Abstract
AIM
To evaluate and compare the efficacy and stability of intrastromal corneal ring segment (ICRs) implantation with cross-linking (CXL) using different sequence and timing.
METHODS
In this single retrospective study, 86 keratoconic eyes subjected the ICRs implantation. We analyzed only 41 eyes that had complete follow-ups. They were divided into three groups: ICRs implantation was applied only (group normal), ICRs first followed by CXL immediately (group CXL-S), CXL first followed by ICRs long after (group CXL-B). The visual acuity, refractive results, keratometry were compared preoperatively and 1y postoperatively. Their differences among the three groups were also analyzed.
RESULTS
Group normal comprised 25 eyes, group CXL-S 8 eyes, and group CXL-B 8 eyes. There were improvements in the mean uncorrected distance visual acuity (UDVA) and the mean corrected distance visual acuity (CDVA) compared preoperatively and 1y postoperatively {UDVA: 0.31 (P=0.030) logarithmic minimum angle of resolution [logMAR] group normal, 0.4 (P=0.020) group CXL-S, 0.45 (P=0.001) group CXL-B; CDVA: 0.21 logMAR (P=0.013) group normal, 0.30 (P=0.036) group CXL-S; 0.26 (P=0.000) group CXL-B}. The refractive and topographic outcomes also showed improvements. In terms of comparisons among the three groups, all the P values were above 0.05, showing no significant difference. But only group CXL-B had improvement in UDVA and CDVA for all the patients.
CONCLUSION
With safety and good visual outcomes, ICRs implantation is a viable alternative for keratoconus. No significant difference was found among these three groups.
Keywords: keratoconus, intrastromal corneal ring segment, cross-linking
INTRODUCTION
Keratoconus is bilateral non-inflammatory degeneration of cornea, resulting in corneal thinning, cone-shaped deformation, irregular astigmatism and decreased vision. The prevalence is 54 per 100 000[1],[2] in the general population. It initiates during puberty and progresses until the third or fourth decade of life. There are spectacles, contact lenses, intrastromal corneal ring segments (ICRs), cross-linking (CXL), lamellar and penetrating keratoplasty to manage the keratoconus. During its progression, about 12% of keratoconus require keratoplasty[3].
CXL is a combined application of riboflavin solution and illumination of ultraviolet A. The activated riboflavin induces free radicals which will cause additional covalent bonds between collagen molecules, stiffening the cornea. Having proved great efficiency to halt the progression of keratoconus and fewer complications[4]–[6], CXL is more frequently recommended to patients which are at high risk to progression of keratoconus.
While the rigid gas permeable lenses are very effective for the irregular astigmatisms[7], ICRs is a good alternative when the patients are intolerant of contact lens. ICRs acts as additional material between corneal lamellaes, which shortens the central arc length of cornea, flattens the central corneal surface and displaces the peripheral area forward. As a consequence, ICRs generates a great change in corneal refraction and astigmatism[8],[9].
In clinical practice, we often encounter the sequential problems of CXL and ICRs: After treated CXL the patients still have poor visual acuity, which often require ICRs. When the condition of keratoconus require both the treatments of CXL and ICRs, should we perform the two treatments at the same time or separately? And which one comes first? Does the combined treatment carry more benefits and less risks of complication than the separate procedure? After several years of implantation of ICRs and application of CXL, we performed this study to investigate the efficiency of ICRs implantation with CXL using different sequence and timing.
Condition of Patients
Before operation, every patient was subjected to complete ocular examination, uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), subjective refraction (auto-refracometry, Nidek ARK-700), slit-lamp biomicroscopy and corneal topography affected by Placido-based systems (TMS-IV Tomey) and by Rotating camera Scheimpflug imagery (Pentacam, Oculus Optikger te GmbH, Wetzlar, Germany). Follow-up visit were scheduled at the 1, 14d and 1, 3, 6, 12mo postoperative. At each visit of follow-up, all the patients were obliged to complete ocular examination, UDVA, CDVA, subjective refraction, and slit-lamp biomicroscopy and corneal topography affected by TMS-IV Tomey to survey the progression of keratoconus.
