Abstract
AIM
To determine the effects of rapamycin on experimental autoimmune uveoretinitis (EAU) and investigate of role of rapamycin on T cell subsets in the disease.
METHODS
EAU was induced in rats using peptides 1169 to 1191 of the interphotoreceptor binding protein (IRBP). Rapamycin (0.2 mg/kg/d) was administrated by intraperitoneal injection for a consecutive 7d after immunization. Th1/Th2/Th17 cytokines, TGF-β1, and IL-6 produced by lymphocyteswere measured by ELISA, while Th17 cells and CD4+CD25+ regulatory T cells (Tregs) from rat spleen were detected by flow cytometry.
RESULTS
Intraperitoneal treatment immediately after immunization dramatically ameliorated the clinical course of EAU. Clinical responses were associated with reduced retinal inflammatory cell infiltration and tissue destruction. Rapamycin induced suppression of Th1/Th2/Th17 cytokines, including IFN-γ, IL-2, IL-17, IL-4, and IL-10 release from T lymphocytes of EAU rats, in vitro. Rapamycin also significantly increased TGF-β1 production but had no effect on IL-6 productionof T lymphocytes from EAU rats in vitro. Furthermore, rapamycin decreased the ratio of Th17 cells/CD4+T cells and upregulated Tregs in EAU, as detected by flow cytometry.
CONCLUSION
Rapamycin effectively interferes with T cell mediated autoimmune uveitis by inhibiting antigen-specific T cell functions and enhancing Tregs in EAU. Rapamycin is a promising new alternative as an adjunct corticosteroid-sparing agent for treating uveitis.
Keywords: experimental autoimmune uveoretinitis, rapamycin, regulatory T cells, Th1 cells, Th2 cells, Th17 cells, uveitis
INTRODUCTION
Experimental autoimmune uveoretinitis (EAU) is a T cell-mediated organ-specific autoimmune disease that has been widely used as a model for human intraocular inflammation[1]. There has been great progress regarding the pathogenic T cells in EAU in the last decade. Early studies implicated Th1 cells as the etiologic agent of EAU; however, subsequent studies revealed that Th17 cell lineage (IL-17-producing CD4+ effector lineage), is another chief contributor to autoimmune diseases[2]–[4]. Although the role of some cytokines produced by these two subsets can be paradoxical, it is currently believed that Th17 and Th1 cells play overlapping as well as differential roles in the pathogenesis of EAU[5]–[7]. It was proposed that Th2 cells, as counter-regulatory to Th1, would be protective; however, research shows that Th2 cells also have the ability to induce uveitis provided that one uses immunodeficient hosts[8]. CD4+CD25+ regulatory T cells (Tregs) are powerful inhibitors of T-cell activation (which play an important role in the regression of EAU[9],[10]. Upregulation and adoptive transfer of Tregs ameliorate EAU[11].
Rapamycin, a product of the bacterium streptomyces hygroscopicus (also known as sirolimus), was first discovered in 1975[12]. It has been used since the late 1990's as an immunosuppressive therapy to prevent rejection of transplanted organ allografts[13],[14]. Although the efficacy of rapamycin on EAU has been evaluated as early as 1993[15],[16], and pilot clinical studies also have shown potential therapeutic effects in uveitis as an adjunct corticosteroid-sparing agent[17]–[20], investigation of immunosuppressive mechanisms of rapamycin on uveitis is scarce.
With the advances of the complicated pathogenesis of uveitis, further study of the impact of rapamycin on lymphocyte subsets in the course of disease is needed. Using the EAU model, we investigated the effect of rapamycin on cytokine profiles of T cell subsets as well as the frequency of Th17 and Treg cells. We observed that rapamycin could dramatically ameliorate both clinical symptoms and pathological manifestations of EAU after injection at the beginning of disease induction. This effect may be caused by inhibition of Th1/Th2/Th17 responses and upregulation of Tregs. The data supports the potential usage of rapamycin to treat ocular autoimmune diseases.
