Abstract
AIM
To investigate the pharmacokinetics and distributions of bevacizumab by intravitreal injection of prepared bevacizumab-poly (L-lactic-co-glycolic acid) (PLGA) microspheres in rabbits, to provide evidence for clinical application of this kind of bevacizumab sustained release dosage form.
METHODS
Bevacizumab was encapsulated into PLGA microsphere via the solid-in-oil-in-hydrophilic oil (S/O/hO) method. Fifteen healthy New Zealand albino-rabbits were used in experiments. The eyes of each rabbit received an intravitreal injection. The left eyes were injected with prepared bevacizumab-PLGA microspheres and the right eyes were injected with bevacizumab solution. After intravitreal injection, rabbits were randomly selected at days 3, 7, 14, 28 and 42 respectively, three animals each day. Then we used immunofluorescence staining to observe the distribution and duration of bevacizumab in rabbit eye tissues, and used the sandwich ELISA to quantify the concentration of free bevacizumab from the rabbit aqueous humor and vitreous after intravitreal injection.
RESULTS
The results show that the concentration of bevacizumab in vitreous and aqueous humor after administration of PLGA formulation was higher than that of bevacizumab solution. The T1/2 of intravitreal injection of bevacizumab-PLGA microspheres is 9.6d in vitreous and 10.2d in aqueous humor, and the T1/2 of intravitreal injection of soluble bevacizumab is 3.91d in vitreous and 4.1d in aqueous humor. There were statistical significant difference for comparison the results of the bevacizumab in vitreous and aqueous humor between the left and right eyes (P<0.05). The AUC0-t of the sustained release dosage form was 1-fold higher than that of the soluble form. The relative bioavailability was raised significantly. The immunofluorescence staining of PLGA-encapsulated bevacizumab (b-PLGA) in rabbit eye tissues was still observed up to 42d. It was longer than that of the soluble form.
CONCLUSION
The result of this study shows the beneficial effects of PLGA in prolonging the residency of bevacizumab in the vitreous. And the drug delivery system may have potential as a treatment modality for related disease.
Keywords: bevacizumab-PLGA microspheres, intravitreal injection, sustained release, pharmacokinetic, immunohistochemistry
INTRODUCTION
Anti-vascular endothelial growth factor (VEGF) therapy has been recently established as an effective treatment for subfoveal neovascular age-related macular degeneration (AMD)[1]–[3]. Two anti-VEGF agents, pegaptanib1 (Macugen; Eyetech Pharmaceuticals, Inc., New York, USA) and ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA) have been demonstrated to be effective in treating neovascular AMD[4],[5]. Bevacizumab (Avastin, Genentech/Roche, USA), a humanized monoclonal VEGF antibody derived from the same murine monoclonal antibody as ranibizumab, is approved for intravenous use[6] in the management of colorectal cancer with a molecular weight of 149 kD, indirectly blocking VEGF and its receptors to inhibit the binding angiogenesis, thus inhibiting the formation of new blood vessels and reducing the vascular permeability[7]. Several laboratory and clinical studies have supported the safety and efficacy of intravitreal bevacizumab[8]–[10]. Unfortunately, its effect is short (its intravitreal half-life time for different animal and human studies was measured about 3-5d) and several intravitreal injections are needed to maintain the therapeutic effect[2],[5].
Poly (L-lactic-co-glycolic acid) (PLGA) is one of the most popular and widely used in the research and development of protein or peptide drug delivery system[11],[12]. Successful examples include leuprorelin depot for delivery of leuprolide acetate for 1 to 3mo, nutropin depot for sustained delivery of human growth hormone for 1mo, and Vitrasert™ for long-term intraocular implant of ganciclovir for 5 to 8mo. Hence, using PLGA for intravitreal injection has been proved to be safe and efficient, so the frequency of intravitreal injection has significantly reduced[13].
In current approach, for improvement of drug availability after intravitreal administration, bevacizumab-encapsulated PLGA microsphere as a novel drug delivery system was prepared and compared with conventional formulas in rabbit's eyes. Fifteen healthy New Zealand albino-rabbits were used in experiments. The eyes of each rabbit received an intravitreal injection. The left eyes were injected with prepared bevacizumab-PLGA microspheres and the right eyes were injected with bevacizumab solution. After intravitreal injection, rabbits were randomly selected at day 3, 7, 14, 28 and 42 respectively, three animals each day. The aqueous humor and vitreous of both eyes were aspirated about 0.05 mL respectively to quantify the free bevacizumab concentrations in order to determine the long-acting potential of the compounds.
