Abstract
AIM
To evaluate and compare aspheric toric intraocular lens (IOL) implantation and aspheric monofocal IOL implantation with limbal relaxing incisions (LRI) to manage low corneal astigmatism (1.0-2.0 D) in cataract surgery.
METHODS
A prospective randomized comparative clinical study was performed. There were randomly recruited 102 eyes (102 patients) with cataracts associated with corneal astigmatism and divided into two groups. The first group received toric IOL implantation and the second one monofocal IOL implantation with peripheral corneal relaxing incisions. Outcomes considered were: visual acuity, postoperative residual astigmatism, endothelial cell count, the need for spectacles, and patient satisfaction. To determine the postoperative toric axis, all patients who underwent the toric IOL implantation were further evaluated using an OPD Scan III (Nidek Co, Japan). Follow-up lasted 6mo.
RESULTS
The mean uncorrected distance visual acuity (UCVA) and the best corrected visual acuity (BCVA) demonstrated statistically significant improvement after surgery in both groups. At the end of the follow-up the UCVA was statistically better in the patients with toric IOL implants compared to those patients who underwent implantation of monofocal IOL plus LRI. The mean residual refractive astigmatism was of 0.4 D for the toric IOL group and 1.1 D for the LRI group (P<0.01). No difference was observed in the postoperative endothelial cell count between the two groups.
CONCLUSION
The two surgical procedures demonstrated a significant decrease in refractive astigmatism. Toric IOL implantation was more effective and predictable compared to the limbal relaxing incision.
Keywords: low corneal astigmatism, toric intraocular lenses, limbal relaxing incisions, cataract surgery, visual acuity
INTRODUCTION
An important aim of cataract surgery has always been a good postoperative visual outcome[1]. It is estimated that approximately 50% of the population over 60y of age has more than 1.0 dioptre (D) of astigmatism and that up to 22% of cataract surgery candidates have pre-existing astigmatism exceeding 1.50 D[1]–[5]. Patients with pre-existing astigmatism of more than 1.00 D may benefit from surgical correction during cataract surgery, in the hope of improving uncorrected visual acuity as well as less image distortion from corneal aberrations[5]–[15].
The limbal relaxing incisions (LRI) technique involves the placement of incisions corresponding to the steep meridian, resulting in corneal flattening and the reduction of astigmatic power. LRI is a safe and inexpensive procedure, simple to perform in expert hands, effective in reducing astigmatism up to 4.0 D and resulting in rapid visual rehabilitation. Recent studies have shown that toric intraocular lens (IOL) improve the predictability of functional results and are associated with limited complications such as inadequate stability of the IOL and biometric error of lens power[1],[16]–[18]. Consequently, toric IOLs seem to achieve high performance in both cases of moderate (1.00-3.00 D) and high (>4.00 D) corneal astigmatism[15]–[20].
These results notwithstanding, corneal astigmatism correction still remains controversial because of the limits connected to the different surgical approaches used. In particular the management of low corneal astigmatism (1.0-2.0 D) opened the possibility of choosing both LRI and toric IOL, depending on the experience of the surgeon and the financial budget of the facility. The aim of this study was to evaluate toric IOL implantation and aspheric monofocal IOL implantation with LRI to manage corneal astigmatism of 1.0-2.0 D during cataract surgery. The study compared the effectiveness, predictability and safety of both techniques in a group of eye with low astigmatism, similar corneal topographic aspect and biometric features. Outcomes included visual and refractive results with specific attention to the residual refractive astigmatism. Follow-up lasted six months.
SUBJECTS AND METHODS
This prospective randomized comparative clinical study included patients having cataract and preoperative anterior corneal astigmatism between 1.0 and 2.0 D.
Patients who had ophthalmologic exams at University Eye Clinic of Trieste between January and June 2013 were included in the study. The inclusion criteria for enrolment of patients were: significant cataract (II-IV group LOCS III- The Lens Opacities Classification System III[21]), regular corneal astigmatism (1.0-2.0 D), with-the-rule (WTR) astigmatism, mean axial length 23-24 mm ±0.81, regular and symmetric astigmatism shape at the corneal topographic map, regular and WTR astigmatism of the posterior corneal surface, pharmacologic mydriasis >6.00 mm diameter to allow intraoperative and postoperative visualization of axis marks on the toric IOLs. The exclusion criteria were: previous surgery in the eye under study, irregular astigmatisms of the anterior or the posterior corneal surfaces, against-the-rule (ATR) astigmatism, ocular diseases (pupil or zonular abnormalities, corneal scaring, uveitis, glaucoma, neuro-ophthalmic diseases, significant macular disease or other retinopathy).
The inclusion criteria were specifically strict in order to select similar topographical and biometrics features in all patients studied to have the more similar comparison between the two surgical techniques.