MATERIALS AND METHODS
Rats and Reagents
Female Lewis rats [6 to 8-week-old purchased from Vital River (Beijing, China)] were housed and maintained under 12-hour light/12-hour dark cycles and specific pathogen-free conditions. All procedures involving rats were approved by the Laboratory Animal Care and Use Committee of the Tianjin Medical University and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Methods
Interphotoreceptor binding protein (IRBP) 1169 to 1191 (PTARSVGAADGSSWEGVGVVPDV, R16) was synthesized by Sangon (Sandon Biotech, Shanghai, China). Complete Freund's adjuvant (CFA) was purchased from Sigma (St. Louis, MO, USA). Mycobacterium tuberculosis strain H37RA was obtained from Difco (Detroit, MI, USA). ELISA kits for the quantitative analysis of interleukin (IL)-2, interferon (IFN)-γ, IL-10, IL-4, transforming growth factor (TGF)-β1, IL-6, and IL-17A were obtained from R&D Systems (Minneapolis, MN, USA). All cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, USA), 5×105 mol/L 2-mercaptoethanol, and penicillin/streptomycin (100 µg/mL).
Immunization and rapamycin administration
EAU was induced in the Lewis rats by active immunization using previously reported procedures[21]. In brief, rats were injected subcutaneously with 100 µL of an emulsion containing 30 g of R16 and 500 g of Mycobacterium tuberculosis H37Ra in CFA into two hind footpads. The immunized rats were then randomly divided into 3 groups (n=10/each), with one group being i.p treated with rapamycin at 0.2 mg/kg/d, one with dimethyl sulfoxide (DMSO)-PBS vehicle, and one without treatment. Rapamycin was dissolved in DMSO to prepare a solution with 1mg/mL concentration and then diluted 10-folds with PBS. The treatment started at the same day of R16 immunization and lasted for 7d.
Clinical and histopathological evaluation of experimental autoimmune uveoretinitis
After immunization, the rats were examined daily for clinical signs of uveitis by slit lamp microscope starting at day 4 post-immunization. Incidence and severity of EAU were graded on a scale of 0 to 4 in half-point increments using previously described criteria[1] based on the type, number, and size of lesions present. For histology, eyes were enucleated on day 14 after immunization, fixed for 1h in 4% glutaraldehyde/PBS, and then transferred to 10% buffered formaldehyde until processing. Fixed and dehydrated tissues were embedded in paraffin, and 4 µm sections were stained with standard hematoxylin and eosin (H&E). The intensity of EAU was scored in a masked fashion from grade 0 to 4[1].
Spleen cell preparation
Single cell suspensions were prepared from the spleens of R16 -immunized rats at day 14 post-immunization. Mononuclear cells (MNCs) were isolated from the splenocyte suspensions by Ficoll gradient (Roche, Switzerland).
Flow cytometry
Aliquots of 1×106 cells were stained with combinations of PE-cy5-conjugated mAb against rat CD4 (BD Biosciences, USA) and/or PE-conjugated mAb against CD25 (eBioscience, USA) for 30min followed by fixation in 2% paraformaldehyde to analyze cell-surface molecular expression. Cells were permeabilized and fixed using a fixation/permeabilization solution (eBioscience, USA), stained with PE-conjugated IL-17A mAbs (BD Biosciences, USA) or FITC-conjugated Foxp3 mAbs (eBioscience, USA) for 1h, and then subjected to FCM analysis. Expression of IL-17A or CD25/FoxP3 was analyzed by gating on a homogenous level of CD4+ cells.
In vitro cell stimulation and cytokine production
MNCs suspended in RPMI 1640 medium were seeded into 96-well, flat-bottomed microtiter plates (Corning, Corning, NY, USA) at a concentration of 5×105 cells in a total volume of 200 µL/well and stimulated with 30 µg/mL IRBP peptide at 37°C in 5% CO2 for 72h. For TGF-β1 detection, medium without FBS was used. Cell-free supernatants were collected after 72h and levels of IFN-γ, IL-2, IL-4, IL-10, TGF-β1, IL-6, and IL-17A were measured using commercially available ELISA kits according to the manufacturer's instructions (R&D Systems, USA).
Statistical Analysis
EAU clinical scores were assessed by repeated-measures ANOVA using mixed models. Histopathological scores were calculated using nonparametric Mann-Whitney U test. The frequency of Tregs and Th17 cells and concentrations of IL-2, IFN-γ, IL-10, IL-4, TGF-β1, IL-6, and IL-17A were evaluated by one-way ANOVA. Data are expressed as mean±SD and P<0.05 was considered significant.
