INTRODUCTION
The prevalence of calcific aortic stenosis increases with age, occurring in 3 to 5% of individuals older than 75 years old (1,2). The narrowing of the aortic valve is driven by a highly complex and intricately regulated process of inflammation, fibrosis and calcification, which eventually results in leaflet immobility and the associated hemodynamic consequences (3). In response to the narrowed valve, left ventricular hypertrophy is initially adaptive to restore wall stress and cardiac performance. Ultimately, adverse events such as symptoms, heart failure and death occur as the left ventricle decompensates. This transition from adaptation to decompensation is driven by progressive myocyte death and myocardial fibrosis (4-6). Therefore, it is important to consider aortic stenosis as a condition that affects both the valve and the myocardium (5,6). Indeed, contemporary guidelines recommend aortic valve replacement in patients with severe aortic stenosis and evidence of advanced ventricular decompensation, defined by either the presence of symptoms or an impaired systolic ejection fraction less than 50% (7,8).
The current strategy relies heavily on the timely identification of symptoms. However, the poor prognosis associated with the development of angina, exertional dyspnea and syncope first described by Ross and Braunwald in 1968 was based on younger patients with bicuspid or rheumatic disease (average age of 63 years at time of death) (4). Establishing symptoms in the more elderly population encountered in current clinical practice is more challenging due to their comorbidities and often sedentary lifestyles. Moreover, it is now widely recognized that left ventricular ejection fraction is not a sensitive marker of myocardial dysfunction (9,10) and impairment in ejection fraction is often a late manifestation that may not be reversible (11,12). Furthermore, there is an urgent need to improve our understanding of the pathogenesis of aortic stenosis, both with respect to the valve and myocardium, so that we can develop novel biomarkers and therapeutic targets for this common condition.
There is considerable interest in novel imaging techniques that can provide key insights into the pathogenesis of aortic stenosis and in improving risk stratification of patients. In this review, we will examine novel imaging biomarkers of the valve and myocardium, with a particular focus on those with prognostic value and potential of translating into clinical practice. Subsequently, we will discuss how we can integrate these techniques to improve the management of patients with aortic stenosis in the near future.
AORTIC VALVE CALCIFICATION AS A MARKER OF DISEASE PROGRESSION AND ADVERSE PROGNOSIS
The risk factors associated with the establishment and incidence of aortic stenosis are remarkably similar to those of atherosclerosis. They include increasing age, smoking and elevated cholesterol concentrations (13,14). Indeed like atherosclerosis, early calcification in the valve appears closely related to lipid deposition and inflammation. However once established, the subsequent progression of aortic valve narrowing appears to be dominated by a self-perpetuating cycle of calcium accumulation (15-17). As a consequence, disease progression and the development of cardiovascular events are more closely associated with markers of calcification rather than lipid or inflammation. For that reason, non-invasive assessments of aortic valve calcification have major potential in risk stratifying patients and improving the patient management.
Assessing the burden of aortic valve calcification
The clinical importance of aortic valve calcification was first described more than a decade ago in a landmark study using echocardiography. In 128 asymptomatic patients with peak aortic jet velocity ≥ 4m/s, moderate or severe aortic valve calcification on echocardiography (defined as aortic valve calcium score of 3 and 4 on a 4-point ordinal scale) was a strong independent predictor of death or aortic valve replacement, outperforming conventional hemodynamic assessment on echocardiography (18). The value of this score with respect to disease progression and adverse cardiovascular events was subsequently demonstrated in patients with mild and moderate aortic stenosis (19). Unfortunately, this 4-point score remains semi-quantitative and limited by poor inter-observer agreement.
Electrocardiography-gated non-contrast computed tomography (CT) is able to provide more detailed information with respect to the density, volume and mass of macroscopic calcium deposits within the aortic valve. CT calcium scoring of the valve is similar to the approach used in the coronary arteries and most commonly expressed as Agatston units (AU). Indeed, aortic valve CT calcium scoring demonstrates high intra-, inter-observer and scan-rescan reproducibility (a limitation with the echocardiographic calcium score) (20,21); and correlates well with the calcium weight of excised valves (20) and other echocardiographic parameters of aortic stenosis severity (17,20-22). Interestingly, patients with more severe disease at baseline experienced more rapid progression of aortic valve calcification on CT (Figure 1B) and multiple studies have also demonstrated a strong relationship between baseline calcium scores and the rate of subsequent disease progression (16,17,23).
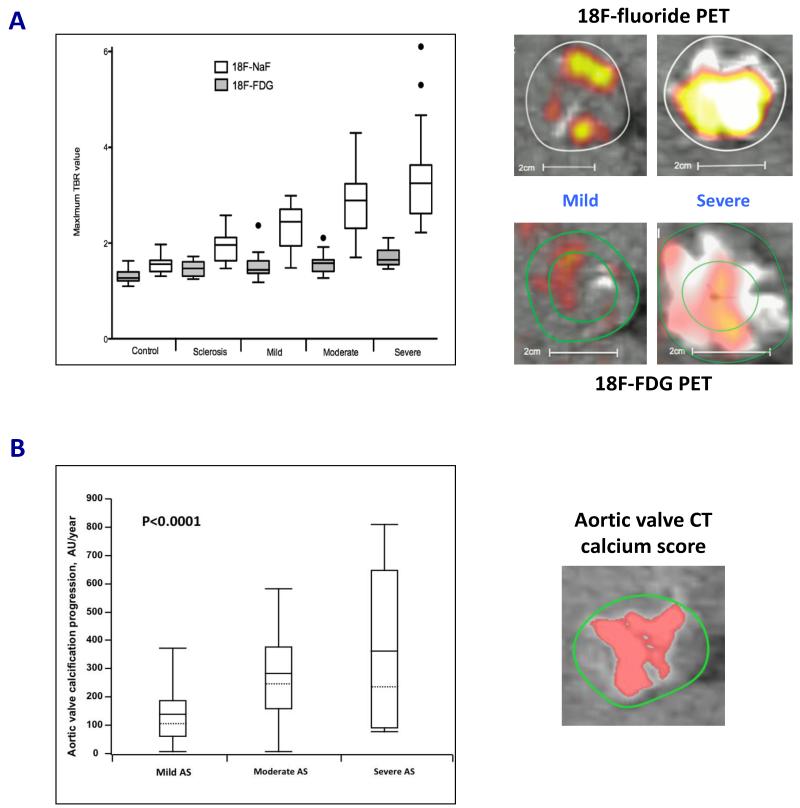
FIGURE 1.