MATERIALS AND METHODS
Materials
Bevacizumab was from Genentech/Roche Ltd. (San Francisco, CA, USA); bevacizumab enzyme-linked immunosorbent assay (ELISA) kit was from Rapidbio Co. (American); donkey anti-human Cy3-labelled IgG was from Jackson Immuno Research (American); bovine serum albumin (BSA) was from Amersco Co. (American); PLGA (LA/GA=50/50, Mw=14 000) was from Biotechnology Co. Ltd. (Jinan Dai Gang, China); Poly vinyl alcohol(PVA), dichloromethane, propenyl alcohol and polyvinyl alcohol were obtained from Sinopharm Chemical Reagent Co. (China).
Preparation of bevacizumab-encapsulated PLGA microspheres
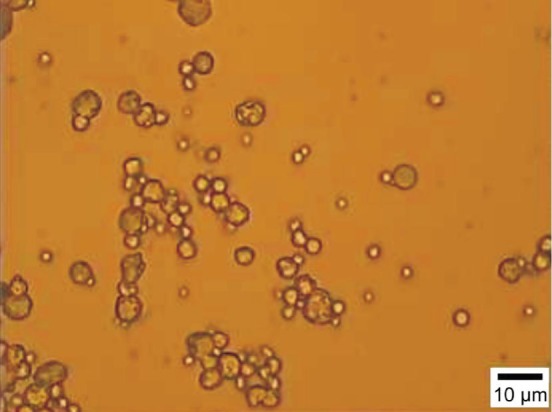
Bevacizumab was encapsulated into PLGA microsphere via the solid-in-oil-in-hydrophilic oil (S/O/hO) method[14]. Briefly, 25 mg dry bevacizumab protein particles were prepared by bevacizumab stock solution (25 mg/mL) through the method of vacuum freeze-drying. And 120 mg PLGA was dissolved in 1.5 mL dichloromethane as the first emulsion. Then, the bevacizumab protein particles were added to the first emulsion while stirring uniformly under 0°C. And then, this emulsion was added to a 5 mL mixed solution (1% PVA+propenyl alcohol+5%NaCl) to undergo second emulsion. Semi-solidified microspheres were formed by high-speed centrifugation (2000 rpm) at 0°C. Finally, the semi-solidified microspheres was added to 300 mL of 10% NaCl solution and was stirred by a low-speed centrifugation (300 rpm) to solidify for three hours at room temperature. The microspheres were separated by centrifugation (500 rpm). The microspheres precipitates washed 5 times using distilled water, freeze dried, and stored under 0°C for further use. The modality of prepared bevacizumab-PLGA microspheres was taken using light microscope and the morphology was shown in Figure 1. From Figure 1 we know that the prepared bevacizumab-PLGA microspheres are smooth sphere with diameters around 2-7 µm.
Figure1. The morphology of prepared bevacizumab-PLGA.
Determination of encapsulation efficiency and drug load
For determination of the encapsulation efficiency and drug load, sandwich ELISA was employed. Twenty millgram of bevacizumab-encapsulated PLGA microspheres was dissolved in 1 mL dichloromethane and then was centrifugation. After discarding the supernatant, the precipitates were washed using dichloromethane for 5 times, 1 mL each time. Finally, the residual precipitates were dissolved in 10 mL PBS buffer solution. From the determination of sandwich ELISA kit, a value of 49% was calculated as the encapsulation efficiency and 98 µg of bevacizumab was loaded in 1 mg of microsphere.