RESULTS
Clinical and Histological Assessment of Experimental Autoimmune Uveoretinitis
Rapamycin has been tested in S-Ag induced EAU in rats[22]–[25] and IRBP161-180 induced EAU in B10RIII mice[26]. To determine whether rapamycin had an effect on R16-induced uveitis, we injected rats with rapamycin on the same day of immunization. As shown in Figure 1, most of R16-immuned rats began to show early signs of uveitis on day 6 (Figure 1G). These included dilated blood vessels in the iris, abnormal pupil contraction, or a hazy anterior chamber (Figure 1A). Severe inflammation with scores of 3 and 4 developed on day 12 (Figure 1G), characterized by an opaque anterior chamber, a dull or absence of red reflex, or an obscured pupil (Figure 1B). These signs diminished on day 14 (Figure 1C, 1G). In contrast, rapamycin treatment strikingly reduced disease severity and yielded only slight signs of anterior chamber inflammation (grade 1 or less) (Figure 1D-1F), reaching statistical significance from day 6 onward when compared with EAU rats (Figure 1G).
Figure 1. Clinical severity of EAU in different groups.
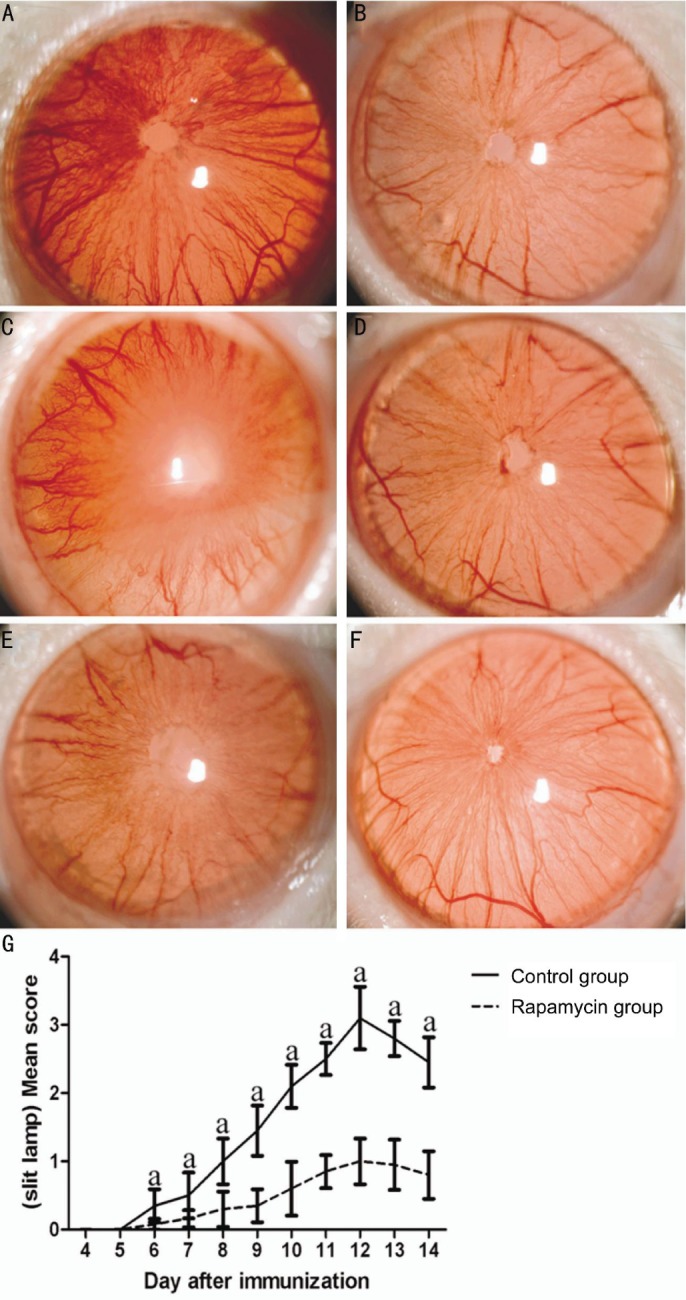
aP<0.05 (n=10).
Consistent with the clinical score, EAU rats without treatment had severe inflammation in the retina with large amounts of infiltrating inflammatory cells and damaged retinal structure (Figure 2A). In contrast, the EAU rats treated with rapamycin showed significantly reduced inflammatory cell infiltration and retinal damage (Figure 2B). Histological scores in the rapamycin group on day 14 were significantly lower than those in the EAU group (Figure 2C).
