Abstract
AIM
To investigate the expression of complement factors in the posterior scleral fibroblasts of guinea pigs with negative lens-defocused myopia.
METHODS
Eighteen guinea pigs were assigned randomly to two groups: the negative lens-defocused group (NLD group, n=9) and the normal control without treatment group (NC group, n=9). The effect of myopic induction was compared in three subgroups: eyes treated with a -10.00 D negative lens in the NLD group (NL group), eyes treated with a plano (0 D) lens in the NLD group (PL group), and untreated right eyes in the NC group (NC group). The following analyses were conducted at four weeks: examination of the refractive error via retinoscopy, assessment of complement C5b-9 expression in the posterior scleral fibroblasts using immunohistochemistry, and measurements of complement C1q and C3 protein levels in the posterior sclera by Western blot.
RESULTS
After an induction period of four weeks, a significant myopic shift was detected in the eyes of the NL group, relative to that of the PL and NC groups (P<0.05). Data analysis showed a significant increase in the percentage of C5b-9 immunopositive fibroblasts in the posterior sclera of the NL group eyes, compared to the PL group (q=11.50, P<0.001). Significantly higher levels of C1q (q=4.94, P=0.01) and C3 (q=4.07, P=0.03) protein were detected in the posterior sclera of NL group eyes, compared to the PL group. There were no significant difference between the PL and NC groups for C5b-9 (q=2.44, P=0.10), C1q (q=1.55, P=0.53) and C3 (q=0.98, P=0.77) in the posterior sclera.
CONCLUSION
The data from present study provide evidence of the up-regulation of C5b-9, C1q and C3 in the posterior scleral fibroblasts in a NLD myopic animal model. The results suggest that the complement system may be involved in the development of myopia.
Keywords: experimental myopia, complement factors, sclera, inflammation
INTRODUCTION
It is evident that genetic and environmental factors are involved in the development of myopia, however, the exact mechanisms are not yet understood[1]–[3]. Animal models have been valuable in investigating the mechanisms involved in the development of myopia. We have previously used negative lens-defocus to successfully induce axial myopia in newborn guinea pigs[4],[5]. Numerous studies have shown that degradation of the visual input using form deprivation or lens defocus can induce axial myopia during the early postnatal period[6]–[8]. During this process, the retina is thought to initiate signal transduction triggered by blurred vision; however, the sclera ultimately facilitates ocular elongation and myopia[4]–[8]. Theoretically, the factors that affect the function of the sclera, more specifically the posterior sclera, may modulate the induction of myopia[9],[10].
We have previously reported that serum concentrations of high sensitivity C-reactive protein (hs-CRP) and complement C3 and CH50 were significantly higher in pathological myopia (PM) patients than in age- and gender-matched normal controls. This suggests that myopia may share features similar to those seen in autoimmune diseases and may represent a state of low-grade systemic or local inflammation[11]. Based on these findings, we hypothesize that the complement system may also contribute to the transformation of the sclera during the development of myopia.
Activation of the complement cascade occurs by three major pathways: classical (triggered by C1q activation), lectin and alternative. This leads to the production of complement C3b, a central component in the complement system, that then joins other complement factors to form C5b, which along with C6, C7, C8 and C9 (referred to as C5b-9), forms the membrane attack complex (MAC), eventually triggering cell lysis or a sublytic attack[12]–[14].
To our knowledge, the relationship between the above complement factors and experimental myopia has not been previously reported. Therefore, the aim of our study is to investigate the expression of complement factors in the posterior sclera of experimentally-induced myopia. The structural organization of the sclera depends largely on the cellular activity of fibroblasts[15],[16]. To test our hypothesis, we examined the expression of complement factors C1q, C3 and C5b-9 in posterior scleral fibroblasts.
MATERIALS AND METHODS
Animals
Eighteen pigmented guinea pigs (Cavia porcellus, approximately 3 days old, weighing 70-90 g) were purchased from the animal center at the Chinese Academy of Medical Sciences (Beijing, China). All animals were treated according to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research.