Animal Studies
Approval was obtained from the Institutional Animal Care and Use Committee of Dalian Medical University. The animal research adhered to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Fifteen healthy New Zealand albino-rabbits (weighing 1.8-2.2 kg) were housed in animal facilities (Dalian Medical University) under standard conditions. Topic ofloxacin (Santen Pharmaceutical Co. Ltd., Osaka, Japan) was applied in the day before operation. Each rabbit was anesthetized by ketamine hydrochloride (0.1 mL/kg) through intramuscular injection and fixed on the operating table. Tropicamide (1%, Santen Pharmaceutical Co. Ltd., Osaka, Japan) was applied topically to dilate the pupils. Before injection, 5% povidone iodine was applied around eye for 2min. The eye was topically anesthetized by 0.4% oxybuprocaine hydrochloride (benoxyl; Santen Pharmaceutical Co. Ltd., Osaka, Japan). After placing a lid speculum on the eye, bevacizumab-PLGA microspheres suspension (containing about 1.25 mg bevacizumab) for the left eye and bevacizumab stock solution (1.25 mg/0.05 mL) for the right eye were injected to intravitreal cavity using a 30-gauge needle at 2.5 mm behind the limbus in the supertemporal quadrant. Topical ofloxacin eye drop and ointment were applied daily within 2wk after operation. Before sacrifice, the rabbits were monitored daily for inflammation, corneal opacity, cataract, vitreous opacity and retinal hemorrhage etc.
Each group of rabbit (three rabbits) was sacrificed at day 3, 7, 14, 28, and 42 respectively by intramuscular ketamine hydrochloride overdose. The aqueous humor and vitreous of each eye were withdrawn into a 1 mL syringe, respectively. The samples were labeled and frozen under -80°C immediately. Then the eyes were immediately enucleated at each time point, fixed in 10% formalin.
Immunohistochemistry
The enucleated eyes, which were fixed in 10% formalin, were embedded in paraffin wax, sectioned and deparaffinized according to general procedures. Donkey anti-human IgG labeled with Cy3 (709-166-149, dilution 1:500; Jackson Immuno Research, West Grove, PA, USA) was used for detection of bevacizumab. This polyclonal antibody binds to epitopes of both Fc and Fab portions of human IgG. The sections were embedded and examined with fluorescence microscope (Zeiss Axioplan2 imaging). All photographs were taken with the same camera settings (brightness, contrast, etc.) to allow a comparison of staining intensity between different time points and the control. The intensity of staining was used to observe the distribution of bevacizumab in the eye tissues.
Enzyme-linked Immunosorbent Assay Studies for Bevacizumab
Before analysis, all the frozen aqueous humor and vitreous were defrosted overnight under 4°C, and then determined by sandwich ELISA using an ELISA kit (R&B Co., America) according to the assay procedures. The lowest detect limit of this method was 0.1 ng/mL. All the data were analyzed by t-test.
Calculation of Pharmacokinetic Parameters
Pharmacokinetic parameters were obtained from the free bevacizumab in the aqueous humor and vitreous including T1/2 (half-life time), Cmax, AUC0–t (the area under drug concentration and time curve) and Fr (relative bioavailability). Results were calculated using software (3p97 software) from mean values of three sample data.
RESULTS
All the experimental rabbits were healthy during the experimental procedures. There were no obvious complications in the eyes after intravitreal injection, such as inflammation, corneal opacity, cataract, vitreous opacity, retinal hemorrhage etc.
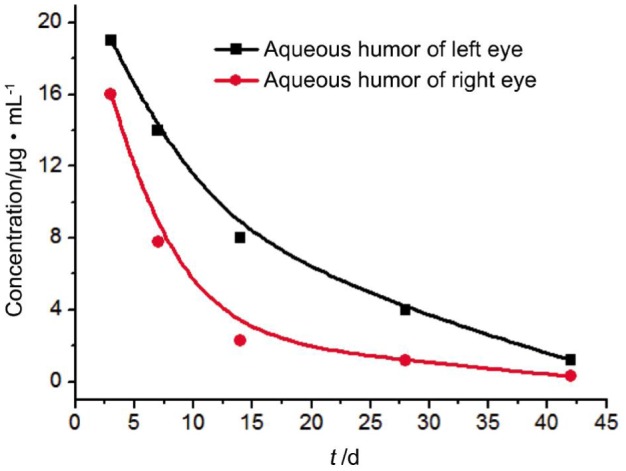
Pharmacokinetic parameters: the vitreous concentration of free bevacizumab reached a peak concentration of 249±0.13 µg/mL (left eyes, received bevacizumab-PLGA microspheres) and 156±0.20 µg/mL (right eyes, received bevacizumab solution) after injection for three days. Vitreous concentration of bevacizumab declined over time. Corresponding concentrations were decreased to 45±0.14 µg/mL and 20±0.23 µg/mL at day 28, and continually decreased to 14±0.22 µg/mL and 3.6±0.19 µg/mL at day 42, respectively. AUC0-t for bevacizumab-PLGA microspheres was 2-fold higher compared with that of bevacizumab solution. These results could be expressed as Figure 2 clearly. The aqueous humor concentrations of bevacizumab also reached a peak concentration of 19±0.22 µg/mL (in left eyes) and 16±0.17 µg/mL (in right eyes) after 3d, respectively. The corresponding values went down to 8±0.17 µg/mL and 2.3±0.04 µg/mL after 14d, and 1.2±0.03 µg/mL and 0.3±0.04 µg/mL after 42d. These results could also be expressed as Figure 3 clearly. The T1/2 of intravitreal injection of bevacizumab-PLGA microspheres is 9.6d in vitreous and 10.2d in aqueous humor, and the T1/2 of intravitreal injection of soluble bevacizumab is 3.91d in vitreous and 4.1d in aqueous humor. There were statistically significant difference for comparison the results of the bevacizumab in vitreous and aqueous humor between the left and right eyes (P<0.05). The AUC0-t of the sustained release dosage form was 1-fold higher than that of the soluble form. The relative bioavailability was raised significantly.