Figure 2. Histopathological changes of the posterior segments of the eyes from the rats in different groups on day 14.
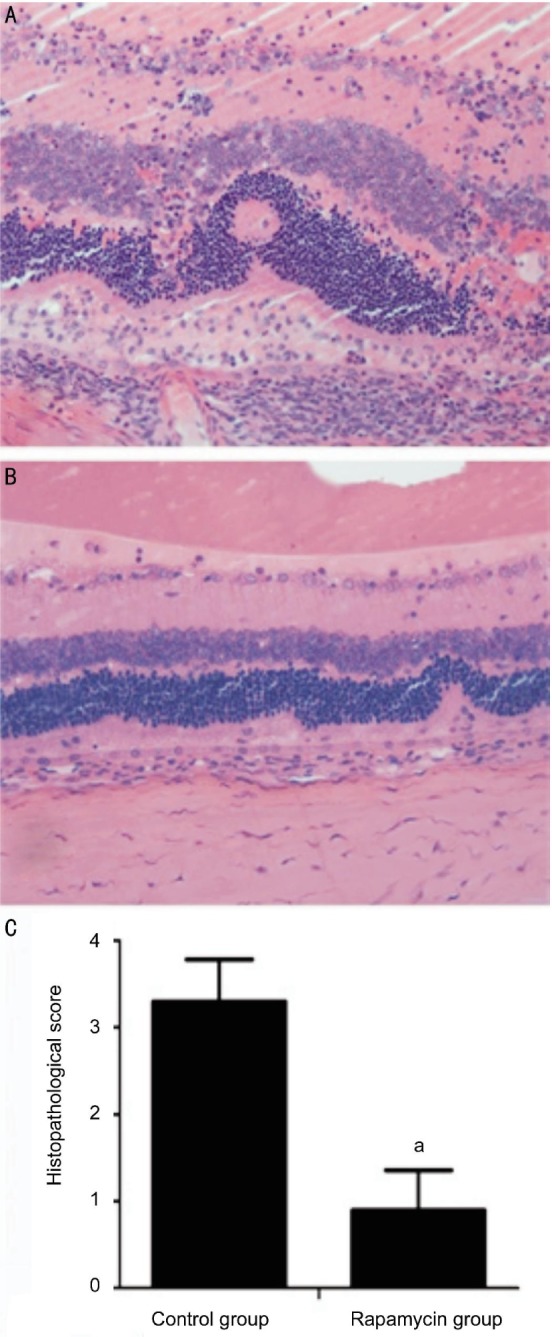
aP<0.05 (n=10).
The Change of Th1/Th2 and Th17 Cytokines in R16-immunized Rats
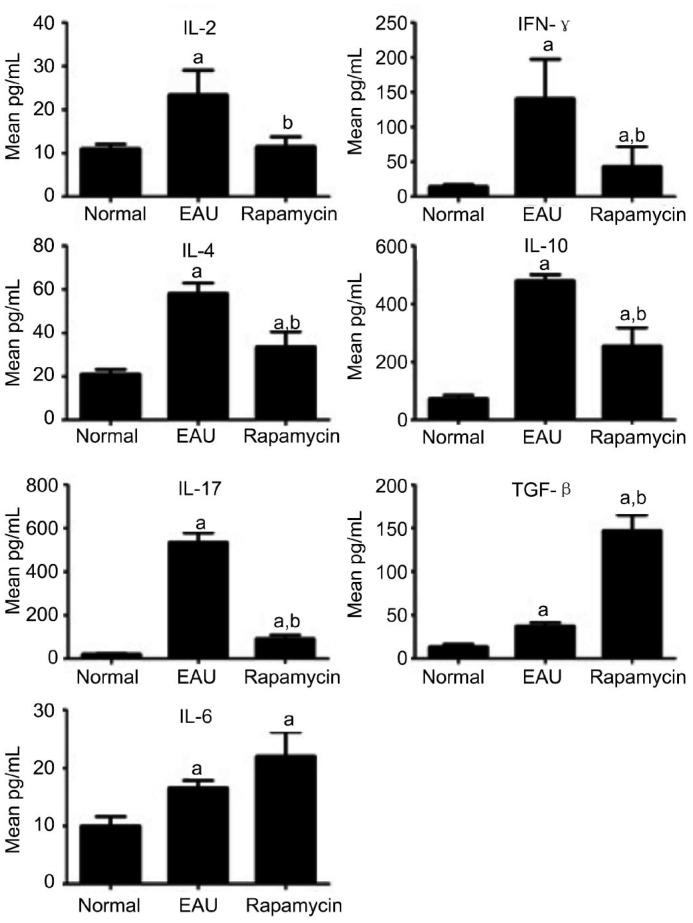
To determine the mechanism by which administration of rapamycin reduced the severity of EAU, we measured the cytokine production by T cells from immunized rats treated with vehicle or rapamycin in response to R16 stimulation. The amounts of IFN-, IL-17, IL-2, IL-4 and IL-10 released into the culture supernatants by T cells from rapamycin-treated rats were markedly lower than those produced by T cells from vehicle-treated disease control rats (Figure 3A). Secretion of TGF-β1, however, was significantly increased by the T cells from the rapamycin treated group. There was no difference in the level of IL-6 between rapamycin-treated and control groups (Figure 3B).