Materials
Custom poly (methyl methacrylate) (PMMA) concave lenses were purchased from Beijing Jingdejiarun Contact Lenses Co. (Beijing, China). The -10.00 diopter (D) lens (confirmed by lensometer) parameters were as follows: overall diameter (including the rim of the lens), 17.00 mm; optical diameter: 11.5 mm; inside optical curve radius, 7.50 mm; outside optical curve radius, 8.927 mm. The plano (0 D) lenses (confirmed by lensometer) parameters were as follows: overall diameter, 17.00 mm; optical diameter: 11.5mm; inside optical curve radius, 7.50 mm; outside optical curve radius, 7.642 mm. There were 6 holes in the rim of each lens enabling suturing to the skin around the eye of the guinea pigs.
Myopic Induction and Ocular Measurement
The guinea pigs were assigned randomly to two groups: the negative lens-defocused group (NLD group, n=9) and the normal control group (NC group, n=9). Animals in the NLD group were treated for four weeks with -10.00 D lenses fitted over one eye and plano (0 D) lenses fitted over the contralateral eye, while the animals in the NC group received no treatment. The effect of myopic induction was compared in three subgroups: the eyes treated with a negative lens in the NLD group (NL group), the eyes treated with a plano (0 D) lens in the NLD group (PL group), and the right eyes in the NC group (NC group). During the experimental period, measurements were taken to prevent form deprivation, such as keeping the lens clean at all times. All animals underwent cycloplegic ocular refraction measurement using streak retinoscopy (Suzhou Medical Equipment Factory, Suzhou, Jiangsu Province, China), prior to the experiment and four weeks after induction.
Immunohistochemistry
At 4wk, guinea pigs were sacrificed by intraperitoneal injection of a lethal dose of thiopental. Both eyes of the guinea pigs in the NLD group (n=6) and right eyes of the guinea pigs in the NC group (n=6) were enucleated, and the remaining muscle and connective tissue were carefully removed. The eyeballs were fixed in 10% formalin at room temperature for 4h, and paraffin sections (3 µm) were cut on a microtome. After deparaffinization and rehydration, antigen retrieval was performed using the microwave oven method with a working solution from BD Pharmingen. Nonspecific binding was prevented by incubating the section with 1% goat serum in PBS for 1h at 37°C, followed by incubation with the primary antibody C5b-9 (1:100 dilution; Calbiochem/204903) overnight at 4°C. After washing, the sections were incubated with the following agents: biotinylated secondary antibodies for 30min at 37°C, Avidin-Biotin Complex reagent for 30min at 37°C, and DAB substrate for 10min. Counterstaining was performed with hematoxylin for 2min. Slides were then dehydrated in gradient alcohol washes, cleared with Xylene, and mounted for analysis. Sections without incubation with the primary antibody were used as negative control. Staining was examined by microscopy (Zeiss) equipped with a digital camera (Nikon). Data analysis of C5b-9 expression was performed by counting the number of immunopositive posterior scleral fibroblasts on each slide using a high powered lens (LM ×400). The data for each eye were calculated by averaging three non-overlapping visual fields in the posterior sclera area. All images were captured using the same light filter settings.