All patients provided written informed consent before surgery in accordance with the Declaration of Helsinki and institutional review board approval was obtained from the hospital ethics committee.
Patients were randomly assigned to one of the two treatments via computer. A randomized number was assigned to each patient when the inclusion and exclusion criteria were satisfied. The patients were randomly divided into two groups which received either toric IOL (AcrySof® IQ Toric IOL, Alcon Inc.) or monofocal IOL (AcrySof® IQ Aspheric IOL, Alcon Inc.) associated with LRI.
Patients had to complete a preoperative ophthalmic evaluation including uncorrected distance visual acuity (UDVA) and best corrected visual acuity (BCVA), slit lamp examination, applanation tonometry and fundoscopy. Keratometry, biometry (IOL Master, Carl Zeiss Meditec), corneal topography (CSO Eye Topographer, Florence, Italy), corneal tomography (CSO Sirius, Florence, Italy) were obtained.
The same experienced surgeon (Tognetto D) performed all surgeries using topical anaesthesia.
The size and location of the LRI according to the Nichamin nomogram[22],[23] were recorded in the surgical plan for each case. The procedures were performed using topical anaesthesia. Patients were instructed to fix their gaze on a microscope light. Prior to surgery, the steep meridian was identified with a surgical marking pen. Based on the procedure described by Langerman[24], a vertical limbal relaxing wound was created with a guarded micrometer diamond blade by making a groove concentric to the limbus. The incision depth was set at 600 µm equal to approximately 85% of the peripheral corneal thickness at the axis to be cut and the incisions were approximately a length of 3 mm. After the paired incision was made, the penetrating clear corneal incision was made along the steepest axis in the upper area for the cataract surgery, along the same axis as the LRI. All monofocal IOLs (AcrySof® IQ Aspheric IOL, Alcon Inc.) were calculated with emmetropia as the goal.
Toric IOL cylinder power and axis placement were determined using the IOL manufacturer's online calculator (Acrysof toric IOL Calculator). Biometry, keratometry, incision location and the surgeon's expected surgically induced astigmatism (SIA) of 0.5 D were entered into the calculator, with emmetropia as the goal. A sterile ink pen was used to mark the corneal limbus at 0° and 180° with the patient sitting upright at the slit lamp to avoid ocular torsion. Intra-operatively, the steepest corneal meridian was marked using a Mendez ring. The toric IOL (AcrySof® IQ Toric IOL, Alcon Inc.) was rotated to align with the planned axis[25]–[27].
In all surgeries, phacoemulsification (Infiniti® Vision System Ozil, Alcon, Inc.) was performed through a 2.2 mm temporal clear corneal incision and was followed by the implantation of a foldable IOL in the posterior capsular bag with a Monarch II injector (Alcon, Inc.).
Patients were evaluated postoperatively at one day, one month, three and six months. Measurement of UDVA, BCVA, intraocular pressure, refraction, keratometry, corneal topography, corneal tomography and endothelial cell count were performed at each visit. All patients who underwent the toric IOL implantation were further evaluated under mydriasis after one, seven and thirty days to determine the toric axis using the “Toric IOL Rotation Summary” software with aberrometer OPD Scan III (Nidek Co, Japan)[28]–[30]. For a misalignment of more than 10 degrees of rotation on the first postoperative day, a repositioning of the IOL was required.
Statistical Analysis
Calculation of the sample dimension is based on reading the data and from the clinical experience of the researchers involved. The computations were done using PASS 2005 (Kaysville, Utah, USA), taking into consideration the biostatic parameter and the dropout rate from the study (about the 20%). There were enrolled 52 patients for the toric IOL Group and 50 patients for the LRI Group.
All quantitative variables considered were reported in summary tables containing average and standard deviation. All data will be analyzed using SPSS® Advanced Statistical™ 19 (Chicago, IL, USA, 2004).
The Wilcoxon signed rank test was used to compare preoperative and postoperative data and the Mann-Whitney U test was used for comparison between groups.
The changes over time of the astigmatism and the value of visual acuity in each treatment group were valued using t-test per pair data. t-test normally requires a normal distribution; any non-parametric statistical calculations were performed using the Wilcoxon and Mann-Whitney test.
In all trials the threshold of statistical significance will be considered at P=0.01.
RESULTS
A total of 102 eyes of 102 patients were included in this study. Data were collected from 52 eyes for the toric IOL group, and 50 eyes for the LRI group.
No statistical differences were demonstrated between the two groups before surgery in terms of demographic characteristics, biometric data (Table 1), visual acuity, keratometric and topographic values, refractive astigmatism and endothelial cell count.