Figure 3. Effect of rapamycin on cytokine production of T cells.
Values are expressed as mean±SD. aP<0.05 when compared with the normal group; bP<0.05 compared with the EAU group (n=10).
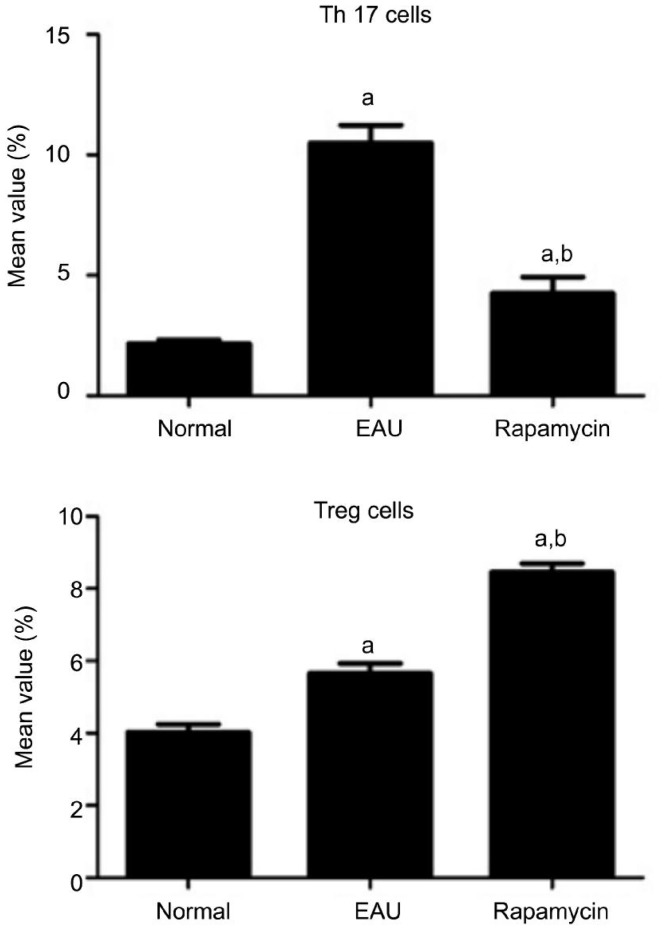
The Change of Th17 Cells in Experimental Autoimmune Uveoretinitis
We then further examined the frequency of Th17 cells in the spleen of both rapamycin-treated and control groups. In the EAU control group, the ratio of Th17 cells to CD4+T cells was 10.50±0.74%, whereas in the rapamycin group, it was 4.25±0.68% significantly lower than those of the control group (P<0.01) suggesting that rapamycin decreased the number of Th17 cells (Figure 4A).
Figure 4. Effect of rapamycin on Th17 and Tregs.
aP<0.05 when compared with the normal group; bP<0.05 compared with the EAU group bP<0.05 (n=10).
The Change of Tregs in Experimental Autoimmune Uveoretinitis
We have also examined the frequency of Treg in the spleen of both rapamycin-treated and control groups. In the EAU group, the ratio of Tregs to CD4+ T cells was 5.65±0.28%, whereas in the rapamycin group, the ratio was 8.46±0.24% -significantly higher than those of the EAU group (P<0.01) -indicating that rapamycin increased the number of Tregs (Figure 4B).
