Abstract
Abstract Secondhand smoke (SHS) exposure has negative effects on maternal and infant health. SHS exposure among pregnant women in Argentina and Uruguay has not been previously described, nor has the proportion of those who have received screening and advice to avoid SHS during prenatal care. Women who attended one of 21 clusters of publicly-funded prenatal care clinics were interviewed regarding SHS exposure during pregnancy at their delivery hospitalization during 2011–2012. Analyses were conducted using SURVEYFREQ procedure in SAS version 9.3 to account for prenatal clinic clusters. Of 3,427 pregnant women, 43.4 % had a partner who smoked, 52.3 % lived with household members who smoked cigarettes, and 34.4 % had no or partial smoke-free home rule. Of 528 pregnant women who worked outside of the home, 21.6 % reported past month SHS exposure at work and 38.1 % reported no or partial smoke-free work policy. Overall, 35.9 % of women were exposed to SHS at home or work. In at least one prenatal care visit, 67.2 % of women were screened for SHS exposure, and 56.6 % received advice to avoid SHS. Also, 52.6 % of women always avoided SHS for their unborn baby's health. In summary, a third of pregnant women attending publicly-funded prenatal clinics were exposed to SHS, and only half of pregnant women always avoided SHS for their unborn baby's health. Provider screening and advice rates can be improved in these prenatal care settings, as all pregnant women should be screened and advised of the harms of SHS and how to avoid it.
Keywords: Environmental tobacco exposure, Pregnancy, Guidelines, Prenatal care
Introduction
Exposure to secondhand smoke (SHS) has negative effects on maternal and infant health [1]. Pregnant women who are exposed to SHS have 20 % higher odds of giving birth to a low birth weight infant compared to women who are not exposed [2]. A meta-analysis of 76 studies found mothers exposed to SHS have small but significantly increased risks of having a lower birth weight infant (60 g difference) than those not exposed and an increased risk of congenital anomalies (OR 1.17; 95 % CI 1.03–1.34) [3]. Furthermore, infants who are exposed to SHS are more likely to die of sudden infant death syndrome (SIDS) compared with infants not exposed, and children exposed to SHS are at increased risk for bronchitis, pneumonia, ear infections, more severe asthma, respiratory symptoms, and slowed lung growth [1]. Thus, preventing SHS exposure during pregnancy could avert poor pregnancy, infant, and child health outcomes.
Globally, 40 % of children and 35 % of female non-smokers were exposed to SHS in 2004 [4]. In 2002, Argentina and Uruguay had the highest median concentration of airborne nicotine, a measure of SHS, in most public places of their capital cities compared to other Latin American capitals in Brazil, Chile, Costa Rica, Paraguay, and Peru [5]. Significantly more men than women smoke cigarettes in Argentina (32 vs 22 %) and Uruguay (30 vs 20 %) [6]. Thus, SHS exposure in households with non-smokers, such as spouses, partners, and children who do not smoke, should be considered. Understanding the burden of SHS exposure may help ministries of health direct appropriate resources to address the problem. Previous studies have reported SHS exposure among women and children in these countries using biochemical measures [7] and self-report [8]. However, the proportion of pregnant women exposed to SHS and sources of these exposures have not been previously estimated in these countries. In 2013, the World Health Organization (WHO) released guidelines recommending that antenatal care providers screen all pregnant women for SHS exposure and provide brief advice on the harms of SHS [9]. To our knowledge, it is not known to what extent providers screen for and provide brief advice on SHS exposure in Argentina and Uruguay. It is also unknown how frequently pregnant women avoid SHS to protect their unborn baby's health.
The study objectives were to estimate the prevalence of pregnant women who were exposed to SHS, identify the sources of their exposure, and describe the characteristics of those exposed to SHS by women's smoking status. We also sought to estimate the prevalence of pregnant women who were screened for SHS exposure and were advised of the harms of SHS during prenatal care. Because advice could vary based on whether pregnant women smoked, we examined SHS exposure and receipt of services by smoking status. These study results may be used to inform tobacco control efforts and antenatal care practices in Argentina and Uruguay.
Methods
Data were collected at baseline as part of a cluster randomized-controlled trial and prior to implementing a brief smoking cessation counseling intervention in prenatal clinics. Detailed methodology of the trial is published elsewhere [10]. Trained interviewers identified consecutively eligible women during their postpartum stay to determine who attended one of 21 clusters of prenatal care clinics and delivered a baby in any of ten public hospitals in Buenos Aires, Argentina or two public hospitals in Montevideo, Uruguay, from October 2011 to May 2012. Consented women were surveyed using a validated questionnaire about tobacco use and SHS exposure during pregnancy and whether they received provider screening and advice on SHS exposure during prenatal care. The study was approved by the ethics committees/institutional review boards of all participating hospitals; the Ministry of Health of the Province of Buenos Aires, Argentina; the Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno”; the Faculty of Medicine, Universidad de la República, Uruguay; Centers for Disease Control and Prevention; and Tulane University.