Western Blot
At 4wk, the eyeballs were obtained as mentioned above. After removing the surrounding connective tissue, the eyeballs were sectioned with a razor blade and the anterior half discarded. The vitreous body, retina, pigment epithelium and choroid were peeled off on ice. The posterior scleral tissue was snap frozen in liquid nitrogen, followed by homogenization. Lysis buffer was added and the samples were centrifuged for 20min at 12 000 rpm at 4°C in a microcentrifuge. The tubes were removed from the centrifuge and placed on ice. The supernatant was transferred into a fresh tube on ice and the pellet was discarded. The protein concentration was determined using the bicinchoninic acid method (Pierce, Rockford, IL, USA). Fifty micrograms of total protein per sample were denatured in SDS with a reducing agent. The samples were loaded into 15% SDS-polyacrylamide gels and subsequently electrophoretically transferred to nitrocellulose membranes in blotting buffer using an 80 V current applied for 60min. Blots were washed for 15min in PBS containing 0.05% Tween 20 (PBST), pre-incubated with blocking solution (5% non-fat milk powder in PBST), washed three times with PBST, and subsequently incubated with primary antibodies, anti-C1q antibody (1:50 dilution; Abcam/ab71940) or anti-C3 antibody (1:50 dilution; Abcam/ab11887) at 4°C overnight. After washing, membranes were incubated with horseradish peroxidase-conjugated goat anti mouse secondary antibody for 1h with agitation and then washed four times for 5min in PBST. Antibody binding was visualized using enhanced chemoluminescence reagents (Amersham Biosciences), exposed on Kodak X-OMAT-R films (Rochester, NY, USA) and quantified using the Image J2×2.1.4.7 software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). Protein loading was assessed by probing the blot for glyceraldehyde phosphate dehydrogenase (GAPDH) at 1:2000 dilution (Proteintech). The densitometry showed no significant differences in loading between the various lanes. The molecular mass of specific bands was determined using the BenchMark pre-stained protein ladder (Invitrogen) electrophoresed alongside the experimental samples.
Statistical Analysis
Data were analyzed using SPSS 11.5 for Windows statistical software (SPSS, Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Between-group analyses of immunostaining data were carried out utilizing one-way analysis of variance (ANOVA) and Student-Newman-Keuls-q test (SNK-q). Numerical variables were expressed as average and standard deviation (mean±SD). A P<0.05 was considered statistically significant.
RESULTS
Myopic Shift in Negative Lens-defocused Eye of the Negative Lens-defocused Group