Inclusion criteria were successful implantation of ICRs with strict visits of follow-up at the 3 and 12mo. Exclusion criteria were explanations of segments caused by severe complications, infections, reposition, keratoplasty, other additional surgery except for CXL, other active ocular disease except for keratoconus.
In reality, it's difficult for patients to complete all the follow-up, as about half of these patients came from other cities to this center of keratoconus to receive ICRs implantation. Finally only 41 consecutive eyes of 86 eyes were analyzed. They were divided into three groups, group Normal without CXL, group CXL-S with ICRs first followed by CXL immediately, group CXL-B with CXL first followed by ICRs long after.
SUBJECTS AND METHODS
The research project has been approved by Ethics Committee of Purpan Hospital and it conforms to the provisions of the Declaration of Helsinki in 2008. All these patients were performed implantation of Ferrara Intrastromal Corneal Ring (Ferrara Ophthalmics, Belo Horizonte, Brazil) by the same experienced surgeon using mechanical procedure during 2010-2012. Ferrara is triangular shape ICRs, with 5 mm diameter (4.4 mm internal diameter and 5.6 mm external diameter), variable thickness (150-300 µm) and 4 arc segments (90°, 120°, 160° and 210°).
The indications of ICRs implantation and CXL followed strictly the protocols[4],[10]. The patients of keratoconus who have limited vision or intolerance to contact lens/spectacle correction, without central opaque, with enough pachymetry in the zone of implantation (>450 µm) and good potential vision (no other severe eye diseases such as amblyopia and uveitis) could have ICRs implantation. If there is one or more of changes during 6mo [an increase of the steepest K-reading ≥1 D; an increase of cylinder ≥1 D; an increase of ≥=0.5 D in refraction spherical equivalent (SE); vision loss or requirement of contact lens], which means progression of keratoconus, CXL can be performed as long as corneal pachymetry >400 µm. If the patients accord to the two indications, we planed ICRs implantation followed immediately by CXL or CXL firstly and ICRs implantation in the next time if the condition of keratoconus required.
Using corneal topographer (Pentacam Oculus) to obtain important corneal data: the type of keratoconus (sag, bowtie or nipple), location (central or paracentral), corneal asphericity (Q), topographic astigmatism and pachymetry, the third Ferrara Ring nomogram designed the strategies of implantation of ICRs.
In an operating room under sterile condition tetracaine was applied to achieve preoperative anesthesia. The surgeon used eyelid speculum to expose the eye and 2.5% povidone iodine eye drops to disinfect the cornea and conjunctival cul-de-sac for 3min. To locate the geometric center of the cornea, the patient was instructed to fixate on the corneal light reflex of the microscope light and then the Sinskey hook was put on cornea. A tinted marker noted the steepest axis and the 5.0 mm optical zone. At the incision site located by the preoperative calculation of topography, a calibrated diamond knife of 1.0 mm depth made an incision at 70% of the thinnest corneal thickness determined by ultrasonic pachymetry. After relocating the depth corneal, one or two pockets were made at the base of incision with a pocketing hook. The semicircular dissecting spatulas was set into the pocket and pushed carefully to make tunnels. The ICRs was inserted and placed rightly on the tunnel with the accompanying forceps. No suture was needed at the incision.
Cross-linking
Topical anesthesia was achieved with tetracaine in an operating room under sterile condition. With a brush, the epithelium of the central corneal 8-9 mm area was removed. Riboflavin 0.1% solution in 20% dextran was instilled on the cornea every 2-3min for 15min. After making sure that the riboflavin had penetrated into the cornea through slit lamp with a blue filter, ultraviolet-A irradiation (3 mW/cm2) was applied with regular instillation of riboflavin per 5min during 30min. Finally, contact lens, sterdex (dexamethasone and oxytetracycline), bandage were successively put on the eye. The bandage was left until the reepithelialization.