Figure 2. Bevacizumab concentration in the vitreous after intravitreal injection of 1.25 mg of bevacizumab.
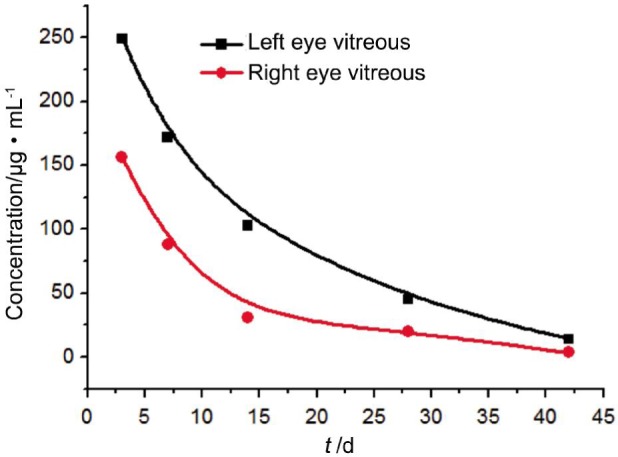
Figure 3. Bevacizumab concentration in the aqueous humor after intravitreal injection of 1.25 mg of bevacizumab.
DISCUSSION
Neovascularization occurs in several ocular diseases, such as exudative AMD, proliferative diabetic retinopathy, and retinopathy of prematurity. One of the major factors inducing formation of new vessels is VEGF, acting via its corresponding receptors during embryonic development and in eye diseases. VEGF therapy has been recently established as an effective treatment for these kinds of diseases. Because of the short half-life time of intravitreal bevacizumab (about 3-5d)[2],[5], multi-intravitreal injections were needed to maintain a therapeutic effect. Unfortunately, repeated intravitreal manipulations may increase the risk of complications and aggravate the pain and economy burden of patients. On the other hand, enhancing the dose in an individual injection to prolong the therapeutic efficacy might expose the patient to toxicity coming from high drug concentration[15],[16]. Many studies demonstrate that chronic retinal neovascularization in diabetics respond to very low dose of bevacizumab (6.2 µg)[17]. So, it is an urgent task to develop a drug delivery system which could provide controllable drug release for treatment of neovascular diseases. It may reduce the frequency and dosage of the drug injection so that to provide convenience for patients as well as doctors.
The use of encapsulated PLGA to produce long-lasting drug concentrations has been proved to be safe and effective over the past few years[13]. The release of the active drug from the polymer mixture dependent on the drug diffusion during PLGA dissolvent. The controllable delivery of drugs with polymers such as implants[18], microspheres[17],[19],[20], nanoparticles[21] and liposomes[18] was widely accepted. An in vivo study in rabbits investigated nanoliposome formulations of bevacizumab and revealed that it could prolong the residency of bevacizumab in the vitreous[18]. Another study investigating an intravitreal injection of poly (ethylene-glycol) (PEG)-bevacizumab conjugate (b-PEG), and PLGA-encapsulated bevacizumab (b-PLGA)[22] has demonstrated that these two kind bevacizumabs could reduce the choroidal neovascularization (CNV) area when laser followed the injection in rat eyes.