No significant differences were observed for the refractive power between eyes in the NL, PL and NC groups before treatment (one-way ANOVA, F=1.50, P>0.05). After four weeks of induction, the refractive error in the eyes of the NL, PL and NC groups were (-1.61±0.49) D, (4.36±1.08) D and (4.78±0.98) D, respectively. A significant myopic shift was detected in eyes of the NL group, relative to that of the PL group (one-way ANOVA, q=20.16, P<0.001). There was no significant difference in refractive error between eyes of the PL and NC groups (one-way ANOVA, q=1.41, P=0.59). This is consistent with the findings of our previous studies[8],[9].
Increased C5b-9 Immunostaining in the Posterior Scleral Fibroblasts of Negative Lens-defocused Eye
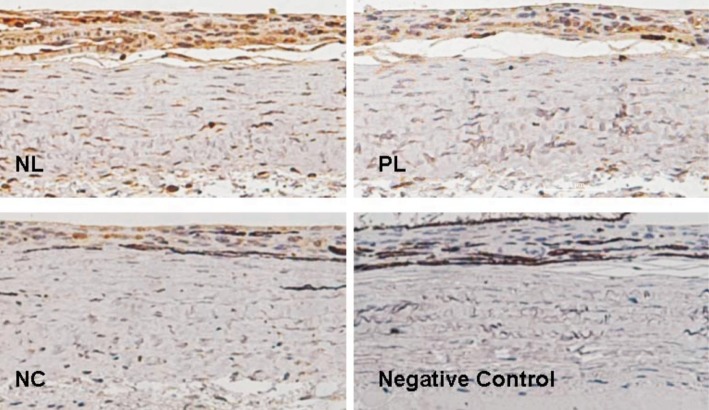
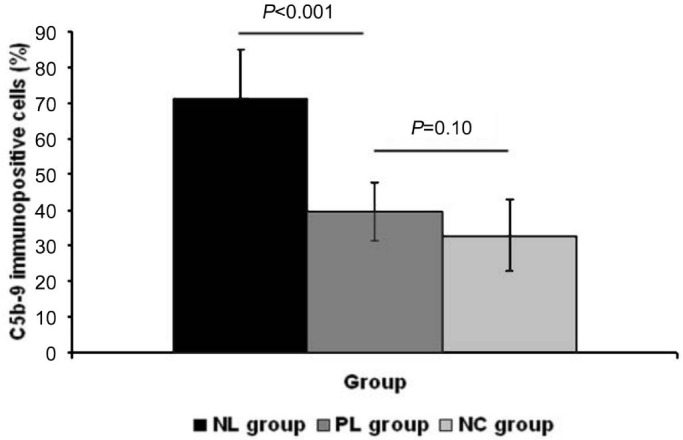
C5b-9 positive cells showed DAB positive brown staining, while negative cells and negative controls were stained with the hematoxylin counterstain only. Immunostaining for C5b-9 revealed little cellular labeling in the posterior sclera of the PL and NC group eyes. However, in the posterior sclera of the NL group eyes, immunopositive staining of C5b-9 was present in many scleral fibroblasts (Figure 1). Data analysis showed a significantly increased percentage of C5b-9 immunopositive fibroblasts in the posterior sclera of NL group eyes, compared to those of the PL group (71.11%±14.10% vs 39.44%±11.62%, one-way ANOVA, q=11.50, P<0.001). The percentage of C5b-9 immunopositive fibroblasts revealed no significant difference between the PL and NC groups (39.44%±11.62% vs 32.78%±8.26%, one-way ANOVA, q=2.44, P=0.10; Figure 2).
Figure 1. C5b-9 immunostaining in the posterior sclera.
Positive cells showed brown staining. Immunopositive staining of C5b-9 was increased in the posterior sclera of NL group eyes, compared to those of the PL and NC groups; no C5b-9 staining was observed when the primary antibody was omitted (negative control).
Figure 2. Data analysis of C5b-9 staining.
C5b-9 staining intensity showed significantly elevated C5b-9 expression in NL group eyes; the staining intensity revealed no significant difference between the PL and NC groups. Magnification: 200×, n=6.
Elevated C1q and C3 Expression in the Posterior Sclera of Negative Lens-defocused Eye
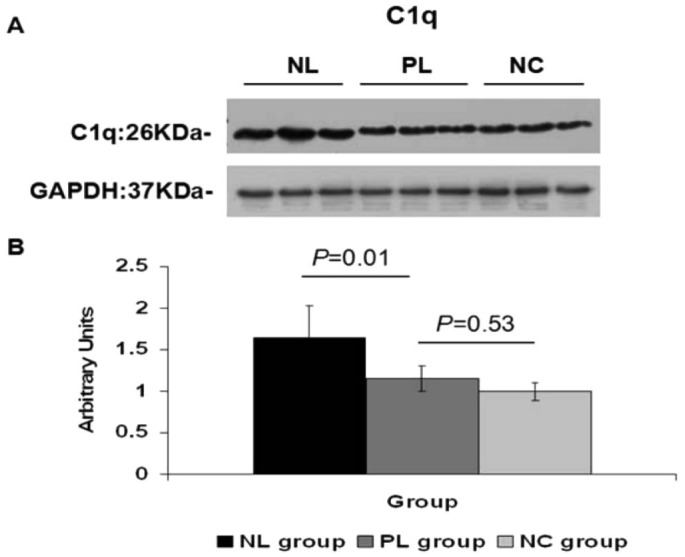
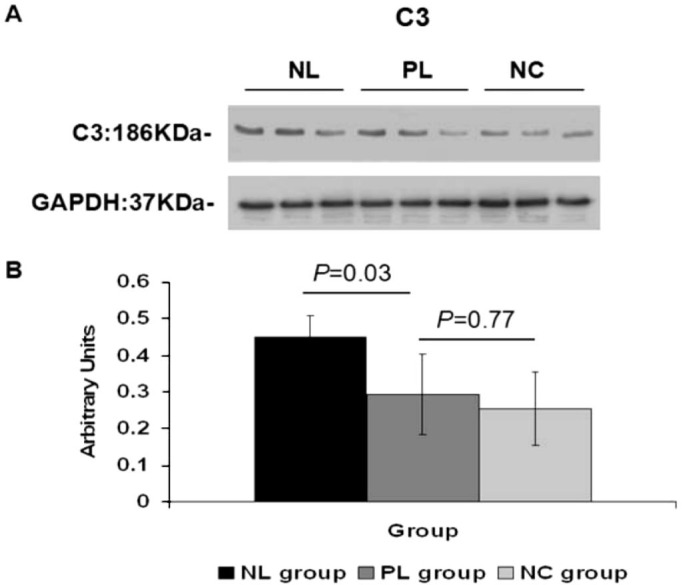
The target bands for C1q and C3 were 26 KDa and 186 KDa, respectively. At four weeks, the densitometric analysis of Western blots showed significantly higher levels of C1q and C3 protein in the posterior sclera of NL group eyes, compared to those of the PL group (C1q:1.65±0.39 vs 1.15±0.15, one-way ANOVA, q=4.94, P=0.01; C3:0.45±0.06 vs 0.29±0.11, one-way ANOVA, q=4.07, P=0.03). No statistical difference in C1q and C3 protein expression was shown between the PL and NC groups in the posterior sclera (C1q:1.15±0.15 vs 0.99±0.10, one-way ANOVA, q=1.55, P=0.53; C3:0.29±0.11 vs 0.25±0.10, one-way ANOVA, q=0.98, P=0.77). A strong band at 37 kDa for GADPH indicated equal amounts of protein in each lane (Figures 3, 4).
Figure 3. C1q increase after negative lens-defocusing for four weeks.