Variables
SHS exposure during pregnancy was determined from questions to each woman on whether her partner or other household members smoked cigarettes, whether smoking was allowed in her home, whether anyone at work smoked in the last 30 days, whether smoking was allowed at her workplace, and how often she was indoors and around other people who were smoking. Composite measures were created of SHS exposure during pregnancy at home, at work, and at home or work. SHS exposure at home was defined from a woman's report about (1) whether smoking was allowed in her home or on certain occasions or in certain rooms and (2) whether a partner or other household member who lived with her smoked. SHS exposure at work was defined from a woman's report about whether anyone at work smoked indoors in the last 30 days.
All women were asked if they received provider screening for SHS exposure. Women were also asked about whether their prenatal care providers advised about the harms of SHS to themselves or to their unborn infant. Composite measures were created of receipt of screening for SHS exposure at home or work, and receipt of advice of harm of SHS to women or unborn infants. These measures were assessed for any and all prenatal care visits. Women were also asked how often they tried to avoid breathing SHS because they thought it was bad for the health of their unborn baby. Response options included: never, rarely, sometimes, and always.
Women's smoking status was categorized as nonsmokers or smokers. Nonsmokers were those who reported never smoking, tried cigarettes but did not smoke regularly, or quit smoking before they found out that they were pregnant. Smokers were those who smoked every day or some days before they found out they were pregnant and included women who quit smoking sometime during pregnancy and those who continued to smoke during pregnancy. Women who self-reported quitting smoking during pregnancy provided saliva samples within 12 h after delivery so that cotinine testing could confirm their quit status.
Additional variables derived from women's interviews and included in the analysis were maternal age, foreign citizenship, marital status, highest level of education completed, work status in past year, and trimester that prenatal care was initiated. Parity was derived from the clinical record.
Analysis
A total of 3,427 were included in the sample. Of these, 1,880 women (54.9 %) gave birth in Argentina and 1,547 women (45.1 %) in Uruguay. Differences in characteristics of SHS exposure, receipt of prenatal care provider screening, advice regarding SHS exposure, and avoidance of SHS during pregnancy by smoking status was assessed using the Wald Chi square test for homogeneity of proportions (significance was set at P < 0.05). As no statistical differences in SHS exposure were observed by country, the data were presented in aggregate. Sample size varied by variable. As the proportion of missing data was small in our study [SHS variables: ranging from 0.6 % (smoke-free home rules) to 5.5 % (partner smokes) and smoking status (4.2 %)] such that bias in any parameter estimates when compared to complete data is likely to be small [11]. Analyses were conducted using the SURVEYFREQ procedure in SAS version 9.3 to account for prenatal clinic care clusters (SAS Institute, Inc., Cary, NC).
Results
Of 3,427 pregnant women, most were aged 20–34 years (70.6 %), were citizens of Argentina or Uruguay (93.3 %), were married or partnered (85.4 %), were unemployed in the past year (76.4 %), were multiparous (65.5 %), and had initiated prenatal care in the first trimester (52.3 %) (Table 1). Almost half of women (46.2 %) had an incomplete secondary education. The majority of women in the sample were nonsmokers (69.8 %). Compared to smokers, nonsmokers were more likely to be of foreign citizenship, be married or partnered, have higher education, and be nulliparous (P < 0.05). No differences were observed between smokers and nonsmokers by maternal age, employment status, and initiation of prenatal care.
Table 1.