For the group CXL-S, the CXL was performed immediately after the implantation of ICRs. In the group CXL-B, it's the patients who still remained poor visual acuity after CXL and required the implantation of ICRs. The mean time between the operation of CXL and ICRs implantation was 21.00±10.52mo, with the minimum 9mo interval and maximum 33mo interval.
Statistical Analysis
Statistical analysis was performed with SPSS of Windows software (version 19.0, SPSS, Inc). Mean value and standard variance were used to describe participants and study variables and A 2-tailed probability of 0.05 or less was considered statistically significant. The Student's t-test for paired data was used to compare the parameters preoperatively and postoperatively in each group. The preoperative parameters were compared with one-way ANOVA to investigate the baseline difference of the three groups. After the homogeneity of variance test for every variances, the differences among groups were investigated using one-way ANOVA or post hoc test with Tamhanes for multiple tests.
RESULTS
These patients were divided into three groups, 25 patients of group normal, 8 patients of group CXL-S, 8 patients of group CXL-B. The degree of keratoconus was classified according grading system based on the limitation of preoperative CDVA[11],[12]. Grade I CDVA≤0.05 logMAR; grade II 0.05 logMAR<CDVA≤0.22 logMAR; grade III 0.22 logMAR<CDVA≤0.4 logMAR; grade IV 0.4 logMAR<CDVA≤0.7 logMAR; grade V CDVA>0.7 logMAR (Table 1).
Table 1. The condition of patients included in this study.
| Groups | Normal | CXL-S | CXL-B | Total |
| Sex | ||||
| M | 12 | 6 | 3 | 21 |
| F | 13 | 2 | 5 | 20 |
| Eye | ||||
| OS | 12 | 4 | 4 | 20 |
| OD | 13 | 4 | 4 | 21 |
| Degree of KC | ||||
| I | 0 | 0 | 0 | 0 |
| II | 7 | 0 | 0 | 7 |
| III | 10 | 3 | 5 | 18 |
| IV | 5 | 1 | 1 | 7 |
| V | 3 | 4 | 2 | 9 |
| Age (a) | ||||
| Mean age | 38.87±10.75 | 29.24±12.18 | 25.96±4.41 | 34.48±11.43 |
| Oldest | 59 | 53.5 | 33.3 | 59 |
| Youngest | 19.5 | 15.5 | 20.6 | 19.5 |
KC: Keratoconus.
Visual Acuity
In the group Normal, the mean improvement of UDVA was 0.31 logMAR (P=0.030) at 12mo, among of which 15/25 patients improved, 6/25 patients maintained stable, 4/25 patients regressed. The mean improvement of CDVA was 0.21 logMAR (P=0.013) at 12mo, among of which 18/25 patients improved, 4/25 patients maintained stable, 3/25 patients regressed. In the group CXL-S, the mean improvement of UDVA was 0.4 logMAR (P=0.020) at 12mo, among of which 6/8 patients improved, 1/8 patients maintained stable, 1/8 patients regressed 0.1 logMAR. The mean improvement of CDVA was 0.3 logMAR (0.036) at 12mo, all of which improved the CDVA. In the group CXL-B, the mean improvement of UDVA was 0.45 logMAR (P=0.001) at 12mo, the mean improvement of CDVA was 0.26 logMAR (P =0.000). All these patients have all improved the UDVA and CDVA at 12mo. For all three groups, a great improvement of visual acuity occurred in the first 3mo and a slight improvement continued from the third month to the end of 12mo (Table 2).