(A) 18F-Fluoride (18F-NaF; a marker of novel calcification) activity increased markedly with severity of aortic stenosis. Conversely, 18F-fluorodeoxyglucose (18F-FDG; a marker of inflammation) activity had a more modest association with aortic stenosis severity. This supports the notion that calcification rather than inflammation predominates in the valve, particularly in the later stages of the disease. Fused positron emission tomography (PET)/computed tomography (CT) scans demonstrated a significant difference in tracer activity (top right panels: 18F-NaF and bottom right panels: 18F-FDG) on co-axial views of the aortic valves in patients with mild and severe aortic stenosis. White and yellow/red areas depict calcium deposits and PET tracer activity, respectively. Results presented in box and whiskers plot (Tukey): the central box represented the interquartile range of tissue-to-background ratios (TBR) with the median indicated by the dark line. The whiskers extended to the most extreme values within the 1.5 interquartile ranges. Adapted with permission from (31).
(B) Aortic valve calcification can be quantified accurately on CT (right panel). Consistent with PET data, the progression of aortic valve calcification on CT was associated with severity of aortic stenosis. Patients with severe aortic stenosis experienced increased progression of aortic valve calcification compared to those with mild or moderate disease. Results presented in box and whiskers plot: the central box represented the interquartile range, with the mean and median indicated by the dark and dotted lines, respectively. The whiskers indicated the 5th and 95th percentiles. Adapted with permission from (17).
CT calcium scoring of the aortic valve holds potential as an alternative assessment of aortic stenosis severity. Indeed, CT assessment holds several potential advantages over and above echocardiography, as it is independent from flow, geometric assumptions and the presence of other cardiovascular conditions such as hypertension and mitral regurgitation. Until recently, we lacked appropriate thresholds to differentiate patients with severe and non-severe disease, thereby limiting the clinical utility of this technique.
These thresholds were recently proposed in a multicenter study involving more than 600 patients with at least moderate aortic stenosis and preserved ejection fraction who had undergone both CT and echocardiography. Using sex-specific thresholds (males ≥2065 AU or ≥476 AU/cm2 when indexed to aortic annular area and females ≥1274 AU or ≥292 AU/cm2), aortic valve CT calcium score defined severe aortic stenosis with high accuracy (sensitivity ≥86% and specificity ≥79%) (24,25). Moreover, the prognostic impact of these calcium score thresholds was subsequently demonstrated, with severe aortic valve calcification demonstrating a strong association with increased all-cause mortality, over and above traditional predictors of outcome in aortic stenosis (Figure 2). Of note, aortic valve replacement was an independent predictor of improved survival in patients with severe aortic valve calcification, but not in those with non-severe disease (26).
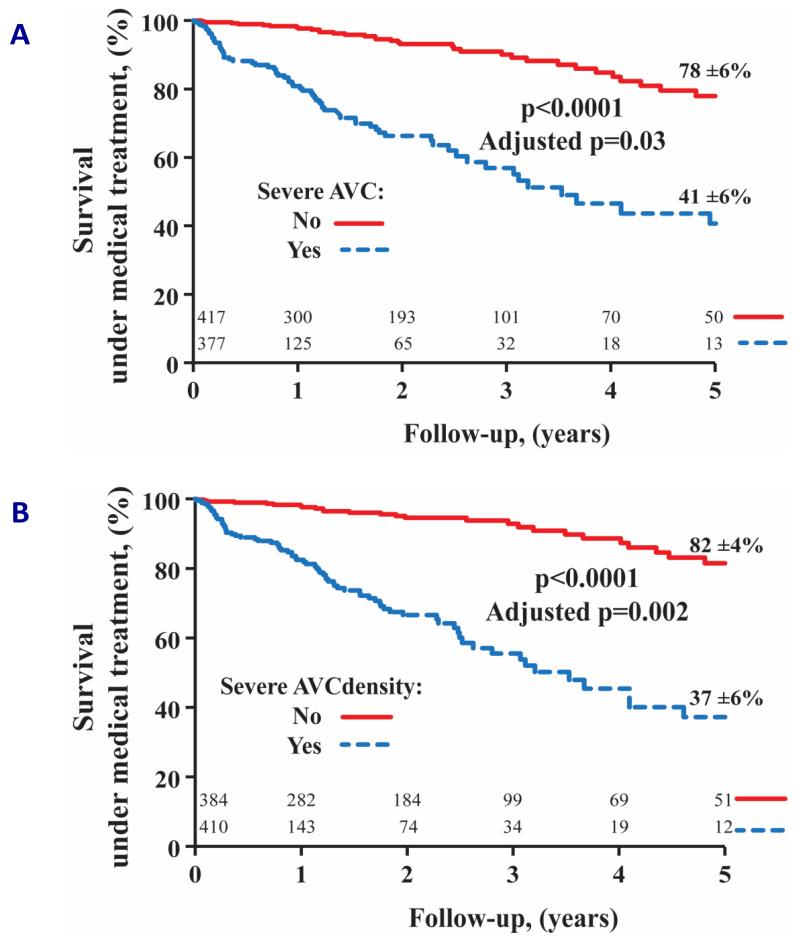
FIGURE 2.
Patients with severe aortic valve calcification based on absolute aortic valve calcium score (A) or aortic valve calcification density (aortic valve calcium score indexed to aortic annular area; B) had increased all-cause mortality compared to those with non-severe aortic valve calcification. Prognostic value remained significant after adjusting for age, sex, NYHA ≥3, diabetes mellitus, coronary artery disease, indexed aortic valve area, mean pressure gradient and systolic ejection fraction. Reproduced with permission from (26).
Furthermore, aortic valve CT calcium score is of particular clinical relevance in patients with discordant small aortic valve area and low mean pressure gradient where the true severity of the disease remains uncertain. This commonly affects up to a third of patients with moderate or severe disease and can be due to either low flow states (as a consequence of a depressed left ventricular ejection fraction or a small ventricle) or inaccuracies in echocardiographic measurements coupled with inconsistent thresholds in current guidelines (27,28). Indeed, when the above sex-specific calcium score thresholds were applied to patients with discordant echocardiographic measures of severity, approximately half were classified as moderate aortic stenosis and the remaining patients as severe disease, underlining the disparate nature of this patient population (24). Although further validation is required in other cohorts, aortic valve CT calcium score holds great promise because of its diagnostic and prognostic impact.