Characteristics of sample by women's smoking status
| Characteristicsa | Total |
Nonsmokerb |
Smokerb |
P valuec | |||
|---|---|---|---|---|---|---|---|
| N % | (95 % CI) | N | % | N | % | ||
| Total | 3,427 | 100 | 2,292 | 69.8 | 991 | 30.2 | |
| Age (years) | |||||||
| ≤19 | 581 | 17.2 (15.0–19.4) | 398 | 17.6 | 159 | 16.3 | 0.5241 |
| 20–34 | 2,390 | 70.6 (68.2–73.1) | 1,584 | 69.9 | 706 | 72.2 | |
| ≥35 | 412 | 12.2 (10.7–13.6) | 284 | 12.5 | 113 | 11.5 | |
| Foreign citizenship | |||||||
| Yes | 228 | 6.7 (3.8–9.6) | 202 | 8.9 | 20 | 2.0 | 0.0010 |
| No | 3,173 | 93.3 (90.4–96.2) | 2,073 | 91.1 | 966 | 98.0 | |
| Marital status | |||||||
| Married or partnered | 2,896 | 85.4 (82.7–88.1) | 1,976 | 87.0 | 764 | 82.2 | 0.0018 |
| Unmarried | 494 | 14.6 (11.9–17.3) | 295 | 13.0 | 129 | 17.8 | |
| Highest level of education | |||||||
| Incomplete primary school or less | 220 | 6.4 (4.4–8.5) | 150 | 6.6 | 64 | 6.5 | 0.0002 |
| Completed primary school | 939 | 27.6 (23.1–32.1) | 586 | 25.7 | 328 | 33.2 | |
| Incomplete secondary school | 1,571 | 46.2 (39.4–52.9) | 1,025 | 45.0 | 465 | 47.1 | |
| Completed secondary or more | 673 | 19.8 (13.0–26.6) | 515 | 22.6 | 131 | 13.2 | |
| Work status in past year | |||||||
| Employed or student | 780 | 23.6 (17.8–29.4) | 507 | 22.9 | 234 | 24.3 | 0.5499 |
| Unemployed | 2,530 | 76.4 (70.6–82.2) | 1,706 | 77.1 | 727 | 75.7 | |
| Parity | |||||||
| 0 | 1,118 | 34.5 (31.4–37.7) | 790 | 36.5 | 282 | 29.9 | 0.0050 |
| ≥1 | 2,118 | 65.5 (62.3–68.6) | 1,375 | 63.5 | 602 | 70.1 | |
| Initiation of prenatal care | |||||||
| First trimester | 1,676 | 52.3 (45.8–58.7) | 1,126 | 52.4 | 480 | 51.8 | 0.7431 |
| Second trimester | 1,269 | 39.5 (34.9–44.2) | 840 | 39.1 | 377 | 40.7 | |
| Third trimester | 262 | 8.2 (5.4–10.7) | 183 | 8.5 | 70 | 7.5 | |
CI confidence intervals
Sample size varied by each item due to missing values, ranging from 0.7 % (education) to 6.4 % (initiation of prenatal care)
Smoking status was missing for 144 women (4.2 %)
Chi square tests were used to assess differences between nonsmokers and smokers
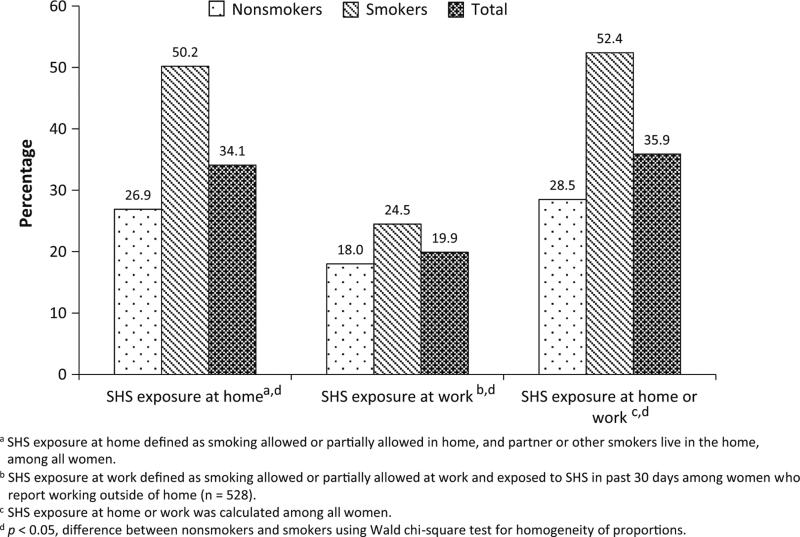
Overall, 43.4 % of pregnant women had a partner who smoked, 52.3 % had smokers living in the household, and 34.4 % reported having no or a partial smoke-free rule in the home (Table 2). Among pregnant women who worked outside of the home (n = 528, 18.2 % of the total sample), 21.6 % were exposed to smoke at work in the past month, and 38.1 % had no or a partial smoke-free policy at work. Sixteen percent of women reported being always exposed to SHS at home, work, or public places. Overall, 34.1 % of pregnant women were exposed to SHS at home, 19.9 % of pregnant women who worked outside of the home were exposed to SHS at work, and 35.9 % percent were exposed at home or work (Fig. 1).
Table 2.