A: Western blot showed elevated C1q in the posterior sclera of NL group eyes, compared to those of the PL and NC groups; B: Graph shows 1.4-fold increased C1q intensity in NL group eyes compared to PL group eyes, as quantified using Image J2×2.1.4.7 software (n=6).
Figure 4. C3 increase after negative lens-defocusing for four weeks.
A: Western blot shows elevated C3 in the posterior sclera of NL group eyes, compared to those of the PL and NC groups; B: Graph shows a 1.5-fold increase in C3 intensity in NL group eyes compared to PL group eyes (n=6).
DISCUSSION
The purpose of this study was to investigate the expression of complement factors in the posterior scleral fibroblasts of guinea pigs with negative lens-defocused myopia. Our results showed that C5b-9 immunostaining was elevated in the posterior scleral fibroblasts of the NL group eyes. This was accompanied by an increase in the expression of proteins C1q and C3 in the posterior sclera of NL group eyes, compared to the PL and NC groups. This corroborates our previous hypothesis that the complement system may be involved in the deformation of the sclera during the development of myopia.
The complement system is an important component of innate immunity and consists of approximately 30 fluid-phase cell-membrane proteins. It plays a key role in driving the immune system and triggering inflammatory responses[17],[18]. Some evidence has suggested a possible role of the complement system in the pathogenesis of myopia. There is a broad consensus that the development of myopia is associated with active remodeling of the scleral extracellular matrix (ECM), evident by the thinning of the posterior sclera[19],[20]. Different aspects have been studied in the search for factors involved in scleral ECM remodeling. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are considered to be the most important enzymes involved in the degradation of the ECM and have been proven to regulate the remodeling of the sclera[5],[21],[22]. Besides MMPs, some growth factors, such as transforming growth factor β (TGF-β) and basic fibroblast growth factor (bFGF), have also been reported to play important roles in the pathogenesis of myopia by modulating the function of either MMPs or scleral fibroblasts[23],[24]. Studies have shown that the complement factors can affect the expression of MMPs, TIMPs, TGF-β and bFGF, which subsequently act on ECM remodeling[25]–[28]. Furthermore, a number of studies have shown associations between the complement system and ECM-related systemic and ocular disorders, such as coronary artery disease, systemic lupus erythematosus (SLE), age-related macular degeneration (AMD) and glaucoma[29]–[32]. Therefore, our current findings, which reveal elevated complement factors in the posterior scleral fibroblasts of lens-defocused myopia indicate the complement system may play a role in the mechanism that cause myopia.