Assessing activity of aortic valve calcification
Positron emission tomography (PET) is a functional imaging technique that, in theory, can measure the activity of any biological process in the body if an appropriate radioactive tracer is available. Recently, the novel application of hybrid PET/CT imaging has been used to study patients with aortic stenosis, providing unique and important insights into its pathophysiology (29).
18F-Fluoride is a PET tracer that is believed to act as a marker of newly developing calcium in the vasculature. It preferentially binds to these regions of newly developing microcalcification due to the very high surface area of nanocrystaline hydroxyapatite crystals compared to established macroscopic calcium deposits, where much of the calcium is internalized and unavailable for binding (30). In the valve, 18F-fluoride also acts as a marker of calcification activity, demonstrating a close association with alkaline phosphatase staining on excised valves (23). In a recent prospective study of 121 patients with aortic sclerosis and stenosis, we have established excellent reproducibility for the quantification of PET activity in the aortic valve and demonstrated a progressive increase in calcification activity with severity of aortic stenosis (31) (Figure 1A). Moreover, a close association was observed between the baseline 18F-fluoride activity and disease progression as assessed by both CT and echocardiography (23). Interestingly, the distribution of 18F-fluoride activity was often distinct from the macroscopic calcium deposits on CT and over time, novel calcification developed in these regions of increased PET activity (Figure 3). We believe 18F-fluoride PET will be of particular use in the research arena where the immediate readout of disease activity will facilitate rapid assessment of the efficacy of novel therapies. Indeed, 18F-fluoride PET is already being employed as an endpoint in just such a study targeting calcium metabolism in patients with aortic stenosis (ClinicalTrials.gov NCT0213206).
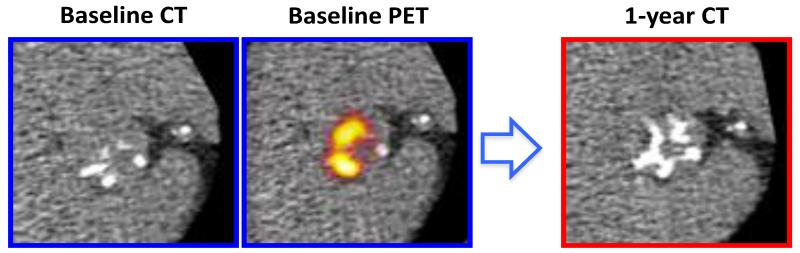
FIGURE 3.
Coaxial short axis views of the aortic valve from a patient with moderate aortic stenosis demonstrated established regions of macrocalcification on baseline computed tomography (CT) scans (left blue panel). Fused 18F-fluoride positron emission tomography (PET) and CT (middle blue panel) showed increased 18F-fluoride activity in the distribution of established calcium deposits and adjacent to regions of calcification. At one year, novel calcification developed in the regions corresponding to baseline 18F-fluoride activity (right red panel).
The utility of 18F-fluorodeoxyglucose has also been investigated in aortic stenosis (31,32). In aortic and carotid atheroma, this glucose analogue acts as a marker of valvular and vascular inflammation on the basis that macrophages have increased metabolic requirements compared to surrounding cells. Its mechanism of action in the valve is less clear given the presence of multiple other cells with increased glucose requirements (33). Nevertheless, 18F-fluorodeoxyglucose activity was higher in patients with aortic stenosis compared to controls and increased modestly with disease activity (Figure 1A). Further work is required to establish the exact mechanism of action of this tracer in the valve and to overcome the problems posed by its uptake in the adjacent myocardium.
MYOCARDIAL FIBROSIS AS A PROGNOSTIC MARKER
The increased afterload associated with aortic stenosis triggers a hypertrophic response in the left ventricle. Whilst initially adaptive, patients ultimately progress to heart failure and other adverse cardiovascular events. (6,34). This transition from adaptation to decompensation is mediated by progressive cell death and two predominant forms of myocardial fibrosis (35,36). Replacement fibrosis occurs later as the disease advances and it is characterized by a more focal distribution corresponding to areas of myocyte loss. On the other hand, interstitial fibrosis is diffusely distributed, reflecting the more uniform and progressive nature of the condition (36). Both represent potential imaging targets and hold promise as objective biomarkers of left ventricular decompensation that might better guide the timing for aortic valve replacement.
Assessing burden of myocardial fibrosis
Myocardial biopsy remains the gold standard for validating myocardial fibrosis but it is invasive, susceptible to sampling errors and unable to assess the fibrotic burden of the whole heart. Multiparametric cardiovascular magnetic resonance is currently the only imaging modality that offers a direct, whole-heart assessment of myocardial fibrosis (37). To date, two approaches are used: late gadolinium enhancement, for direct visualization and quantification of focal replacement fibrosis, and novel myocardial T1 mapping, for assessing more diffuse patterns of interstitial fibrosis.
Late gadolinium enhancement requires the administration of extracellular gadolinium-based contrast agents. After intravenous injection, gadolinium diffuses into the extracellular space. As a result of redistribution and renal excretion, the blood concentration of gadolinium falls and the contrast is ‘washed out’ from the extracellular space into the blood pool. In the normal myocardium, contrast concentration in the extracellular space equilibrates rapidly with the blood pool. Conversely, in regions of myocardial fibrosis, the extracellular space is expanded as a consequence of excessive collagen deposition. As a result, gadolinium accumulates in these regions and contrast wash out is delayed, thereby producing differences in signal intensities between normal and abnormal myocardium. Focal regions of replacement fibrosis (frequently in the mid-wall of the myocardium) therefore appear white in contrast to the surrounding normal myocardium that is black (36,38,39). Of note, this distinctive pattern of mid-wall fibrosis must be differentiated from late gadolinium enhancement due to prior myocardial infarction, which is also common in patients with aortic stenosis. Late gadolinium enhancement due to a previous myocardial infarction typically involves the subendocardium and then it extends transmurally towards the epicardium. Unlike mid-wall myocardial fibrosis, regions of myocardial infarction correspond to specific epicardial coronary distribution (39).