Sources of secondhand smoke exposure and policies during pregnancy by women's smoking status
| Indicatora | Total |
Nonsmokerb |
Smokerb |
P valuec | |||
|---|---|---|---|---|---|---|---|
| N % | (95 % CI) | N | % | N | % | ||
| Smoke-free home rule | |||||||
| Yes | 2,236 | 65.6 (61.5–69.8) | 1,667 | 73.0 | 485 | 48.2 | <.0001 |
| No | 1,170 | 34.4 (30.2–38.5) | 617 | 27.0 | 501 | 50.8 | |
| Partner smokes | |||||||
| Yes | 1,407 | 43.4 (41.1–45.8) | 743 | 34.1 | 589 | 63.5 | <.0001 |
| No | 1,832 | 56.6 (54.2–58.9) | 1,438 | 65.9 | 338 | 36.5 | |
| Number of smokers in householdd | |||||||
| None | 1,634 47.7 (44.2–51.2) | 1,288 | 56.2 | 293 | 29.6 | <.0001 | |
| 1 | 1,188 38.9 (36.1–11.7) | 755 | 32.9 | 514 | 51.9 | ||
| 2 | 299 8.7 (7.5–10.0) | 169 | 7.4 | 113 | 11.4 | ||
| 3+ | 161 4.5 (3.8–5.6) | 80 | 3.5 | 71 | 7.2 | ||
| Smoke-free workplace policye | |||||||
| Yes | 327 | 61.9 (57.4–66.4) | 240 | 64.3 | 87 | 56.1 | 0.1858 |
| No | 201 | 38.1 (33.6–42.6) | 133 | 35.7 | 68 | 43.9 | |
| Exposure to smoke at work in the last 30 dayse | |||||||
| Yes | 105 | 21.6 (16.9–26.2) | 67 | 19.5 | 38 | 26.4 | 0.0343 |
| No | 382 | 78.4 (73.8–83.1) | 276 | 80.5 | 106 | 73.6 | |
| How often around smokers (home, work, public places) | |||||||
| Never | 788 | 23.7 (16.9–30.5) | 627 | 28.2 | 142 | 14.7 | 0.0001 |
| Rarely | 903 | 27.2 (21.0–33.3) | 710 | 31.9 | 153 | 15.9 | |
| Sometimes | 1,102 | 33.1 (30.4–35.9) | 655 | 29.4 | 385 | 40.0 | |
| Always | 534 | 16.1 (13.6–18.5) | 235 | 10.6 | 283 | 29.4 | |
CI confidence interval
Sample size varied by each item due to missing values, ranging from 0.6 % (smoke-free home rule) to 5.5 % (partner smokes)
Smoking status was missing for 144 women (4.2 %)
Chi square tests were used to assess differences between nonsmokers and smokers
Excludes the pregnant smoker
Exposure to smoke at work was calculated only among women who reported working outside of the house (n = 528)
Fig. 1.
Secondhand smoke (SHS) exposure during pregnancy at home or work by women's smoking status
Compared to smokers, nonsmokers were more likely to report having a 100 % smoke-free rule at home, having nonsmoking partners, living with nonsmokers in their household, and never being around other smokers at home, work, and public places (Table 2). There were also differences by smoking status among working women exposed to SHS at work in the past month. When examining characteristics of nonsmokers who were exposed to SHS at home, compared to those not exposed, a higher proportion of nonsmokers exposed to SHS at home were aged ≤19 years (23.8, 14.4 %) and nulliparous (45.1, 32.4 %) (data not shown).
In at least one prenatal visit, the majority of women (65.9 %) were asked by their prenatal care provider about SHS exposure at home, 26.8 % were asked about SHS exposure at work, and 67.2 % were asked about SHS exposure at home or work (Table 3). A lower percentage of women were asked about SHS at home (10.5 %), at work (4.2 %), or at home or work (11.1 %) at all prenatal care visits. Half of pregnant women were advised that SHS was not good for their health (53.7 %) or their baby's health (51.2 %) in at least one visit. About 56.6 % were advised that SHS was not good for her or her baby's health in at least one visit.
Table 3.