C5b-9, also known as MAC, is a terminal complex of the complement cascade. Its assembly is considered to be a hallmark feature of innate immunity and the inflammatory response[14]. Although it is capable of inducing cell lysis in many nucleated cell types, C5b-9 formation can also activate various signaling pathways and inflammatory processes without cell lysis (sub-lytic)[33],[34]. It has previously been reported that sub-lytic C5b-9 can generate a pro-inflammatory microenvironment to promote the expression of MMP-2 and MMP-9[35]. As discussed above, an increase in metalloproteinases may lead to a shift in ECM composition. Since C5b-9 is a compound of C5b, C6, C7, C8 and C9 with different molecular weights, we used immunohistochemistry instead of Western blot to detect expression. In this study, we found significantly higher levels of C5b-9 immunostaining in the posterior sclera of the NL group eyes, compared to controls. This provides evidence that complement activation occurs during the development of induced myopia and may serve as a role in the active remodeling of the posterior scleral ECM.
Another important finding that supports the correlation between complement activation and myopic induction is the significant increase in C3 protein levels in the posterior sclera of NL induced eyes, which accompanies the elevated C5b-9 staining. As mentioned above, the complement system can be activated via three different pathways: the classical, lectin or alternative pathway. Each pathway eventually leads to the activation of a central protein, C3[13]. To date, attention has been focused on inhibiting the complement system by suppressing the activation of C3, thereby limiting local MAC production[36],[37]. Therefore, our findings not only indicate a possible role of complement activation in the development of induced myopia, but also provide an insight into future therapies for clinical myopia.
Although the elevated expression of C5b-9 and C3 in the posterior sclera of induced myopic eyes suggests an association between the complement system and scleral ECM remodeling, the precise mechanisms involved are not clearly defined. To address this issue, we investigated C1q, an initiating component of the classical complement cascade that belongs to a family of proteins called defense collagens[12],[38]. It has been shown to serve as a bridging molecule to enhance the phagocytosis of antigen-antibody complexes and apoptotic cells through its collagen-like domain[39],[40]. In addition, C1q plays an important role in mediating the adhesion of fibroblasts to ECM proteins such as collagen and fibronectin[41],[42]. Evidence indicates that patients with high myopia have elevated serum levels of circulating immune complexes and autoantibodies to collagen[43],[44]. This may explain our finding of increased C1q expression in the posterior sclera of myopia induced eyes, suggesting that the classical pathway may, at least in part, contribute to scleral ECM remodeling.
In summary, the results from our in vivo studies provide preliminary evidence showing an increase in complement in the sclera of myopic eyes. The data suggest that the complement system may be involved in the development of myopia and would be further confirmed by enlarging the sample size in a future study. As a powerful defense mechanism of the immune system, complement activation is a double-edged sword. Not only can it damage host tissue by causing cell lysis or sublytic attack, it can also promote the clearance of antigen-antibody complexes and enhance cell survival. Our observations support and encourage further investigation into the dynamic changes within the complement system during the various stages of myopic induction and suggest using specific complement inhibitors to elucidate the relationship between complement factors and negative lens-defocused myopia. A thorough understanding of these changes could lead to potential complement-targeted treatments in clinical myopia to ensure a balance between the helpful and harmful activities of the complement system.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No.81070755).
Conflicts of Interest: Gao TT, None; Long Q, None; Yang X, None.