Focal mid-wall myocardial fibrosis has been demonstrated in 19 to 62% of patients with aortic stenosis (40-43) and it is associated with impaired cardiac function and adverse cardiovascular events (42,44,45). In 143 patients with moderate to severe aortic stenosis, focal mid-wall fibrosis was an independent predictor of cardiovascular and all-cause mortality. Whilst patients without myocardial fibrosis had extremely good prognosis, those with mid-wall fibrosis had more than an eight-fold increase in all-cause mortality despite similar severity of aortic stenosis (Figure 4). Of note, the prognosis worsened with an increasing burden of fibrosis (42). Similar findings were also observed in patients following aortic valve replacement. Mid-wall fibrosis was associated with adverse ventricular remodelling, incomplete left ventricular functional recovery and worse cardiovascular outcomes following aortic valve replacement (43,44,46-48). Recently, mid-wall fibrosis on cardiovascular magnetic resonance provided further validation for other biomarkers of left ventricular decompensation. In separate studies, high sensitivity cardiac troponin I concentrations and electrocardiographic left ventricular hypertrophy and strain pattern not only demonstrated a close association with the presence of mid-wall late gadolinium enhancement but also provided incremental prognostic information in asymptomatic patients with aortic stenosis (49,50). These data provide increasing support that mid-wall fibrosis is a marker of left ventricular decompensation in aortic stenosis and several on-going prospective studies will confirm the prognostic value of this technique (ClinicalTrials.gov NCT01755936, NCT01658345, NCT02174471), which can prove useful in identifying patients who may benefit from early aortic valve replacement.
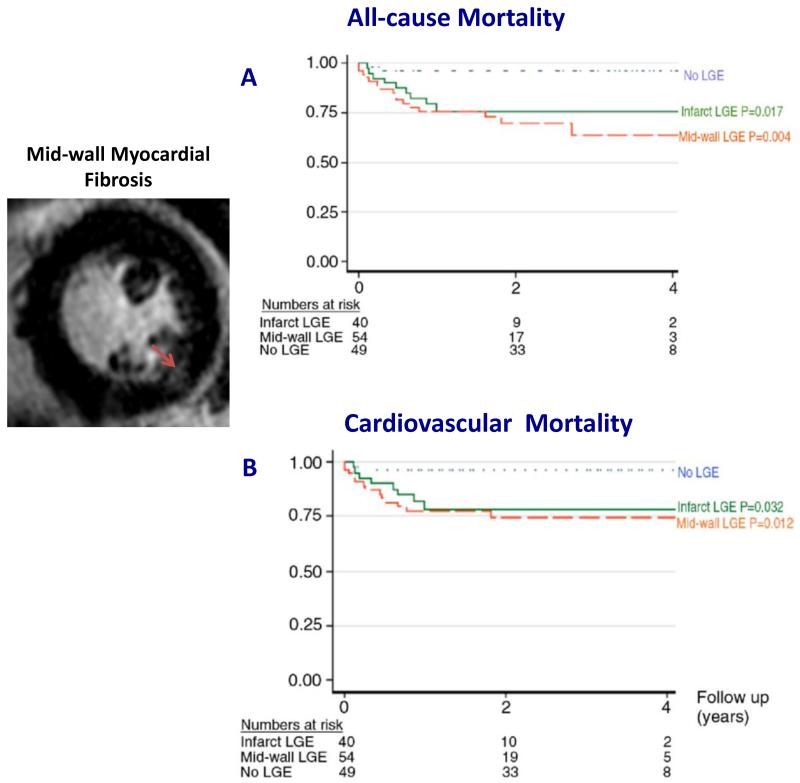
FIGURE 4.
The presence of mid-wall myocardial fibrosis on cardiovascular magnetic resonance was associated with increased all-cause mortality (A) and cardiovascular mortality (B) in patients with at least moderate aortic stenosis. Adapted with permission from (42).
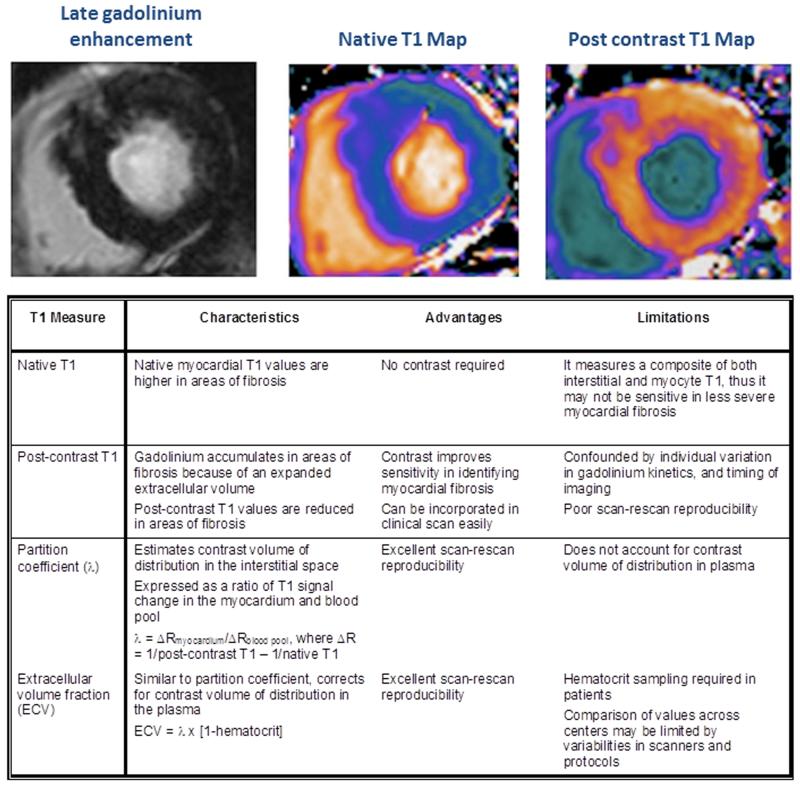
Unlike focal replacement fibrosis that is believed to be irreversible, there is increasing interest in diffuse interstitial fibrosis as a potential treatment target (51-53). Late gadolinium enhancement relies upon a difference in signal intensity between normal and focal regions of myocardial fibrosis. It is therefore not an optimal technique for assessing interstitial fibrosis, which is more uniformly distributed and does not provide the necessary signal contrast for visualization. Instead, myocardial T1 mapping has emerged as a novel cardiovascular magnetic resonance technique to assess this form of diffuse fibrosis (54,55). T1 mapping improves myocardial characterization by its ability to quantify signal intensity for each voxel in the myocardium, generating a parametric T1 map (Figure 5). Indeed, the role of myocardial T1 mapping has been well studied in aortic stenosis, with many studies demonstrating a correlation between this marker of diffuse fibrosis and aortic stenosis severity, left ventricular mass and cardiac performance (56-60). To date four different T1 mapping approaches have been investigated and validated against the extent of myocardial fibrosis on histology (56,61-66). Each has its own potential advantages and limitations (Figure 5). In our experience, extracellular volume fraction, which corrects for blood pool and the plasma gadolinium volume of distribution, offers the best reproducibility (including scan-rescan repeatability) and the ability to differentiate patients from healthy volunteers. Furthermore, increasing extracellular volume fraction values were associated with worse diastolic function (59). Yet even with this parameter, we have observed a substantial overlap in extracellular volume fraction values between patients with aortic stenosis and healthy volunteers, highlighting a potential limitation of myocardial T1 mapping as a diagnostic marker or clinical decision making tool. This has also been observed in other studies (57,58,60). Although the prognostic value of extracellular volume fraction has been demonstrated in other patient populations (53,67,68), similar outcome data are currently lacking in aortic stenosis but results from the above-mentioned studies may provide further insights in the near future. Despite its potential limitations, myocardial T1 mapping may still be useful in assessing treatment responses and disease progression because of its low scan-rescan variability and greater sensitivity (59,60,69,70).