In current approach, we use S/O/hO method to synthesize b-PLGA microsphere, use immunofluorescence staining (Figure 4) to observe the distribution and duration of bevacizumab in rabbit eye tissues[23],[24], and use the sandwich ELISA to quantify the concentration of free bevacizumab from the rabbit aqueous humor and vitreous after intravitreal injection[16],[18],[25]. The results show that the concentration of bevacizumab in aqueous humor and vitreous after administration of PLGA formulation was higher than that of bevacizumab solution. The immunofluorescence staining of b-PLGA in rabbit eye tissues was still observed up to 42d. It was longer than that of in soluble form. These results demonstrated that the long-acting effect of bevacizumab formulations compared with control and reduced CNV area[22].
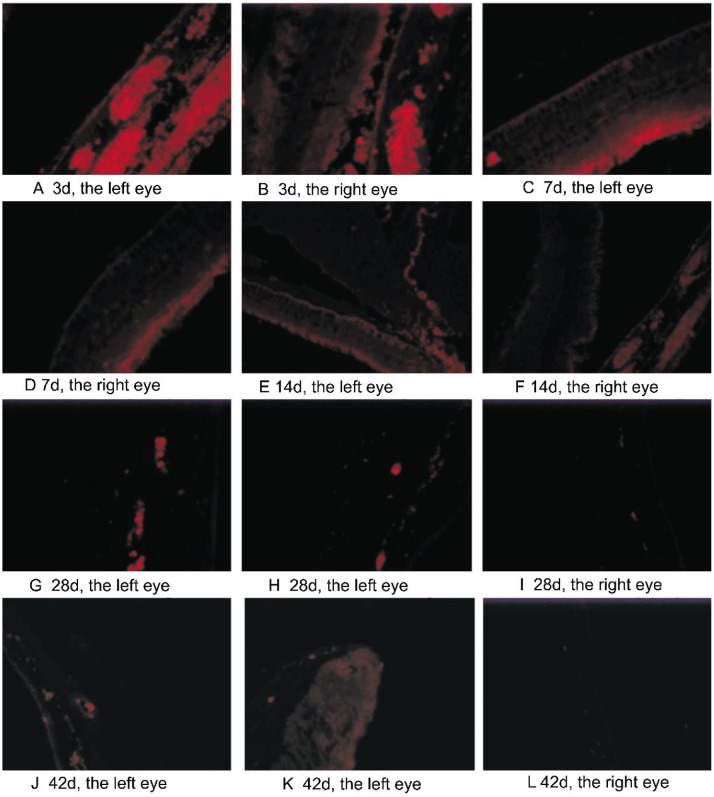
Figure 4. The distribution of bevacizumab in the eye tissues. The immunofluorescence staining can be observed in the retina, choroid, iris, ciliary body and anterior chamber angle, especially in the vascular tissues after intravitreal injection.
Strongest staining was seen at day 3 and day 7 (A-D) in both eyes. The intensity of staining in the left eyes was weakened to bright staining at day 14 and day 28 (E, G). Faint staining could also be detected at day 42 (J, K). Contrastively, in the right eyes, bright staining was seen at day 14 (F) which was weaker than the left eyes. Only very weak staining was detected at day 28 (I), and almost no staining can be seen at day 42 (L).
However, there are still some limitations in our study. First, it is difficult to separate the empty particles completely, which need precisely controlling the microsphere diameter. Second, the damage and denaturation of bevacizumab activity during the synthesis step is unclear. Third, the influence of the PLGA diameter and molecular mass on Drug Controlled Release System need to be further researched.
In conclusion, the clearance of this drug in vitreous from PLGA formulations was slower than the soluble form. Since the eye-ball is a closed organ, novel therapeutic molecules such as bevacizumab for neovascular AMD and diabetic retinopathy, have been investigated. Certainly, we should further consider the most efficacious combinations of optimal dose, route, and drug release pattern according to the targeted disease. Therefore, additional studies with different animal model, dosage, route and other control system are warranted to study.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.30973263, No.81370869)
Conflicts of Interest: Ye Z, None; Ji YL, None; Ma X, None; Wen JG, None; Wei W, None; Huang SM, None.