Table 2. Comparisons respective of preoperative and postoperative visual acuity, refractive results, topographic results in the three groups.
| Groups | CXL-S |
Normal |
CXL-B |
||||||
| Preop. | 3mo follow-up | 1a follow-up | Preop. | 3mo follow-up | 1a follow-up | Preop. | 3mo follow-up | 1a follow-up | |
| UDVA | 0.97±0.28 | 0.61±0.39 | 0.57±0.37 | 0.99±0.55 | 0.71±0.39 | 0.68±0.41 | 1.04±0.30 | 0.71±0.38 | 0.59±0.19 |
| CDVA | 0.60±0.31 | 0.32±0.27 | 0.30±0.21 | 0.52±0.59 | 0.36±0.27 | 0.31±0.30 | 0.58±0.34 | 0.32±0.27 | 0.32±0.32 |
| SE | -7.19±6.07 | -6.97±7.86 | -5.22±6.13 | -4.97±5.54 | -3.18±4.97 | -3.23±5.97 | -4.44±4.01 | -3.41±3.82 | -2.88±3.28 |
| CE | -6.91±2.42 | -3.32±2.42 | -3.75±1.48 | -4.84±2.18 | -3.71±2.15 | -3.34±2.05 | -4.84±2.22 | -3.53±2.36 | -2.25±2.13 |
| Kmax | 56.81±7.48 | 52.49±5.85 | 50.81±3.78 | 53.37±5.38 | 50.17±4.71 | 50.38±4.64 | 52.68±3.93 | 49.89±4.55 | 48.33±4.69 |
| Kmin | 48.24±6.41 | 48.87±6.48 | 49.23±5.30 | 47.04±4.57 | 46.06±4.59 | 46.29±4.02 | 47.25±3.99 | 46.24±3.82 | 44.52±4.75 |
| Kave | 52.61±6.43 | 50.91±6.07 | 49.81±4.37 | 50.25±4.80 | 48.31±4.51 | 48.48±4.30 | 50.04±3.58 | 48.15±4.07 | 46.79±4.42 |
UDVA: Uncorrected distance visual acuity; CDVA: Corrected distance visual acuity; SE: Spherical equivalent; CE: Cylinder equivalent; Kmax: Maximum keratometry; Kmin: Minimum keratometry; Kave: Average keratometry.
Refractive Outcomes
In the group Normal, the mean SE and the cylinder equivalent (CE) decreased 1.74 D (P=0.029) and 1.49 D (P=0.028) respectively at 12mo after ICRs implantation. In the group CXL-S, the values decreased 1.97 D (P=0.317) and 3.16 D (P=0.021) respectively. In the group CXL-B, The values decreased1.56 D (P=0.060) and 2.59 D (P=0.047) respectively (Table 2).
Topography
In the group normal, the mean maximum (Kmax), minimum (Kmin), and average (Kave) Keratometry reduced by 2.99 D (P=0.000), 0.85 D (P=0.058), 1.77 D (P=0.000) respectively at 12mo after ICRs implantation. In the group CXL-S, the Kmax and Kave reduced by 6.0 D (P=0.024), 2.8 D (P=0.142) respectively, Kmin increased 0.99 D (P=0.378). In the group CXL-B, Kmax, Kmin, Kave reduced by 4.35 D (P=0.09), 2.73 D (P=0.049), 3.25 D (P=0.012) respectively (Table 2).
Among Groups
With one-way ANOVA, the preoperative 7 parameters showed no significant difference among the three groups (all P>0.05). The homogeneity of variance tests showed P>0.05 for variances of the UDVA, CDVA, SE, CE (Figure 1), and P<0.05 for decrease of the Kmax, Kmin, Kave (Figure 2). The P values of one-way ANOVA (UDVA, CDVA, SE, CE) and the P values of multiple tests of the post hoc test with Tamhanes (Kmax, Kmin, Kave) were above 0.05, showing no difference of among the three groups.
Figure 1. P value of homogeneity of variance test >0.05.