FIGURE 5.
To date, there are four different myocardial T1 mapping techniques used to assess diffuse interstitial fibrosis. Each technique has its own unique merits and limitations. In aortic stenosis, extracellular volume fraction appears to be the most promising technique in assessing diffuse fibrosis. Extracellular volume fraction demonstrates excellent scan-rescan reproducibility, which is necessary when assessing change related to treatment response and disease progression.
Assessing activity of myocardial fibrosis
Similar to the relationship between 18F-fluoride PET and CT calcium scoring, a marker of fibrosis activity would complement current assessments of fibrosis burden by cardiovascular magnetic resonance (6,29). 18F-Fluciclatide is a PET tracer that has a high affinity for extracellular integrin receptors, which are upregulated in regions of myocardial fibrosis (71,72). Recently, we have demonstrated increased 18F-Fluciclatide activity in regions of prior myocardial infarction and the potential of this radioactive tracer as marker of fibrosis activity in aortic stenosis is currently being investigated (ClinicalTrials.gov NCT01837160). Other metabolic markers of fibrosis and cell death are in development and might similarly provide useful information with respect to disease activity in the myocardium.
Assessing the functional consequences of myocardial fibrosis
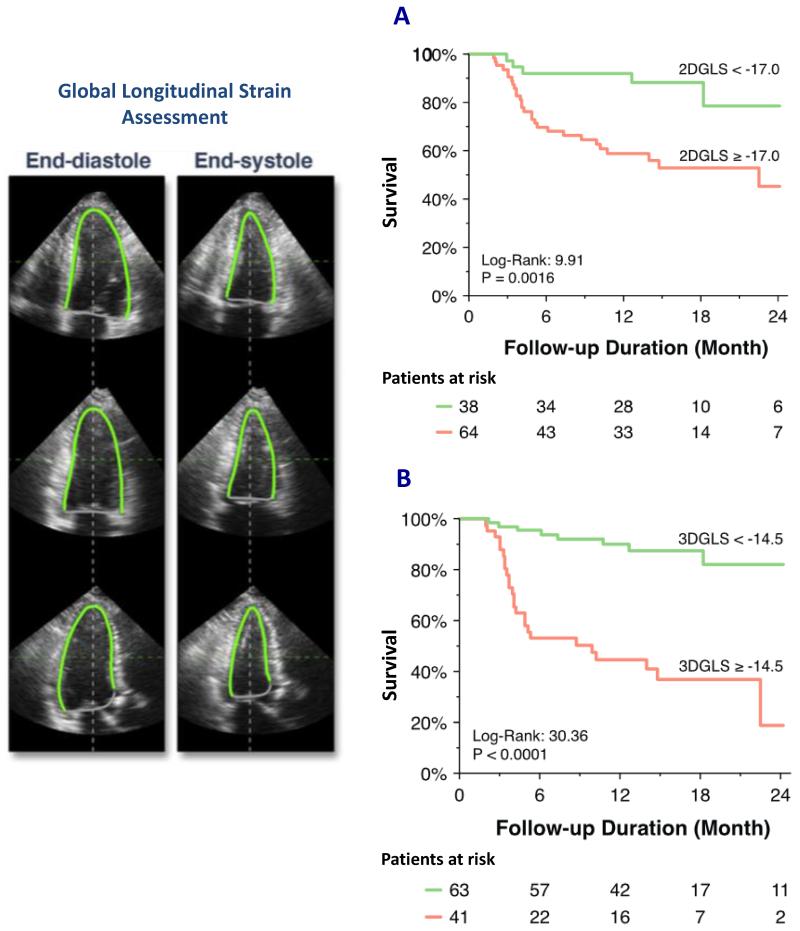
Although impaired left ventricular systolic ejection fraction is the conventional marker of global systolic dysfunction and a current indication for aortic valve replacement (7,8), it is a very late manifestation of ventricular decompensation and myocardial fibrosis. In fact, left ventricular ejection fraction in aortic stenosis can overestimate myocardial function in the presence of advanced concentric hypertrophy because of increased myocardial wall thickness and reduced ventricular volumes (73-75). By contrast, novel myocardial deformation imaging (strain and strain rate imaging) using speckle tracking echocardiography has been proposed as an alternative and a more sensitive approach of assessing intrinsic myocardial contractility (76,77). In particular, longitudinal deformation (motion from base to apex) assessed using 2D (and more recently 3D) speckle tracking echocardiography has demonstrated incremental prognostic value in patients with aortic stenosis (78-82). In a recent study involving 104 asymptomatic patients with severe aortic stenosis and preserved left ventricular ejection fraction, both 2D and 3D global longitudinal strain was associated with increased adverse events but the latter provided a higher accuracy in predicting adverse events (Figure 6). Future studies will be needed to confirm the emerging role of 3D speckle tracking echocardiography, and to investigate the complementary roles of these novel markers that reflect the functional consequences of myocardial fibrosis and cardiovascular magnetic resonance that directly assess the burden of myocardial fibrosis.
FIGURE 6.
Global longitudinal strain (GLS) analysis by speckle tracking echocardiography is assessed in the 3 apical views. In this case, the endocardial borders were manually contoured and both 2D and 3D volumetric longitudinal strain can be measured. Impaired GLS assessed by either 2D or 3D speckle tracking (A and B, respectively) was associated with increased adverse cardiovascular events (cardiac death, sustained ventricular tachyarrhythmia, aortic valve replacement and hospital admission for heart failure). Reproduced with permission from (82).