Prevalence of providers' asking about secondhand smoke (SHS) exposure and advising about adverse health effects of SHS during pregnancy, by women's smoking status
| Indicators | Total |
Nonsmokera |
Smokera |
Difference | P valuec | |||
|---|---|---|---|---|---|---|---|---|
| N % | (95% CI) | N | % | N | % | (95 % CI)b | ||
| At least one visit | ||||||||
| Ask | ||||||||
| Provider asked about SHS exposure at home | 2,235 | 65.9 (55.5–76.3) | 1,478 | 65.1 | 673 | 68.6 | –0.04 (–0.09, 0.02) | 0.2161 |
| Provider asked about SHS exposure at work | 868 | 26.8 (18.7–34.8) | 551 | 25.2 | 291 | 31.6 | –0.06 (–0.11, –0.02) | 0.0107 |
| Provider asked about SHS exposure at home or work | 2,257 | 67.2 (56.7–77.7) | 1,494 | 66.2 | 678 | 70.3 | –0.04 (–0.10, 0.02) | 0.1549 |
| Advise | ||||||||
| Provider advised women that SHS was not good for their health | 1,821 | 53.7 (43.6–63.8) | 1,107 | 48.7 | 632 | 64.4 | –0.16 (–0.20, –0.12) | <.0001 |
| Provider advised women that SHS was not good for their babieś health | 1,737 | 51.2 (40.6–61.7) | 1,065 | 46.8 | 594 | 60.5 | –0.14 (–0.18, –0.09) | <.0001 |
| Provider advised women that SHS was not for their health or their babies' health | 1,923 | 56.6 (46.5–66.8) | 1,181 | 51.9 | 657 | 66.8 | –0.15 (–0.20, –0.10) | <.0001 |
| In all prenatal visits | ||||||||
| Ask | ||||||||
| Provider asked about SHS exposure at home | 354 | 10.5 (5.2–15.7) | 196 | 8.6 | 145 | 14.8 | –0.06 (–0.11, –0.02) | 0.0151 |
| Provider asked about SHS exposure at work | 136 | 4.2 (1.6–6.8) | 77 | 3.5 | 56 | 6.1 | –0.03 (–0.05, –0.00) | 0.0313 |
| Provider asked about SHS exposure at home or work | 361 | 11.1 (5.4–16.8) | 200 | 9.1 | 147 | 15.9 | –0.07 (–0.12, –0.02) | 0.0154 |
| Advise | ||||||||
| Provider advised women that SHS was not good for their health | 294 | 8.7 (4.3–13.0) | 140 | 6.2 | 141 | 14.4 | –0.08 (–0.13, –0.04) | 0.0044 |
| Provider advised women that SHS was not good for their babieś health | 275 | 8.1 (4.1–12.2) | 128 | 5.6 | 134 | 13.7 | –0.08 (–0.12, –0.04) | 0.0032 |
| Provider advised women that SHS was not for their health or their babies' health | 320 | 9.5 (5.0–14.0) | 153 | 6.7 | 152 | 15.6 | –0.09 (–0.13, –0.04) | 0.0023 |
SHS secondhand smoke, CI confidence interval
Smoking status was missing for 144 women (4.2 %)
Difference in prevalence between nonsmokers and smokers
Chi square tests were used to assess differences between nonsmokers and smokers
There were differences between smokers and nonsmokers on whether their provider asked about SHS exposure at work in any prenatal care visits, but smokers were more likely to be asked about SHS at home or work in all visits (Table 3). Smokers were more likely to be advised about the harms of SHS exposure at any visit or in all visits compared to nonsmokers. Among the 35.9 % of women (n = 1,231) who were exposed to SHS, 59.8 % received provider advice to avoid SHS.
Approximately 52.6 % of women reported they always avoided SHS during pregnancy because of concerns about their babies’ health (data not shown). Nonsmokers were significantly more likely to always avoid SHS for their babies’ health compared to smokers (63.5, 28.6 %).
Discussion
Overall, 67.2 % of women reported being screened for SHS exposure either at home or in the workplace and 56.6 % reported receiving advice to avoid SHS during prenatal care. We found that one-third of pregnant women reported exposure to SHS at home or at work, with the majority exposed to SHS at home by their partner or other household members who smoked. Yet, little more than half of these women reported receiving any information from their prenatal care provider on the harms of SHS to themselves or their babies. Furthermore, only half of pregnant women (52.6 %) reported that they always avoided SHS because of concerns about their babies’ health.
The percentages (67.2 and 56.6 %) of screening and brief advice for SHS in our study are lower than the WHO recommendation that during antenatal care visits all pregnant women be routinely screened and provided brief advice on the harms of SHS exposure [9]. Best practices for treatment of tobacco dependence during pregnancy have focused on screening for active maternal smoking and providing assistance to pregnant smokers who want to quit [12, 13] but screening and advice on SHS exposure have not been recommended broadly for both smokers and nonsmokers during pregnancy. National guidelines, clinical protocols, and training can support the goal that during prenatal care all pregnant women be informed of the harms of SHS and advised to avoid it [14, 15].