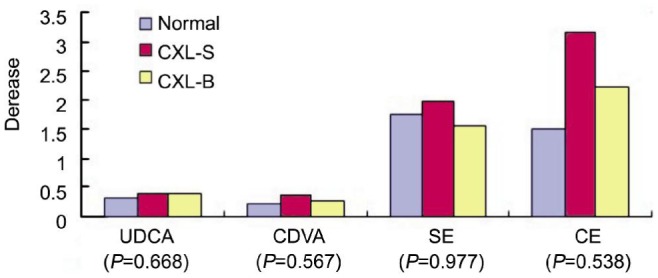
The P values (P=0.668 for UDVA, P=0.567 for CDVA, P=0.977 for SE, P=0.391 for CE) of one-way ANOVA showed no difference among three groups.
Figure 2. P value of homogeneity of variance test <0.05. All the P value of post hoc test with Tamhanes for multiple tests were above 0.05.
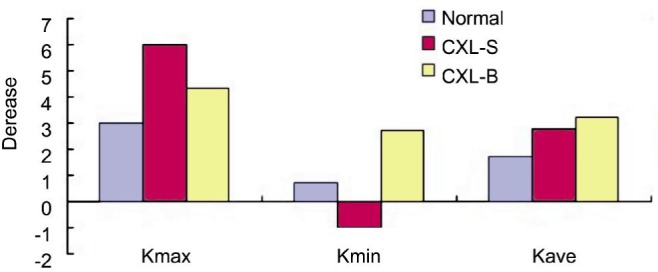
Complications
Among these 86 eyes, there were 8 (6.3%) ring segment explantations 5 (4.0%) eyes for extrusion, 2 (1.6%) eyes for corneal melting, 1 (0.8%) for infectious keratitis. All of them were performed reposition in several months later. The eyes of 4 extrusions, 1 corneal melting and 1 infectious keratitis had only implantation of ICRs. The eyes of 1 extrusion and 1 corneal melting had ICRs followed by CXL immediately. After 8mo of ICRs implantation without CXL, 1 (0.8%) eye was performed keratoplasty because of photophobia and no improvement for the very poor visual acuity.
DISCUSSION
Although the previous studies[4]–[6] concerning CXL all demonstrated decreases of Kmax, SE, and improvement of visual acuity, the variances are so small that we prefer to consider them as good signs of halting the evolution of keratoconus rather than solving refractive problems and gaining visual acuity. With different sequence and timing, we investigate if CXL influences the refractive treatment of ICRs in this study. It's a great pity that we included only 41 eyes for 3y, as the prevalence of keratoconus is very low and only small part of them received the implantation of ICRs.
In this study, ICRs implantation proved to be a safe and effective therapeutic treatment for keratoconus. The significant improvements of visual acuity, refractive and topographic results were noted in all the three groups, as confirmed by the other previous studies[10],[13]–[15]. After ICRs implantation, all the parameters of the three groups showed great improvement in the first 3mo and slight but continuous improvement from 3mo to 12mo. Torquetti et al[16] and Guell et al[17] also showed the continuous improvement after 18mo of ICRs implantation. This was explained by the long-term biomechanical effect of ICRs.
Vega-Estrada et al[12] did a multicenter and retrospective study. They concluded that the different degree of keratoconus based on limitation of preoperative visual acuity (CDVA) influenced greatly the rate of success of ICRs implantation. In the three groups, there was no difference of degree of keratoconus preoperatively. The patients of group normal were older than group CXL-B and CXL-S, and the patients of CXL-B were a bit younger than that of CXL-S. Because of the small number of each group, no subgroup of age was executed. In general, the patients of three groups were comparative preoperatively. Comparing the improvements of the 7 parameters among the three groups, no significant difference (all the P>0.05) was observed in the efficiency of ICRs implantation. This showed that ICRs combined with CXL was not better than ICRs alone as treatment of keratoconus. But the statistics indicated that the group CXL-B and the group CXL-S both had greater improvements than the group Normal in terms of visual acuity, manifest cylinder, Kmax, Kave in our study (Figures 1, 2). Cakir et al[18] did a retrospective study with 166 eyes of two groups treated by combined ICRs-CXL or separate ICRs, showing no statistical difference between the two groups.