THE FUTURE ROLE OF MODERN IMAGING TECHNIQUES IN AORTIC STENOSIS
Currently, the assessment and management of patients with aortic stenosis rely heavily on hemodynamic assessments by echocardiography. Whilst echocardiography will remain the mainstay of imaging in aortic stenosis, modern non-invasive imaging provides important complementary information that has the potential to inform how we manage our patients.
Aortic valve CT calcium score will likely emerge as an alternative method for assessing the severity of aortic stenosis, particularly in evaluating disease progression and when echocardiographic measurements are discordant. PET imaging may have the potential of refining the prediction of disease progression offered by CT and for rapidly assessing the efficacy of novel therapeutic strategies. As aortic stenosis progresses in the later stages, the detection of replacement fibrosis by cardiovascular magnetic resonance as an objective marker of left ventricular decompensation will help guide the optimum timing of aortic valve replacement.
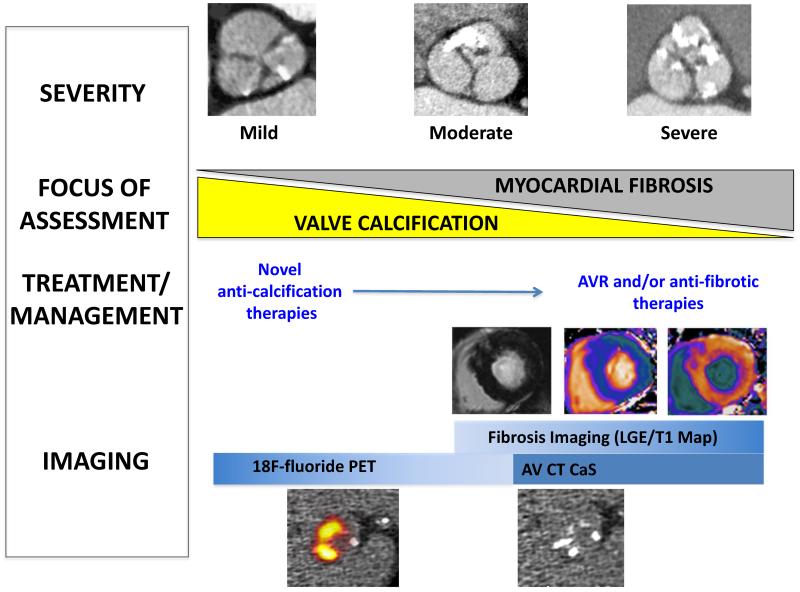
Whilst we are progressing in the early stages of this journey, an integrated multi-modality approach of assessing the valve and myocardium in aortic stenosis will ultimately facilitate a stratified-medicine approach to the management of patients with aortic stenosis (Figure 7).
FIGURE 7.
Echocardiography will remain the mainstay of imaging in the initial assessment. Aortic valve computed tomography (CT) calcium score is an alternative method in assessing aortic stenosis severity, particularly in patients with discordant echocardiographic findings. In the early stages of aortic stenosis, hybrid positron emission tomography/computed tomography (PET/CT) may complement assessment of disease progression by aortic valve CT calcium score and to monitor efficacy of novel anti-calcification therapies. In the later stages, the focus shifts to the myocardium with the aims of identifying patients with myocardial fibrosis using cardiovascular magnetic resonance, to help guide the timing of aortic valve replacement.
CONCLUSION
Aortic valve CT calcium score, PET/CT, cardiovascular magnetic resonance and echocardiographic strain analyses are novel imaging techniques that have not only advanced our understanding of the natural history of aortic stenosis, but also improved our ability to risk stratify patients with this condition. In combination, they offer the ability to predict adverse events related to the valve and the myocardium. Future prospective studies and clinical trials are now needed to investigate their clinical utility and whether their potential might translate into improved patient care.
Acknowledgments
SOURCES OF FUNDING
Drs Newby and Dweck are supported by the British Heart Foundation and Dr Newby holds a Wellcome Trust Senior Investigator Award (WTf103782AIA). Dr Chin is supported by the National Research Foundation-Ministry of Health, Singapore.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Carabello BA. Introduction to aortic stenosis. Circ Res. 2013;113:179–85. doi: 10.1161/CIRCRESAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 3.Rajamannan NM, Evans FJ, Grande-Allen EAK, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for Research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group Executive Summary: Calcific aortic valve disease - 2011 Update. Circulation. 2011;124:1783–91. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38:V61–V67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 5.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–63. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 6.Chin CW, Vassiliou V, Jenkins WS, Prasad SK, Newby DE, Dweck MR. Markers of left ventricular decompensation in aortic stenosis. Expert Rev Cardiovasc Ther. 2014;12:901–12. doi: 10.1586/14779072.2014.923307. [DOI] [PubMed] [Google Scholar]
- 7.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, 3rd, Thomas JD, ACC/AHA Task Force Members 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 9.Lancellotti P, Donal E, Magne J, Moonen M, O’Connor K, Daubert JC, Pierard LA. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364–71. doi: 10.1136/hrt.2009.190942. [DOI] [PubMed] [Google Scholar]
- 10.Marwick TH. Methods used for the assessment of LV systolic function: common currency or tower of Babel? Heart. 2013;99:1078–86. doi: 10.1136/heartjnl-2012-303433. [DOI] [PubMed] [Google Scholar]
- 11.Connolly HM, Oh JK, Orszulak TA, Osborn SL, Roger VL, Hodge DO, Bailey KR, Seward JB, Tajik AJ. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation. 1997;95:2395–400. doi: 10.1161/01.cir.95.10.2395. [DOI] [PubMed] [Google Scholar]
- 12.Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–6. doi: 10.1161/01.cir.101.16.1940. [DOI] [PubMed] [Google Scholar]
- 13.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O’Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kalsch H, Muhleisen TW, Nothen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathlresan S, Melander O, Gudnason V, O’Donnell CJ, Post WS, CHARGE Extracoronary Calcium Working Group Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stritzke J, Linsel-Nitschke P, Markus MRP, Mayer B, Lieb W, Luchner A, Doring A, Koenig W, Keil U, Hense HW, Schunkert H, MONICA/KORA Investigators Association between degenerative aortic valve disease and long-term exposure to cardiovascular risk factors: results of the longitudinal population-based KORA/MONICA survey. Eur Heart J. 2009;30:2044–53. doi: 10.1093/eurheartj/ehp287. [DOI] [PubMed] [Google Scholar]
- 15.Yoon H-C, Emerick AM, Hill JA, Gjertson DW, Goldin JG. Calcium begets calcium: progression of coronary artery calcification in asymptomatic subjects. Radiology. 2002;224:236–41. doi: 10.1148/radiol.2241011191. [DOI] [PubMed] [Google Scholar]
- 16.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–8. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen V, Cimadevilla C, Estellat C, Codogno I, Huart V, Benessiano J, Duval X, Pibarot P, Clavel MA, Enriquez-Sarano M, Vahanian A, Messika-Zeitoun D. Haemodynamic and anatomic progression of aortic stenosis. Heart. 2015;101:943–7. doi: 10.1136/heartjnl-2014-307154. [DOI] [PubMed] [Google Scholar]