DISCUSSION
Although corticosteroids are still recommended as the first line of therapy for patients with active uveitis, numerous adverse effects associated with long-term usage of corticosteroids have pushed the development of new therapies to decrease the corticosteroid burden on patients and to manage refractive uveitis. New immunosuppressants such as rapamycin, biologic agents[22], and mesenchymal stem cells[21] are among these new corticosteroid-sparing agents that may provide better alternatives as monotherapy or combined therapy with less side effects and more responsiveness for chronic cases. The potential use of rapamycin in uveitis was first explored in 1993[15],[16], demonstrating that it efficiently inhibits EAU induction by reducing the number of T cells in the immunization site-draining lymph nodes, and lowering the peak of the lymphocyte proliferative response curve. Subsequent studies revealed that rapamycin has synergistic effects with dexamethasone, tacrolimus, and cyclosporine A in EAU, allowing the use of reduced doses of each drug to achieve a therapeutic effect[23]–[25]. Several recent pilot clinical experiences with rapamycin also demonstrate its effectiveness in non-infectious uveitis by intravitreal injection and systemic administration[17]–[20]. The mechanism of the immunosuppressive effect of rapamycin on uveitis, however, remains largely unknown.
Our study was consistent with the previous reports showing that rapamycin therapy with the dose of 0.2 mg/kg/d potently reduced the severity of R16-induced EAU when administered at the same day of disease induction. Clinical efficacy for all treatments was demonstrated by a decreased mean maximum score with reduced cellular infiltrates and milder uveal and retinal impairment. Since in a clinical setting, treatment usually is adopted after uveitis has developed, it is of therapeutic importance that in further study rapamycin should also be tested in animals with established disease. A recent study reported that the daily intraperitoneal injection of low dose rapamycin (about 0.05 mg/kg) exacerbated ocular inflammation. In our study, we didn't show the dose-effect relationship in the treatment with rapamycin. The paradoxical role of rapamycin in uveitis underscores the need to validate dosing and pharmacokinetics during rapamycin therapy[26].
Now it is believed that either Th1 or/and Th17 responses drive the pathology of EAU, and Th2 cells may play a pathogenic or a protective role under different conditions[6],[7]. IL-2 and IFN-γ are the main signature cytokines of Th1 cells. IL-2 is a key Th1-inducing cytokine cells, and IFN-γ can enhance the phagocytosis of neutrophil and macrophages, and also increase the cytotoxicity of NK cells[7]. IL-17A is the hallmark of Th17 lineage that plays a pro-inflammatory and pathogenic role in EAU by inducing the expression of different chemokines[7]. IL-4 and IL-10 are main cytokines produced by Th2 cells. IL-4 is an important differentiation factor of Th2, while IL-10 is an important anti-inflammatory cytokine[7]. In our study, rapamycin therapy significantly decreased the production of IL-2, IFN-γ, IL-17A, IL-4 and IL-10 of T cells isolated from EAU-inducing rats. Moreover, rapamycin therapy significantly decreased Th17 cells in the spleen. These data suggest that rapamycin treatment inhibited the Th1/Th17/Th2 cell responses simultaneously in EAU, thereby decreasing the severity of EAU in rats.
Tregs play a critical role in preventing immune aggression. The effector function of T helper cells, including Th1, Th2, and Th17, is controlled by Tregs. It has recently been elucidated that Th17 cells and Tregs have a common induced pathway, and disturbed Th17/Treg balance has been found in several autoimmune diseases[27]–[30]. TGF-β and IL-6 are the most important cytokines that, in a concentration-dependent manner, can orchestrate the differentiation of Tregs and Th17 cells. High concentrations of TGF-β favor the development of Foxp3+ Tregs, while in low TGF-β concentrations, IL-6 suppresses Foxp3 expression, and the development of Th17 cells prevails[31],[32]. Rapamycin has been reported to inhibit the proliferation and differentiation of Th17 cells by blocking IL-6 signal transduction and decreasing the production of IL-6[33]. In our study, we found that rapamycin therapy significantly increased production of TGF-β1 of T lymphocytes isolated from EAU rats, but had no effect on the expression of IL-6 on day 14 after immunization. Tregs in the spleen of EAU rats were also significantly upregulated by rapamycin. These results suggest that rapamycin treatment may activate a cascade of Tregs by upregulating the production of TGF-β, thereby inhibiting EAU in rats. Determination of whether or not the inhibitory effect of rapamycin on IL-6 occurs at early stages of EAU requires further studies.