Compared to group CXL-B, group CXL-S had greater mean improvement in CDVA (0.30 vs 0.26 logMAR), spherical equivalent (1.97 vs 1.56), manifest cylinder (3.16 vs 2.69), Kmax (6.00 vs 4.35 D), but fewer mean improvement in UDVA (0.40 vs 0.45 logMAR), Kave (2.80 vs 3.25 D), Kmin (-0.99 vs 2.73 D) after 1y postoperatively. No significant differences were observed between the two groups. But in terms of the benefits of individual, the group CXL-B was more certain with improvement in UDVA and CDVA for all the patients, while in the group CXL-S 6/8 patients improved, 1/8 patients maintained stable, 1/8 patients regressed 1 line for UDVA and all patients improved for CDVA. In the group normal, 15/25 patients improved, 6/25 patients maintained stable, 4/25 patients regressed for UDVA and 18/25 patients improved, 4/25 patients maintained stable, 3/25 patients regressed for CDVA. Kling and Marcos[19] designed a simulated keratoconic cornea. They reported that the changes of refraction were due to changes in curvature of both the anterior (75.4%) and posterior corneal surfaces (12.3%), as well as changes in the relative position of the corneal apex (13.4%). As the keratometry is the indicator of curvature of the surface anterior, the Kmax, Kmin, and Kave can indicate the condition and progression of keratoconus in a great measure. In group CXL-S, the patient with 1line regression of UDVA showed increases in Kmin (5.77 D) and Kave (3.10 D), but decrease in Kmax (-0.58 D), and the patient with stable UDVA also showed increases in Kmin (3.65 D) and Kave (1.35 D), but decrease in Kmax (-2.58 D). The other patients who had improvements in UDVA and CDVA all showed decreases in Kmax, Kmin, Kave.
Henriquez et al[20] prefer the CXL long before ICRs implantation. They executed a prospective study including 9 patients who had cross CXL 6mo before FR implantation. There was a mean reduction from 0.75 logMAR to 0.23 logMAR (P<0.001) for UDVA, from 0.24 logMAR to 0.12 logMAR (P=0.05) for CDVA, after 6mo insertion of ICRs. The results were similar to that of the group CXL-B in our study. In their experience, patients with a history of previous incisional surgery can develop moderate to severe haze after CXL. With CXL first to stop or slow the progression of keratoconus, the residual refractive error can be treated by the best alternative.
Kanellopoulos[21] confirmed that topography-guided laser ablation with simultaneous CXL had better outcomes than that with sequential CXL later. Topography-guided laser ablation generates anatomical changes of cornea, which induces unpredictable influences in the visual acuity and refraction. In corneal stroma, keratocytes remain quiescent. Exposed to corneal injury, they response either apoptosis or regenerative and fibrotic corneal repair[22]. In vivo confocal microscopy, Kymionis et al[23] confirmed immediate keratocyte apoptosis after cross-linking and visible repopulation of keratocytes 1mo after CXL, reaching to the preoperative level in the anterior segment of stroma 6mo later. As the new population of keratocytes renew the cross-likened collgen, so the effect of CXL is not life-time and can decrease with the time. The different interval of CXL can explain why the simultaneous CXL was more effective than the sequential CXL later in topography-guided laser ablation.
But insertion of ICRs is required to induce predictable corneal astigmatism. Good results depend on precise induced astigmatism. Though the monograms for ICRs implantation have developed, it is not easy to predict the induced astigmatism. On the other hand, CXL can also change corneal anterior surface[4]–[6]. In the group CXL-S, ICRs implantation was followed immediately by CXL, making the induced astigmatism less predictable. While in group CXL-B, the mean time between the operation of CXL and ICRs implantation was 21.00±10.52mo. So the CXL did not interfere the effect of ICRs. What's more, the progression of keratoconus has been halted and the cornea was stable. The insertion of ICRs was more precise and predictable.