- 18.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–7. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 19.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. doi: 10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Messika-Zeitoun D, Aubry M-C, Detaint D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Breen JF, Scott C, Taijk AJ, Enriquez-Sarano M. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004;110:356–62. doi: 10.1161/01.CIR.0000135469.82545.D0. [DOI] [PubMed] [Google Scholar]
- 21.Cowell SJ, Newby DE, Burton J, White A, Northridge DB, Boon NA, Reid J. Aortic valve calcification on computed tomography predicts the severity of aortic stenosis. Clin Radiol. 2003;58:712–6. doi: 10.1016/s0009-9260(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 22.Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A, Messika-Zeitoun D. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–6. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 23.Dweck MR, Jenkins WSA, Vesey AT, Pringle MAH, Chin C, Malley TS, Cowie WJ, Tsampasian V, Richardson H, Fletcher A, Wallace WA, Pessotto R, van Beek EJ, Boon NA, Rudd JH, Newby DE. 18F-Sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–8. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 24.Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal S, Malouf JF, Araoz P, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M. The complex nature of discordant severe calcified aortic valve disease grading. J Am Coll Cardiol. 2013;62:2329–38. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 25.Dweck MR, Chin C, Newby DE. Small valve area with low-gradient aortic stenosis. Beware of the hard hearted. J Am Coll Cardiol. 2013;62:2339–40. doi: 10.1016/j.jacc.2013.08.1620. [DOI] [PubMed] [Google Scholar]
- 26.Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf JF, Aggraval S, Araoz PA, Michelena HI, Cueff C, Larose E, Miller JD, Vahanian A, Enriquez-Sarano M. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202–13. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–53. doi: 10.1016/j.jacc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 28.Chin CW, Khaw J, Luo E, Tan SW, White A, Newby DE, Dweck MR. Echocardiography underestimates stroke volume and aortic valve area: implications for patients with small-area low-gradient aortic stenosis. Can J Cardiol. 2014;30:1064–72. doi: 10.1016/j.cjca.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins W, Chin C, Rudd J, Newby DE, Dweck MR. What can we learn about valvular heart disease from PET/CT? Future Cardiol. 2013;9:657–67. doi: 10.2217/fca.13.47. [DOI] [PubMed] [Google Scholar]
- 30.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular micro-calcification by 18F-sodium fluoride positron emission tomography. Nat Commun. 2015 doi: 10.1038/ncomms8495. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace MA, Salter DM, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Assessment of Valvular calcification and inflammation by Positron Emission Tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 32.Marincheva-Savcheva G, Subramanian S, Qadir S, Figueroa A, Truong Q, Vijayakumar J, Brady TJ, Hoffman U, Tawakol A. Imaging of the aortic valve using fluorodeoxyglucose Positron Emission Tomography. J Am Coll Cardiol. 2011;57:2507–15. doi: 10.1016/j.jacc.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 33.Dweck MR, Khaw HJ, Sng GK, Luo EL, Baird A, Williams MC, Makiello P, Mirsadraee S, Joshi NV, van Beek EJ, Boon NA, Rudd JH, Newby DE. Aortic stenosis, atherosclerosis, and skeletal bone: is there a common link with calcification and inflammation? Eur Heart J. 2013;34:1567–74. doi: 10.1093/eurheartj/eht034. [DOI] [PubMed] [Google Scholar]
- 34.Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, Krayenbuehl HP. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–84. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- 35.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 36.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambale-Venkatesh B, Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol. 2015;12:18–29. doi: 10.1038/nrcardio.2014.159. [DOI] [PubMed] [Google Scholar]
- 38.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 39.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 40.Debl K, Djavidani B, Buchner S, Lipke C, Nitz W, Feuerbach S, Riegger G, Luchner A. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart. 2006;92:1447–51. doi: 10.1136/hrt.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–91. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 42.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 43.Barone-Rochette G, Piérard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, Pouleur AC, Vancraeynest D, Pasquet A, Vanoverschelde JL, Gerber BL. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144–54. doi: 10.1016/j.jacc.2014.02.612. [DOI] [PubMed] [Google Scholar]
- 44.Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Erti G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–84. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 45.Lee SP, Park SJ, Kim YJ, Chang SA, Park EA, Kim HK, Lee W, Lee SC, Park SW, Sohn DW, Choe YH. Early detection of subclinical ventricular deterioration in aortic stenosis with cardiovascular magnetic resonance and echocardiography. J Cardiovasc Magn Reson. 2013;15:72. doi: 10.1186/1532-429X-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quarto C, Dweck MR, Murigu T, Joshi S, Melina G, Angeloni E, Prasad SK, Pepper JR. Late gadolinium enhancement as a potential marker of increased perioperative risk in aortic valve replacement. Interact Cardiovasc Thorac Surg. 2012;15:45–50. doi: 10.1093/icvts/ivs098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–87. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 48.Milano AD, Faggian G, Dodonov M, Golia G, Tomezzoli A, Bortolotti U, Mazzucco A. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg. 2012;144:830–7. doi: 10.1016/j.jtcvs.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–21. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah AS, Chin CW, Vassiliou V, Cowell SJ, Doris M, Kwok TC, Semple S, Zamvar V, White AC, McKillop G, Boon NA, Prasad SK, Mills NL, Newby DE, Dweck MR. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. 2014;130:1607–16. doi: 10.1161/CIRCULATIONAHA.114.011085. [DOI] [PubMed] [Google Scholar]
- 51.Stuckey DJ, McSweeney SJ, Thin MZ, Habib J, Price AN, Fiedler LR, Gsell W, Prasad SK, Schneider MD. T1 mapping detects pharmacological retardation of diffuse cardiac fibrosis in mouse pressure-overload hypertrophy. Circ Cardiovasc Imaging. 2014;7:240–9. doi: 10.1161/CIRCIMAGING.113.000993. [DOI] [PubMed] [Google Scholar]