Regulation of the development of T cell subsets by rapamycin is through its mammalian target of rapamycin C2 (mTORC2) pathway[34]. Rapamycin binds to FKBP12, a cytoplasmic receptor, and the drug-protein complex inhibits the function of mTOR kinase, an important cellular regulator of translation and transcription[35]. Such inhibition blocks intracellular signaling in response to T cell growth factors, such as IL-2. In addition, since rapamycin inhibits differentiation of Th17 cells and favors the expansion of Tregs in vitro and in vivo[36],[37], rapamycin has been reported to be much stronger than dexamethasone in inhibiting the production of IL-17 of peripheral blood mononuclear cells from Vogt-Koyanagi-Harada patients[38].
In summary, rapamycin as an immunosuppressive antibiotic can effectively ameliorate EAU. Its action apparently occurs through the inhibition of pathogenic T cell responses, including Th1/Th2/Th17, and activation of Tregs. Given the success of application of rapamycin in EAU and early clinical studies, we believe that rapamycin is a promising new alternative as an adjunct corticosteroid-sparing agent for treating uveitis. However, our R16-immunized uveitis is a single-phase model and has self-limited nature, which are different from human chronic and recurrence uveitis, and futher study therefore should be conducted with rapamycin on the treatment of recurrent uveitis.
Acknowledgments
Li-Fei Yuan carried out all of the experiments, did statistical analysis, and wrote most parts of the manuscript. Guang-Da Li helped in carrying out the experiments, and wrote some parts of the manuscript. Xin-Jun Ren and Hong Nian gave technique support in experiments. Xiao-Min Zhang conceived and coordinated the study and endorse the data and conclusions. Xiao-Rong Li designed the study.
Foundation: Supported by National Natural Science Foundation of China (No.81371005).
Conficts of Interest: Yuan LF, None; Li GD, None; Ren XJ, None; Nian H, None; Li XR, None; Zhang XM, None.
REFERENCES
- 1.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. 2003;Chapter 15:Unit 15.6. doi: 10.1002/0471142735.im1506s53. [DOI] [PubMed] [Google Scholar]
- 2.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 4.Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, Chen L, Zhou H, Huang X, Kijlstra A. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119(5):1218–1224. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann. N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horai R, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011;31(10):733–744. doi: 10.1089/jir.2011.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Zhang M, Vistica BP, Chan CC, Shen DF, Wawrousek EF, Gery I. Induction of ocular inflammation by T-helper lymphocytes type 2. Invest Ophthalmol Vis Sci. 2002;43(3):758–765. [PubMed] [Google Scholar]
- 9.Ke Y, Jiang G, Sun D, Kaplan HJ, Shao H. Ocular regulatory T cells distinguish monophasic from recurrent autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49(9):3999–4007. doi: 10.1167/iovs.07-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, Yang P, Du L, Zhou H, Ren X, Kijlstra A. Contribution of CD4+CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2010;51(1):383–389. doi: 10.1167/iovs.09-3514. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Ma J, Takeuchi M, Usui Y, Hattori T, Okunuki Y, Yamakawa N, Kezuka T, Kuroda M, Goto H. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of arylhydrocarbon receptor. Invest Ophthalmol Vis Sci. 2010;51(4):2109–2017. doi: 10.1167/iovs.09-3993. [DOI] [PubMed] [Google Scholar]
- 12.Sehgal SN, Baker H, Vezina C. Rapamycin, a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28(10):727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 13.Morris RE, Wu J, Shorthouse R. A study of the contrasting effects of cyclosporine, FK 506, and rapamycin on the suppression of allograft rejection. Transplant Proc. 1990;22(4):1638–1641. [PubMed] [Google Scholar]
- 14.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Roberge FG, Xu D, Chan CC, de Smet MD, Nussenblatt RB, Chen H. Treatment of autoimmune uveoretinitis in the rat with rapamycin, an inhibitor of lymphocyte growth factor signal transduction. Curr Eye Res. 1993;12(2):197–203. doi: 10.3109/02713689308999487. [DOI] [PubMed] [Google Scholar]