As the complications occur almost before the first three month postoperative, so the comparison of the risks about complication was impossible among the three groups. But there was no difference of the risks of complication among the eyes subjected only ICRs implantation and these which had ICRs first followed by CXL immediately. Coskunseven et al[24] did a retrospective study of 531 patients (850 eyes) to investigate the complications after ICRs implantation using femtosecond laser for channel creation. They reported 1.3% of extrusion and 0.2% of corneal melting, caused by superficial placement of segment. It was lower than that of our study using mechanical channel creation.
With safety and good visual outcomes, ICRs implantation is a viable alternative to treat the keratoconus. No significant difference was found in the three groups of ICRs implantation with CXL using different sequence and timing.
Acknowledgments
Conflicts of Interest: Liu XL None; Li PH, None; Fournie P, None; Malecaze F, None.
REFERENCES
- 1.Xu L, Wang YX, Guo Y, You QS, Jonas JB, Bejing Eye Study Group Prevalence and associations of steep cornea/keratoconus in Greater Beijing. The Beijing Eye Study. PLoS One. 2012;7(7):e39313. doi: 10.1371/journal.pone.0039313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157–166;quiz205. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Vazirani J, Basu S. Keratoconus: current perspectives. Clin Ophthalmol. 2013;7:2019–2030. doi: 10.2147/OPTH.S50119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournié P, Galiacy S, Arné JL, Malecaze F. Corneal collagen cross-linking with ultraviolet-A light and riboflavin for the treatment of progressive keratoconus. J Fr Ophtalmol. 2009;32(1):1–7. doi: 10.1016/j.jfo.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Goldich Y, Barkana Y, Wussuku Lior O, Marcovich AL, Hirsh A, Avni I, Zadok D. Corneal collagen cross-linking for the treatment of progressive keratoconus: 3-year prospective outcome. Can J Ophthalmol. 2014;49(1):54–59. doi: 10.1016/j.jcjo.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Razmjoo H, Nasrollahi AP, Salam H, Karbasi N, Najarzadegan MR, Razmjoo H. Topographic corneal changes after collagen cross-linking in patients with corneal keratoconus. J Res Med Sci. 2013;18(10):882–886. [PMC free article] [PubMed] [Google Scholar]
- 7.Kazanci B, Ozek D, Anayol A, Balikçi A, Ileri D, Yilmazbas P. Applications of different types of gas-permeable contact lenses in keratoconus and their visual results. Eur J Ophthalmol. 2014;24(6):835–841. doi: 10.5301/ejo.5000449. [DOI] [PubMed] [Google Scholar]
- 8.Fleming JF, Lee Wan W, Schanzlin DJ. The theory of corneal curvature change with the intra-stromal corneal ring. CLAO J. 1989;15(2):146–150. [PubMed] [Google Scholar]
- 9.Patel S, Marshall J, Fitzke FW., 3rd Model for deriving the optical performance of the myopic eye corrected with an intracorneal ring. J Refract Surg. 1995;11(4):248–252. doi: 10.3928/1081-597X-19950701-08. [DOI] [PubMed] [Google Scholar]