- 52.Roubille F, Busseuil D, Merlet N, Kritikou EA, Rhéaume E, Tardif JC. Investigational drugs targeting cardiac fibrosis. Expert Rev Cardiovasc Ther. 2014;12:111–25. doi: 10.1586/14779072.2013.839942. [DOI] [PubMed] [Google Scholar]
- 53.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–64. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kellman P, Hansen MS. T1 mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:1–20. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins DM, Moon JC. Review of T1 mapping methods: comparative effectiveness including reproducibility issues. Curr Cardiovasc Imaging Rep. 2014;7:1–10. [Google Scholar]
- 56.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–7. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA, McKenna WJ, Taylor AM, Hausenloy DJ, Hawkins PN, Elliott PM, Moon JC. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–41. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 58.Flett AS, Sado DM, Quarta G, Mirabel M, Pellerin D, Herrey AS, Hausenloy DJ, Ariti C, Yap J, Kolvekar S, Taylor AM, Moon JC. Diffuse myocardial fibrosis in severe aortic stenosis: An equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2012;13:819–26. doi: 10.1093/ehjci/jes102. [DOI] [PubMed] [Google Scholar]
- 59.Chin CW, Semple S, Malley T, White A, Mirsadraee S, Weale PJ, Prasad S, Newby DE, Dweck MR. Optimization and comparison of myocardial T1 mapping techniques at 3T in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2014;15:556–65. doi: 10.1093/ehjci/jet245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh A, Horsfield MA, Bekele S, Khan J, Greiser A, McCann GP. Myocardial T1 and extracellular volume fraction measurement in asymptomatic patients with aortic stenosis: reproducibility and comparison with age-matched controls. Eur Heart J Cardiovasc Imaging. 2015;16:763–70. doi: 10.1093/ehjci/jev007. [DOI] [PubMed] [Google Scholar]
- 61.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–80. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 62.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, Mudd JO, van der Geest RJ, Lima JA, Halushka MK, Bluemke DA. T1 mapping in cardiomyopathy at cardiac MR: Comparison with endomyocardial biopsy. Radiology. 2012;265:724–32. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–78. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 65.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC. T1 mapping for myocardial extracellular volume measurement by CMR: Bolus only versus primed infusion technique. JACC Cardiovasc Imag. 2013;6:955–62. doi: 10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–83. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 67.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutia SR, Simon MA, Schelbert EB. Association Between Extracellular Matrix Expansion Quantified by Cardiovascular Magnetic Resonance and Short-Term Mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindler TH, Lima JA. Assessment of myocardial matrix expansion with cardiac magnetic resonance: entering a new area of cardiac risk stratification in type 2 diabetes mellitus? Eur Heart J. 2014;35:608–11. doi: 10.1093/eurheartj/eht245. [DOI] [PubMed] [Google Scholar]
- 69.Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, Sibley CT, Bluemke DA. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: Sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012;14:90–8. doi: 10.1186/1532-429X-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doltra A, Messroghli D, Stawowy P, Hassel JH, Gebker R, Leppanen O, Grafe M, Schneewels C, Schnackenburgh B, Fleck E, Kelle S. Potential Reduction of Interstitial Myocardial Fibrosis With Renal Denervation. J Am Heart Assoc. 2014;3:e001353. doi: 10.1161/JAHA.114.001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lygoe KA, Norman JT, Marshall JF, Lewis MP. AlphaV integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 2004;12:461–70. doi: 10.1111/j.1067-1927.2004.12402.x. [DOI] [PubMed] [Google Scholar]
- 72.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: A sticky business. Exp Cell Res. 2006;312:651–8. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 73.Dumesnil JG, Shoucri RM. Effect of the geometry of the left ventricle on the calculation of ejection fraction. Circulation. 1982;65:91–98. doi: 10.1161/01.cir.65.1.91. [DOI] [PubMed] [Google Scholar]
- 74.Pibarot P, Dumesnil JG. Improving assessment of aortic stenosis. J Am Coll Cardiol. 2012;60:169–180. doi: 10.1016/j.jacc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 75.Lancellotti P, Donal E, Magne J, O’Connor K, Moonen ML, Cosyns B, Pierard LA. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr. 2010;11:537–543. doi: 10.1093/ejechocard/jeq014. [DOI] [PubMed] [Google Scholar]
- 76.Manovel A, Dawson D, Smith B, Nihoyannopoulos P. Assessment of left ventricular function by different speckle-tracking software. Eur J Echocardiogr. 2010;11:417–421. doi: 10.1093/ejechocard/jep226. [DOI] [PubMed] [Google Scholar]
- 77.Blessberger H, Binder T. Non-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart. 2010;96:716–722. doi: 10.1136/hrt.2007.141002. [DOI] [PubMed] [Google Scholar]
- 78.Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging. 2012;5:719–25. doi: 10.1161/CIRCIMAGING.112.977348. [DOI] [PubMed] [Google Scholar]
- 79.Dahou A, Bartko PE, Capoulade R, Clavel MA, Mundigler G, Grondin SL, Bergler-Klein J, Burwash I, Dumesnil JG, Senechai M, O’Connor K, Baumgartner H, Pibarot P. Usefulness of global left ventricular longitudinal strain for risk stratification in low ejection fraction, low gradient aortic stenosis: results from the multicenter True or Pseudo-Severe Aortic Stenosis Study. Circ Cardiovasc Imaging. 2015;8:e002117. doi: 10.1161/CIRCIMAGING.114.002117. [DOI] [PubMed] [Google Scholar]
- 80.Delgado V, Ng CT. Assessment of left ventricular systolic function in aortic stenosis and prognostic implications. Eur Heart J Cardiovasc Imaging. 2012;13:805–7. doi: 10.1093/ehjci/jes136. [DOI] [PubMed] [Google Scholar]
- 81.Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM. Global longitudinal strain is a strong predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012;13:827–33. doi: 10.1093/ehjci/jes115. [DOI] [PubMed] [Google Scholar]
- 82.Nagata Y, Takeuchi M, Wu VC, Izumo M, Suzuki K, Sato K, Seo Y, Akashi YJ, Aonuma K, Otsuji Y. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. JACC Cardiovasc Imaging. 2015;8:235–45. doi: 10.1016/j.jcmg.2014.12.009. [DOI] [PubMed] [Google Scholar]