Beyond brief advice to avoid harms of SHS, it is unknown whether additional provider intervention, such as counseling women to avoid SHS and providing assistance to partners to quit smoking, may be more effective in eliminating SHS exposure during pregnancy. There have been a limited number of studies that have evaluated interventions to reduce SHS among nonsmoking pregnant women, and though some reported positive effects on reducing SHS, the majority of studies are based on self-reported SHS exposure, which may be unreliable [16]. Thus, additional trials are needed that biochemically verify intervention effect of reducing SHS exposure at the end of pregnancy.
For all measures of SHS exposure at home and at work, smokers were more likely to be exposed to SHS than nonsmokers, and smokers were significantly more likely to receive advice about the harms of SHS than nonsmokers. As noted earlier, national clinical guidelines exist in Argentina and Uruguay that promote intervention with pregnant smokers and, often, SHS exposure is addressed to protect the health of infants. Given that four out of ten nonsmokers in our study lived in households that included smokers, more attention is needed to ensure that routine screening and advice occur for all pregnant women regardless of smoking status.
We found that 65.6 % of women reported that they had a smoke-free rule in their home during pregnancy. Nonsmokers most likely to be exposed to SHS at home were young, first-time mothers. Increased implementation of voluntary smoke-free home rules can be achieved through educating pregnant women and their families, and may be encouraged by the adoption of smoke-free policies in worksites and public places [17]. As public settings increasingly become smoke-free, awareness of the harms of SHS becomes more prevalent in communities, which can encourage changes in the social environment and increase the unacceptability of smoking indoors [1]. In addition to their effectiveness in reducing smoking prevalence among the general population, public smoke-free policies may also reduce SHS exposure among pregnant nonsmokers [18, 19] and reduce smoking among pregnant smokers [20–22], thereby improving birth outcomes. In 2006, Uruguay was the first country in the Americas to pass comprehensive national smoke-free legislation [23]. In 2011, Argentina passed legislation to prohibit smoking in public places. Thus, to target young pregnant women and those who live with them, including families and partners, educational materials in these countries can be developed with simple health messages about the harms of SHS and the importance of smoke-free homes.
This is the first study to assess prenatal provider practices regarding screening and brief advice for SHS exposure during pregnancy in two Latin America countries using the new WHO recommendations. Given the high and increasing rates of smoking in this region and potential for SHS exposure [4], these findings can be used to improve obstetrical practice and inform the development of educational materials to eliminate SHS exposure in pregnant women. Second, the study used validated and standardized questions to assess SHS exposure; thus allowing our data to be compared with SHS data from other countries and settings [24].
This study is not without limitations. First, smoke-free home status and whether smokers lived with the pregnant woman were self-reported by women at delivery, and there was no biochemical verification of SHS exposure. Recall and nondisclosure bias could result in underre-porting of SHS exposure, but it is unknown the degree to which underreporting occurs among pregnant women in these countries. However, a Japanese study found that pregnant women's reports of smoking among partners and other household members correlated with cotinine levels to indicate the woman's SHS exposure [25]. In the current study, both women's reports of partner smoking and other household smokers were used to indicate exposure at home. Second, women were asked whether co-workers smoked indoors to assess workplace SHS exposure, but we were unable to assess the proximity to which the co-workers smoked near them. SHS can infiltrate many areas of a building or home even with designated smoking areas [26, 27], thus indicating any smoking indoors may result in exposure. Finally, these findings may not be general-izable beyond the study participants in these two countries.
In conclusion a third of pregnant women attending public prenatal clinics in Buenos Aires and Montevideo are exposed to SHS, predominantly at home. Because pregnancy may be an opportune time to make positive behavior change, such as avoiding SHS exposure, more work is needed in these countries to encourage prenatal care providers to screen all pregnant women for SHS exposure, to promote smoke-free home environments, and to counsel women to always avoid SHS exposure. Whenever possible, providers can encourage partners and family members to quit smoking to protect the health of pregnant women and their babies. Further research is needed to identify interventions beyond brief advice which may be effective for eliminating SHS exposure during pregnancy.