- 10.Ancèle E, Malecaze F, Arné JL, Fournié P. Predictive factors for successful Ferrara intracorneal ring segment implantation in keratoconus. J Fr Ophtalmol. 2011;34(8):513–520. doi: 10.1016/j.jfo.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Alió JL, Piñero DP, Alesón A, Teus MA, Barraquer RI, Murta J, Maldonado MJ, Castro de Luna G, Gutiérrez R, Villa C, Uceda-Montanes A. Keratoconus-integrated characterization considering anterior corneal aberrations, internal astigmatism, and corneal biomechanics. J Cataract Refract Surg. 2011;37(3):552–568. doi: 10.1016/j.jcrs.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Vega-Estrada A, Alio JL, Brenner LF, Javaloy J, Plaza Puche AB, Barraquer RI, Teus MA, Murta J, Henriques J, Uceda-Montanes A. Outcome analysis of intracorneal ring segments for the treatment of keratoconus based on visual, refractive, and aberrometric impairment. Am J Ophthalmol. 2013;155(3):575–584.e1. doi: 10.1016/j.ajo.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Hamdi IM. Optical and topographic changes in keratoconus after implantation of Ferrara intracorneal ring segments. J Refract Surg. 2010;26(11):871–880. doi: 10.3928/1081597X-20100114-05. [DOI] [PubMed] [Google Scholar]
- 14.Hamdi IM. Preliminary results of intrastromal corneal ring segment implantation to treat moderate to severe keratoconus. J Cataract Refract Surg. 2011;37(6):1125–1132. doi: 10.1016/j.jcrs.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 15.Miranda D, Sartori M, Francesconi C, Allemann N, Ferrara P, Campos M. Ferrara intrastromal corneal ring segments for severe keratoconus. J Refract Surg. 2003;19(6):645–653. doi: 10.3928/1081-597X-20031101-06. [DOI] [PubMed] [Google Scholar]
- 16.Torquetti L, Berbel RF, Ferrara P. Long-term follow-up of intrastromal corneall ring segments in keratoconus. J Cataract Refract Surg. 2009;35(10):1768–1773. doi: 10.1016/j.jcrs.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Güell JL, Morral M, Salinas C, Elies D, Gris O, Manero F. Intrastromal corneal ring segments to correct low myopia in eyes with irregular or abnormal topography including forme fruste keratoconus: 4-year follow-up. J Cataract Refract Surg. 2010;36(7):1149–1155. doi: 10.1016/j.jcrs.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Cakir H, Pekel G, Perente I, Genç S. Comparison of intrastromal corneal ring segment implantation only and in combination with collagen crosslinking for keratoconus. Eur J Ophthalmol. 2013;23(5):629–634. doi: 10.5301/ejo.5000250. [DOI] [PubMed] [Google Scholar]
- 19.Kling S, Marcos S. Finite-element modeling of intrastromal ring segment implantation into a hyperelastic cornea. Invest Ophthalmol Vis Sci. 2013;54(1):881–889. doi: 10.1167/iovs.12-10852. [DOI] [PubMed] [Google Scholar]
- 20.Henriquez MA, Lzquierdo L, Jr, Bernilla C, McCarthy M. Corneal collagen cross-linking before Ferrara intrastromal corneal ring implantation on for treatment of progressive keratoconus. Cornea. 2012;31(7):740–745. doi: 10.1097/ICO.0b013e318219aa7a. [DOI] [PubMed] [Google Scholar]
- 21.Kanellopoulos AJ. Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided PRK for treatment of keratoconus. J Refract Surg. 2009;25(9):S812–S818. doi: 10.3928/1081597X-20090813-10. [DOI] [PubMed] [Google Scholar]
- 22.West-Mays JA, Dwivedi DJ. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kymionis GD, Diakonis VF, Kalyvianaki M, Portaliou D, Siganos C, Kozobolis VP, Pallikaris AI. One-year follow-up of corneal confocal microscopy after corneal cross-linking in patients with post laser in situ keratosmileusis ectasia and keratoconus. Am J Ophthalmol. 2009;147(5):774–778. doi: 10.1016/j.ajo.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Coskunseven E, Kymionis GD, Tsiklis NS, Atun S, Arslan E, Siganos CS, Jankov M, Pallikaris IG. Complications of intrastromal corneal ring segment implantation using a femtosecond laser for channel creation: a survey of 850 eyes with keratoconus. Acta Ophthalmol. 2011;89(1):54–57. doi: 10.1111/j.1755-3768.2009.01605.x. [DOI] [PubMed] [Google Scholar]