REFERENCES
- 1.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Op. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Maduka Okafor FC, Okoye OI, Eze BI. Myopia: a review of literature. Niger J Med. 2009;18(2):134–138. doi: 10.4314/njm.v18i2.45051. [DOI] [PubMed] [Google Scholar]
- 3.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 4.Long Q, Ai FR, Li Y. Collagen density and morphologic change of sclera in guinea pigs with negative lens-defocused myopia. Chin Opthal Res. 2009;27(6):449–452. [Google Scholar]
- 5.Yang SR, Ye JJ, Long Q. Expressions of collagen, matrix metalloproeinases-2, and tissue inhibitor of matrix metalloproteinase-2 in the posterior sclera of newborn guinea pigs with negative lens-defocused myopia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32(1):55–59. doi: 10.3881/j.issn.1000-503X.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Amedo AO, Norton TT. Visual guidance of recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Ophthalmic Physiol Opt. 2012;32(2):89–99. doi: 10.1111/j.1475-1313.2011.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Hung LF, Smith EL. Effects of foveal ablation on the pattern of peripheral refractive errors in normal and form-deprived infant rhesus monkeys (Macaca mulatta) Invest Ophthalmol Vis Sci. 2011;52(9):6428–6434. doi: 10.1167/iovs.10-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Q, Chen DH, Chu RY. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol. 2009;28(4):176–180. doi: 10.3109/15569520903178364. [DOI] [PubMed] [Google Scholar]
- 9.Ritchey ER, Zelinka C, Tang J, Liu J, Code KA, Petersen-Jones S, Fischer AJ. Vision-guided ocular growth in a mutant chicken model with diminished visual acuity. Exp Eye Res. 2012;102:59–69. doi: 10.1016/j.exer.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBrien NA, Jobling AI, Truong HT, Cottriall CL, Gentle A. Expression of muscarinic receptor subtypes in tree shrew ocular tissues and their regulation during the development of myopia. Mol Vis. 2009;15:464–475. [PMC free article] [PubMed] [Google Scholar]
- 11.Long Q, Ye J, Li Y, Wang S, Jiang Y. C-reactive protein and complement components in patients with pathological myopia. Optom Vis Sci. 2013;90(5):501–506. doi: 10.1097/OPX.0b013e31828daa6e. [DOI] [PubMed] [Google Scholar]
- 12.Nayak A, Pednekar L, Reid KB, Kishore U. Complement and non-complement activating functions of C1q: a prototypical innate immune molecule. Innate Immun. 2012;18(2):350–363. doi: 10.1177/1753425910396252. [DOI] [PubMed] [Google Scholar]
- 13.Ricklin D. Manipulating the mediator: modulation of the alternative complement pathway C3 convertase in health, disease and therapy. Immunobiology. 2012;217(11):1057–1066. doi: 10.1016/j.imbio.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosbrink M, Niculescu F, Rus H. The role of c5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol Res. 2005;31(1):37–46. doi: 10.1385/IR:31:1:37. [DOI] [PubMed] [Google Scholar]
- 15.Christian PG, Harkin DG, Rayner C, Schmid KL. Comparative effects of posterior eye cup tissues from myopic and hyperopic chick eyes on cultured scleral fibroblasts. Exp Eye Res. 2013;107:11–20. doi: 10.1016/j.exer.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Chen BY, Ma JX, Wang CY, Chen WY. Mechanical behavior of scleral fibroblasts in experimental myopia. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):341–348. doi: 10.1007/s00417-011-1854-y. [DOI] [PubMed] [Google Scholar]
- 17.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trouw LA, Daha MR. Role of complement in innate immunity and host defense. Immunol Lett. 2011;138(1):35–37. doi: 10.1016/j.imlet.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Long Q, Chu RY. Recent findings on scleral extracellular matrix and matrix metalloproteinases in the development of myopia. Zhonghua Yan Ke Za Zhi. 2005;41(11):1047–1049. [PubMed] [Google Scholar]
