Evans, PhD, Rebecca Torrance, RN, MSN, Rebekah Van Raaphorst, MPH, and Susan Yanovski, MD, at the National Institute of Diabetes and Digestive and Kidney Diseases.
Additional Contributions: We acknowledge the generous contribution of methodological details from the LABS consortium. The consortium is grateful for the important work done by the adjudication committee.
References
- 1.Inge TH, Zeller MH, Jenkins TM, et al. Teen-LABS Consortium Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168(1):47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inge TH, Miyano G, Bean J, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123(1):214–222. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 3.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9(5):322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157(1):26–31.e2. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimm SY, Barton BA, Obarzanek E, et al. NHLBI Growth and Health Study Obesity development during adolescence in a biracial cohort: the NHLBI Growth and Health Study. Pediatrics. 2002;110(5):e54. doi: 10.1542/peds.110.5.e54. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17.e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang CY, Fulton JE. Cardiorespiratory fitness levels among US youth aged 12–15 years: United States 1999–2004 and 2012. NCHS Data Brief. 2014;(153):1–8. [PubMed] [Google Scholar]
- 8.Teeple EA, Teich S, Schuster DP, Michalsky MP. Early metabolic improvement following bariatric surgery in morbidly obese adolescents. Pediatr Blood Cancer. 2012;58(1):112–116. doi: 10.1002/pbc.23370. [DOI] [PubMed] [Google Scholar]
- 9.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42(11):1969–1971. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apovian CM, Baker C, Ludwig DS, et al. Best practice guidelines in pediatric/adolescent weight loss surgery. Obes Res. 2005;13(2):274–282. doi: 10.1038/oby.2005.37. [DOI] [PubMed] [Google Scholar]
- 11.Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. ASMBS pediatric committee best practice guidelines: surgery for obesity and related diseases. J Am Soc Bariatric Surg. 2012;8(1):1–7. doi: 10.1016/j.soard.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring) 2009;17(5):901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belle SH, Berk PD, Courcoulas AP, et al. Longitudinal Assessment of Bariatric Surgery Consortium Writing Group Safety and efficacy of bariatric surgery: longitudinal assessment of bariatric surgery. Surg Obes Relat Dis. 2007;3(2):116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackey RH, Belle SH, Courcoulas AP, et al. Longitudinal Assessment of Bariatric Surgery Consortium Writing Group Distribution of 10-year and lifetime predicted risk for cardiovascular disease prior to surgery in the longitudinal assessment of bariatric surgery-2 study. Am J Cardiol. 2012;110(8):1130–1137. doi: 10.1016/j.amjcard.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cresanta JL, Srinivasan SR, Webber LS, Berenson GS. Serum lipid and lipoprotein cholesterol grids for cardiovascular risk screening of children. Am J Dis Child. 1984;138(4):379–387. doi: 10.1001/archpedi.1984.02140420045016. [DOI] [PubMed] [Google Scholar]
- 17.Burns SF, Lee SJ, Arslanian SA. Surrogate lipid markers for small dense low-density lipoprotein particles in overweight youth. J Pediatr. 2012;161(6):991–996. doi: 10.1016/j.jpeds.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Executive summary: standards of medical care in diabetes. 2013. Diabetes Care. 2013;36(suppl1):S4–S10. doi: 10.2337/dc13-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 20.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 21.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age. EPIRCE Cross-sectional Study. BMC Endocr Disord. 2013;13:47. doi: 10.1186/1472-6823-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin J, Li M, Xu L, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5(1):71. doi: 10.1186/1758-5996-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 24.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 25.Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 26.Berenson GS, Srnivasan SR, Bogalusa Heart Study Group Cardiovascular risk factors in youth with implications for aging: the Bogalusa Heart Study. Neurobiol Aging. 2005;26(3):303–307. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Michalsky MP, Raman SV, Teich S, Schuster DP, Bauer JA. Cardiovascular recovery following bariatric surgery in extremely obese adolescents: preliminary results using cardiac magnetic aesonance (CMR) imaging. J Pediatr Surg. 2013;48(1):170–177. doi: 10.1016/j.jpedsurg.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Brandt ML, Harmon CM, Helmrath MA, Inge TH, McKay SV, Michalsky MP. Morbid obesity in pediatric diabetes mellitus: surgical options and outcomes. Nat Rev Endocrinol. 2010;6(11):637–645. doi: 10.1038/nrendo.2010.167. [DOI] [PubMed] [Google Scholar]
- 29.Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248(5):763–776. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]
- 30.Ippisch HM, Inge TH, Daniels SR, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51(14):1342–1348. doi: 10.1016/j.jacc.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 31.Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G. Effects of bariatric surgery on cardiac structure and function: a systematic review and meta-analysis. Am J Hypertens. 2014;27(2):146–156. doi: 10.1093/ajh/hpt215. [DOI] [PubMed] [Google Scholar]
- 32.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103(6, pt 1):1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 33.Steinberger J, Daniels SR, Eckel RH, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119(4):628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]