REFERENCES
- 1.Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I, Loewenstein A. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26(3):262–269. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rich RM, Rosenfeld PJ, Puliafito CA, Dubovy SR, Davis JL, Flynn HW, Jr, Gonzalez S, Feuer WJ, Lin RC, Lalwani GA, Nguyen JK, Kumar G. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006;26(5):495–511. doi: 10.1097/01.iae.0000225766.75009.3a. [DOI] [PubMed] [Google Scholar]
- 3.Bashshur ZF, Haddad ZA, Schakal A, Jaafar RF, Saab M, Noureddin BN. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol. 2008;145(2):249–256. doi: 10.1016/j.ajo.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Beer PM, Wong SJ, Hammad AM, Falk NS, O'Malley MR, Khan S. Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina. 2006;26(8):871–876. doi: 10.1097/01.iae.0000233327.68433.02. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevaeizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 7.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114(5):855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF, Laud K, Fine HF, Klancnik JM, Jr, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL, Cooney MJ. Intravitreal bevacizumab treatment of choroidal neovasularization secondary to age-related macular degeneration. Retina. 2006;26(4):383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 9.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab(Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695–1705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevaeizumab (Avastin) as treatment for subfoveal choroidal ncovaseularisation secondary to pathological myopia. Br J Ophthalmol. 2007;91(2):157–160. doi: 10.1136/bjo.2006.096776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microsphere. Adv Drug Deliv Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 12.Noriyuki Kuno, Hinobu Fujii. Recent advances in ocular drug delivery systems. Polymers. 2011;3:193–221. [Google Scholar]
- 13.Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26(5):1025–1058. doi: 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Jin T. Effect of formulation process of sustained-release PLGA microspheres on the protein activity and immunogenicity. Master Thsis, Shanghai Jiao Tong University. 2008 [Google Scholar]
- 15.Spitzer MS, Wallenfels-Thilo B, Sierra A. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol. 2006;90(10):1316–1321. doi: 10.1136/bjo.2006.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T. Pharmacokinetics of Bevacizumab after Topical, Subconjunctival, and Intravitreal Administration in Rabbits. Invest Ophthalmol Vis Sci. 2009;50(10):4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 17.Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm Res. 2010;27(10):2043–2053. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Chen TN, Chen HL, Wang WJ. Microfabrication of biodegradable (PLGA) honeycomb-structures and potential applications in implantable drug delivery. Sens Actuators B. 2005;106(2):506–551. [Google Scholar]
- 19.Abrishami M, Zarei-Ghanavati S, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh-Nikouei B. Preparation, characterization, and in vivo evalution of nanoliposomes-encapsulated bevacizumab (Avastin) for intravitreal administration. Retina. 2009;29(5):699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Sancho C, Herrero-Vanrell R, Negro S. Vitamin A palmitate and acyclovir biodegradable microspheres for intraocular sustained release. Int J Pharm. 2006;326(1–2):100–106. doi: 10.1016/j.ijpharm.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Moshfeghi AA, Peyman GA. Micro- and nanoparticulates. Adv. Drug Delivery Rev. 2005;57(14):2047–2052. doi: 10.1016/j.addr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Pan CK, Durairaj C, Kompella UB, Agwu O, Oliver SC, Quiroz-Mercado H, Mandava N, Olson JL. Comparison of Long-Acting Bevacizumab Formulations in the Treatment of Choroidal Neovascularization in a Rat Model. J Ocul Pharmacol Ther. 2011;27(3):219–224. doi: 10.1089/jop.2010.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heiduschka P, Fietz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, Ziemssen F, Niggemann B, Julien S, Bartz-Schmidt KU, Schraermeyer U, Tübingen Bevacizumab Study Group Penetration of bevacizumab through the retinal after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007;48(6):2814–2823. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- 24.Peters S, Heiduschka P, Julien S, Bartz-Schmidt KU, Schraermeyer U. Immunohistochemical localization of intravitreally injected bevacizumab in the anterior chamber angle,iris and ciliary body of the primate eye. Br J Ophthalmol. 2008;92(4):541–544. doi: 10.1136/bjo.2007.133496. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Xu JS, Sanders VM, Letson AD, Roberts CJ, Xu RX. Multifunctional microbubbles for image-guided antivascular endothelial growth factor therapy. J Biomed Opt. 2010;15(3):1–3. doi: 10.1117/1.3457669. [DOI] [PubMed] [Google Scholar]