- 20.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86(1):E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 21.Frost MR, Norton TT. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci. 2012;53(1):322–336. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46(10):3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schippert R, Brand C, Schaeffel F, Feldkaemper MP. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res. 2006;82(4):710–719. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen BY, Wang CY, Chen WY, Ma JX. Altered TGF-β2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1133–1144. doi: 10.1007/s00417-013-2269-8. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179(2):838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, Maurer G, Huber K, Badimon JJ, Wojta J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2011;25(1):35–44. doi: 10.1096/fj.10-156083. [DOI] [PubMed] [Google Scholar]
- 27.Qing X, Koo GC, Salmon JE. Complement regulates conventional DC-mediated NK-cell activation by inducing TGF-β1 in Gr-1+ myeloid cells. Eur J Immunol. 2012;42(7):1723–1734. doi: 10.1002/eji.201142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakiyama H, Kaji K, Nakagawa K, Nagino K. Inhibition of bFGF activity by complement C1s: covalent binding of C1s with bFGF. Cell Biochem Funct. 1998;16(3):159–163. doi: 10.1002/(SICI)1099-0844(199809)16:3<159::AID-CBF779>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Széplaki G, Varga L, Füst G, Prohászka Z. Role of complement in the pathomechanism of atherosclerotic vascular diseases. Mol Immunol. 2009;46(14):2784–2793. doi: 10.1016/j.molimm.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Mevorach D. Clearance of dying cells and systemic lupus erythematosus: the role of C1q and the complement system. Apoptosis. 2010;15(9):1114–1123. doi: 10.1007/s10495-010-0530-8. [DOI] [PubMed] [Google Scholar]
- 31.Khandhadia S, Cipriani V, Yates JR, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2012;217(2):127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Ren L, Danias J. A role for complement in glaucoma? Adv Exp Med Biol. 2010;703:95–104. doi: 10.1007/978-1-4419-5635-4_7. [DOI] [PubMed] [Google Scholar]
- 33.Niculescu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24(2):191–199. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- 34.Cybulsky AV, Takano T, Papillon J, Bijian K, Guillemette J. Activation of the extracellular signal-regulated kinase by complement C5b-9. Am J Physiol Renal Physiol. 2005;289(3):F593–603. doi: 10.1152/ajprenal.00066.2005. [DOI] [PubMed] [Google Scholar]
- 35.Lueck K, Wasmuth S, Williams J, Hughes TR, Morgan BP, Lommatzsch A, Greenwood J, Moss SE, Pauleikhoff D. Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration. Eye (Lond) 2011;25(8):1074–1082. doi: 10.1038/eye.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potempa M, Potempa J, Kantyka T, Nguyen KA, Wawrzonek K, Manandhar SP, Popadiak K, Riesbeck K, Eick S, Blom AM. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5(2):e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(7):3056–3064. doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44(1–3):33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Galvan MD, Greenlee-Wacker MC, Bohlson SS. C1q and phagocytosis: the perfect complement to a good meal. J Leukoc Biol. 2012;92(3):489–497. doi: 10.1189/jlb.0212099. [DOI] [PubMed] [Google Scholar]
- 40.Daha NA, Banda NK, Roos A, Beurskens FJ, Bakker JM, Daha MR, Trouw LA. Complement activation by (auto-) antibodies. Mol Immunol. 2011;48(14):1656–1665. doi: 10.1016/j.molimm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 41.Bing DH, Almeda S, Isliker H, Lahav J, Hynes RO. Fibronectin binds to the C1q component of complement. Proc Natl Acad Sci U S A. 1982;79(13):4198–4201. doi: 10.1073/pnas.79.13.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordin S, Ghebrehiwet B, Page RC. Participation of C1q and its receptor in adherence of human diploid fibroblast. J Immunol. 1990;145(8):2520–2526. [PubMed] [Google Scholar]
- 43.Lazuk AV, Slepova OS. Study of immune reactions to collagen in patients with myopia. Vestn Oftalmol. 1995;111(2):14–16. [PubMed] [Google Scholar]
- 44.Stukalov SE, Shchepetneva MA, Kurolap SA. Clinical-immunological and epidemiological studies in high complicated myopia. Vestn Oftalmol. 1995;111(2):16–18. [PubMed] [Google Scholar]