Acknowledgments
The study was supported through CDC cooperative agreement 5U48DP001948-04 (SIP09-18) to Tulane University. The authors report no competing interests. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC. We would like to acknowledge participating coordinators and hospitals: Argentina—D. Fernández at Hospital Municipal Materno Infantil “Dr. Carlos Gianantonio”; J. Antón at Hospital Zonal General deAgudos“Héroesde Malvinas”; E. Macagno at Hospital Zonal General de Agudos “Dr. Carlos Bocalandro”; M. Ferrary at Hospital Zonal General de Agudos “Magdalena Villegas de Martínez”; V. Nicolaci at Instituto de Maternidad Santa Rosa; M.T. Moreno Hospital Zonal General de Agudos “San Roque”; M.R. Sabbadín at Hospital Zonal General de Agudos “Dr. Narciso López”; S.M. Souza at Hospital Zonal General de Agudos “Evita Pueblo”; C. Muzzio at Hospital Municipal “Ostaciana B. de Lavignolle”; L. Frías at Hospital Zonal General de Agudos “Dr. Lucio Meléndez.” Uruguay—O. Graña and E. Gomez, at Administración de los Servicios de Salud del Estado; A. Raggio at Banco de Previsión Social.
Footnotes
Publisher's Disclaimer: Your article is protected by copyright and all rights are held exclusively by Springer Science+Business Media New York (outside the USA). This e-offprint is for personal use only and shall not be self-archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: “The final publication is available at link.springer.com”.
Contributor Information
Van T. Tong, Division of Reproductive Health/NCCDPHP, Centers for Disease Control and Prevention, 4770 Buford Hwy, NE, MS-F74, Atlanta, GA 30341, USA
Paola Morello, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina.
Alicia Alemán, Montevideo Clinical and Epidemiological Research Unit, Montevideo, Uruguay.
Carolyn Johnson, Tulane School of Public Health and Tropical Medicine, New Orleans, LA, USA.
Patricia M. Dietz, Division of Reproductive Health/NCCDPHP, Centers for Disease Control and Prevention, 4770 Buford Hwy, NE, MS-F74, Atlanta, GA 30341, USA
Sherry L. Farr, Division of Reproductive Health/NCCDPHP, Centers for Disease Control and Prevention, 4770 Buford Hwy, NE, MS-F74, Atlanta, GA 30341, USA
Agustina Mazzoni, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina.
Mabel Berrueta, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina.
Mercedes Colomar, Montevideo Clinical and Epidemiological Research Unit, Montevideo, Uruguay.
Alvaro Ciganda, Montevideo Clinical and Epidemiological Research Unit, Montevideo, Uruguay.
Ana Becú, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina.
Maria G. Bittar Gonzalez, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay.
Laura Llambi, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay.
Luz Gibbons, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina.
Ruben A. Smith, Division of Reproductive Health/NCCDPHP, Centers for Disease Control and Prevention, 4770 Buford Hwy, NE, MS-F74, Atlanta, GA 30341, USA
Pierre Buekens, Tulane School of Public Health and Tropical Medicine, New Orleans, LA, USA.
José M. Belizán, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina
Fernando Althabe, Institute for Clinical Effectiveness and Health Policy, Buenos Aires, Argentina.
References
- 1.Centers for Disease Control and Prevention . The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. US Department of Health and Human Services; Atlanta, GA.: 2006. [PubMed] [Google Scholar]
- 2.Windham GC, Eaton A, Hopkins B. Evidence for an association between environmental tobacco smoke exposure and birthweight: A meta-analysis and new data. Paediatric and Perinatal Epidemiology. 1999;13(1):35–57. doi: 10.1046/j.1365-3016.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 3.Salmasi G, Grady R, Jones J, et al. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):423–441. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Report of the global tobacco epidemic: Implementing smoke-free environments. World Health Organization; Geneva: 2009. [July 23, 2014]. http://www.who.int/tobacco/mpo wer/2009/gtcr_download/en/. [Google Scholar]
- 5.Navas-Acien A, Peruga A, Breysse P, et al. Secondhand tobacco smoke in public places in Latin America, 2002–2003. JAMA. 2004;291(22):2741–2745. doi: 10.1001/jama.291.22.2741. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Report of the global tobacco epidemic: Warning about the dangers of tobacco. World Health Organization; Geneva: 2011. [Google Scholar]