- 16.Roberge FG, Kozhich A, Chan CC, Martin DF, Nussenblatt RB, De Smet MD. Inhibition of cellular transfer of experimental autoimmune uveoretinitis by Rapamycin. Ocul Immunol Inflamm. 1993;1(3):269–273. doi: 10.3109/09273949309085028. [DOI] [PubMed] [Google Scholar]
- 17.Shanmuganathan VA, Casely EM, Raj D, Powell RJ, Joseph A, Amoaku WM, Dua HS. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89(6):666–669. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussenblatt RB, Coleman H, Jirawuthiworavong G, Davuluri G, Potapova N, Dahr SS, Ragheb JA, Levy-Clarke G. The treatment of multifocal choroiditis associated choroidal neovascularization with sirolimus (rapamycin) Acta Ophthalmol Scand. 2007;85(2):230–231. doi: 10.1111/j.1600-0420.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips BN, Wroblewski KJ. A retrospective review of oral low-dose sirolimus (rapamycin) for the treatment of active uveitis. J Ophthalmic Inflamm Infect. 2010;1(1):29–34. doi: 10.1007/s12348-010-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mudumba S, Bezwada P, Takanaga H, Hosoi K, Tsuboi T, Ueda K, Kawazu K, Ali Y, Naor J. Tolerability and Pharmacokinetics of Intravitreal Sirolimus. J Ocul Pharmacol Ther. 2012;28(5):507–514. doi: 10.1089/jop.2011.0226. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Ren X, Li G, Jiao C, Zhang L, Zhao S, Wang J, Han ZC, Li X. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest Ophthalmol Vis Sci. 2011;52(6):3143–3152. doi: 10.1167/iovs.10-6334. [DOI] [PubMed] [Google Scholar]
- 22.Servat JJ, Mears KA, Black EH, Huang JJ. Biological agents for the treatment of uveitis. Expert Opin Biol Ther. 2012;12(3):311–328. doi: 10.1517/14712598.2012.658366. [DOI] [PubMed] [Google Scholar]
- 23.Martin DF, DeBarge LR, Nussenblatt RB, Chan CC, Roberge FG. Synergistic effect of rapamycin and cyclosporin A in the treatment of experimental autoimmune uveoretinitis. J Immunol. 1995;154(2):922–927. [PubMed] [Google Scholar]
- 24.Roberge FG, Martin DF, Xu D, Chen H, Chan CC. Synergism between corticosteroids and Rapamycin for the treatment of intraocular inflammation. Ocul Immunol Inflamm. 1995;3(3):195–202. doi: 10.3109/09273949509069112. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda E, Hikita N, Eto K, Mochizuki M. Tacrolimus-rapamycin combination therapy for experimental autoimmune uveoretinitis. Jpn J Ophthalmol. 1997;41(6):396–402. doi: 10.1016/s0021-5155(97)00083-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Wu X, Duan J, Hinrichs D, Wegmann K, Zhang GL, Hall M, Rosenbaum JT. Low dose rapamycin exacerbates autoimmune experimental uveitis. PLoS One. 2012;7(5):e36589. doi: 10.1371/journal.pone.0036589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Ferraro A, Socci C, Stabilini A, Valle A, Monti P, Piemonti L, Nano R, Olek S, Maffi P, Scavini M, Secchi A, Staudacher C, Bonifacio E, Battaglia M. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes. 2011;60(11):2903–2913. doi: 10.2337/db11-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu Q, Cai B, Huang ZC, Shi YY, Wang LL. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32(9):2731–2736. doi: 10.1007/s00296-011-1984-x. [DOI] [PubMed] [Google Scholar]
- 30.Khoury SJ. Th17 and Treg balance in systemic sclerosis. Clin Immunol. 2011;139(3):231–232. doi: 10.1016/j.clim.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7(13):1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91(8):1420–1424. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donia M, Mangano K, Amoroso A, Mazzarino MC, Imbesi R, Castrogiovanni P, Coco M, Meroni P, Nicoletti F. Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmun. 2009;33(2):135–140. doi: 10.1016/j.jaut.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Esposito M, Ruffini F, Bellone M, Gagliani N, Battaglia M, Martino G, Furlan R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J Neuroimmunol. 2010;220(1–2):52–63. doi: 10.1016/j.jneuroim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Yang K, Wen J, Liu X, Kijlstra A, Chen L, Chi W, Zhou H, Huang X, Yang P. Inhibitory effect of rapamycin and dexamethasone on production of IL-17 and IFN-gamma in Vogt-Koyanagi-Harada patients. Br J Ophthalmol. 2009;93(2):249–253. doi: 10.1136/bjo.2008.142489. [DOI] [PubMed] [Google Scholar]