- 7.Wipfli H, Avila-Tang E, Navas-Acien A, et al. Secondhand smoke exposure among women and children: Evidence from 31 countries. American Journal of Public Health. 2008;98(4):672–679. doi: 10.2105/AJPH.2007.126631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Maio FG, Konfino J, Ondarsuhu D, et al. Sexstratified and age-adjusted social gradients in tobacco in Argentina and Uruguay: Evidence from the Global Adult Tobacco Survey (GATS). Tobacco Control. 2014 doi: 10.1136/tobaccocontrol-2013-051525. doi: 10.1136/tobaccocontrol-2013-051525. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO recommendations for the prevention and management of tobacco use and second-hand smoke exposure in pregnancy. World Health Organization; Geneva: 2013. [November 30, 2013]. http://www.who.int/tobacco/publications/pregnancy/guidelinestobaccosmokeexposure/en/index.html. [PubMed] [Google Scholar]
- 10.Althabe F, Aleman A, Mazzoni A, et al. Tobacco cessation intervention for pregnant women in Argentina and Uruguay: Study protocol. Reproductive Health. 2013;10(1):44. doi: 10.1186/1742-4755-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz KF, Grimes DA. Sample size slippages in randomised trials: Exclusions and the lost and wayward. The Lancet. 2002;359(9308):781–785. doi: 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- 12.Ministerio de Salud de la Nación . Programa Nacional para el control del tabaco, Ministerio de Salud Publica (MSP) Guia Nacional para el Abordaje del Tabaquimo; Uruguay: 2009. [Google Scholar]
- 13.Ministerio de Salud de la Nación . Guía de Práctica Clínica Nacional de Tratamiento a la Adicción al Tabaco. Buenos Aires; Argentina: 2011. [Google Scholar]
- 14.Farquhar CM, Kofa E, Power ML, et al. Clinical practice guidelines as educational tools for obstetrician–gynecologists. Journal of Reproductive Medicine. 2002;47(11):897–902. [PubMed] [Google Scholar]
- 15.Flenady V, Macphail J, New K, et al. Implementation of a clinical practice guideline for smoking cessation in a public antenatal care setting. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;48(6):552–558. doi: 10.1111/j.1479-828X.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 16.Tong VT, Dietz PM, Rolle IV, et al. Clinical interventions to reduce secondhand smoke exposure among pregnant women: A systematic review. Tobacco Control. 2014 doi: 10.1136/tobaccocontrol-2013-051200. doi:10. 1136/tobaccocontrol-2013-051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mons U, Nagelhout GE, Allwright S, et al. Impact of national smoke-free legislation on home smoking bans: Findings from the international tobacco control policy evaluation project Europe surveys. Tobacco Control. 2013;22(e1):e2–e9. doi: 10.1136/tobaccocontrol-2011-050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charrier L, Serafini P, Giordano L, et al. Smoking habits in Italian pregnant women: Any changes after the ban? Journal of Public Health Policy. 2010;31(1):51–58. doi: 10.1057/jphp.2009.43. [DOI] [PubMed] [Google Scholar]
- 19.Puig C, Vall O, Garcia-Algar O, et al. Assessment of prenatal exposure to tobacco smoke by cotinine in cord blood for the evaluation of smoking control policies in Spain. BMC Pregnancy Childbirth. 2012;12:26. doi: 10.1186/1471-2393-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams EK, Markowitz S, Kannan V, et al. Reducing prenatal smoking: The role of state policies. American Journal of Preventive Medicine. 2012;43(1):34–40. doi: 10.1016/j.amepre.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Cox B, Martens E, Nemery B, et al. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: Analysis of routinely collected birth data. BMJ. 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay DF, Nelson SM, Haw SJ, et al. Impact of Scotland’s smoke-free legislation on pregnancy complications: Retrospective cohort study. PLoS Medicine. 2012;9(3):e1001175. doi: 10.1371/journal.pmed.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco-Marquizo A, Goja B, Peruga A, et al. Reduction of secondhand tobacco smoke in public places following national smoke-free legislation in Uruguay. Tobacco Control. 2010;19(3):231–234. doi: 10.1136/tc.2009.034769. [DOI] [PubMed] [Google Scholar]
- 24.Global Adult Tobacco Survey Collaborative Group . Tobacco questions for surveys: A subset of key questions from the Global Adult Tobacco Survey (GATS) 2nd ed. Centers for Disease Control and Prevention; Atlanta, GA: 2011. [February 12, 2014]. http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. [Google Scholar]
- 25.Sasaki S, Braimoh TS, Yila TA, et al. Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy—A validation study in Northern Japan. Science of the Total Environment. 2011;15(412–413):114–118. doi: 10.1016/j.scitotenv.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Van Deusen A, Hyland A, Travers MJ, et al. Secondhand smoke and particulate matter exposure in the home. Nicotine & Tobacco Research. 2009;11(6):635–641. doi: 10.1093/ntr/ntp018. [DOI] [PubMed] [Google Scholar]
- 27.King BA, Travers MJ, Cummings KM, et al. Secondhand smoke transfer in multiunit housing. Nicotine & Tobacco Research. 2010;12(11):1133–1141. doi: 10.1093/ntr/ntq162. [DOI] [PMC free article] [PubMed] [Google Scholar]