Table 1. Demographic and biometric data.
| Characteristics | Groups |
1P | |
| LRI | Toric IOL | ||
| Eyes (R/L; n) | 26/24 | 23/29 | - |
| Age (range; a) | 70.9±7.3 (62-88) | 69.6±5.9 (53-85) | 0.29 |
| Sex (M/F; n) | 22/28 | 26/26 | - |
| AL (mm) | 22.90±1.15 | 23.04±0.97 | 0.13 |
| Spherical IOL power (D) | 21.90±3.2 | 20.5±2.9 | 0.10 |
| Topographic cylinder (D) | 1.27±0.58 | 1.32±0.55 | 0.49 |
| LRI length (degrees) | 47.8±9.1 | - | |
| IOL cylinder power (D) | - | 1.39±0.56 | |
AL: Axial length; IOL: Intraocular lens; LRI: Limbal relaxing incisions; 1Mann-Whitney U test.
x±s
There were no intraoperative and postoperative ocular or systemic complications. No surgeries performed required suturing or repositioning for a misalignment greater than 10 degrees of rotation.
Visual Acuity
Table 2 shows the mean visual acuity variations for the Toric IOL and LRI groups. Both groups considered had a significant increase in UCVA and BCVA during the follow-up period (P<0.01). UCVA was statistically higher in the group of the toric IOLs compared to LRI, while BCVA did not demonstrate statistically significant differences between the two groups.
Table 2. Preoperative and postoperative visual acuity (logMAR).
| Groups | Pre-operative | Postoperative follow-up |
1P | |||
| 1d | 1mo | 3mo | 6mo | |||
| Uncorrected visual acuity | ||||||
| Toric IOL | 0.75±0.27 | 0.28±015 | 0.21±0.11 | 0.18±0.14 | 0.15±0.08 | P<0.01 |
| LRI | 0.79±0.31 | 0.32±0.19 | 0.19±0.14 | 0.23±0.09 | 0.22±0.12 | P<0.01 |
| 2P | 0.44 | 0.28 | 0.37 | P<0.01 | P<0.01 | |
| Best corrected visual acuity | ||||||
| Toric IOL | 0.35±0.20 | 0.15±0.12 | 0.07±0.05 | 0.05±0.03 | 0.04±0.03 | P<0.01 |
| LRI | 0.39±0.13 | 0.22±0.14 | 0.07±0.6 | 0.07±0.06 | 0.05±0.04 | P<0.01 |
| 2P | 0.59 | 0.72 | 0.64 | 0.87 | 0.83 | |
IOL: Intraocular lens; LRI: Limbal relaxing incisions; 1Wilcoxon Test; 2Mann-Whitney U test.
Topographic and Keratometric Changes
Anterior and posterior variations of the corneal surfaces were evaluated during the follow-up at 3 different points in time: 1, 3 and 6mo after surgery (Table 3). At the end of the follow-up a statistically significant reduction of the mean keratometric and topographic anterior cylinder were observed in the LRI group. The toric group did not present a significant change in keratometric and topographic astigmatism over the follow-up period. The topographical posterior cornea surfaces did not demonstrate variations after surgery in both groups.
Table 3. Topographic and keratometric development (Cyl).
| Variables | Pre-operative | Postoperative follow-up |
1P | ||
| 1m | 3mo | 6mo | |||
| Keratometric data | |||||
| Toric IOL (D) | 2.35±0.36 | 1.92±0.49 | 1.93±0.40 | 1.85±0.42 | n.s. |
| LRI (D) | 2.16±0.40 | 1.44±0.57 | 1.29±0.53 | 0.84±0.46 | P<0.01 |
| 2P | n.s. | P<0.01 | P<0.01 | P<0.01 | |
| Topographic anterior surface data | |||||
| Toric IOL (D) | 2.05±0.30 | 1.99±0.32 | 1.85±0.34 | 1.84±0.29 | n.s. |
| LRI (D) | 1.89 ± 0.26 | 1.34 ± 0.45 | 1.10±0.36 | 1.04±0.40 | P<0.01 |
| 2P | n.s. | P<0.01 | P<0.01 | P<0.01 | |
| Topographic posterior surface data | |||||
| Toric IOL (D) | -0.18±0.10 | -0.15±0.06 | -0.15±0.08 | -0.14±0.11 | n.s. |
| LRI (D) | -0.20±0.12 | -0.17 ± 0.17 | -0.16±0.18 | -0.16±0.09 | n.s. |
| 2P | n.s. | n.s. | n.s. | n.s. | |
IOL: Intraocular lens; LRI: Limbal relaxing incisions; n.s.: Not significant; 1Wilcoxon Test; 2Mann-Whitney U test.
Refractive Evaluation and Residual Astigmatism
The refractive astigmatism variation from baseline were statistically significant (P<0.01) in the two groups. Both groups presented a reduction of the refractive astigmatism at the end of the follow-up resulting in 0.4 D ± 0.20 for the toric group and 1.1 D ± 0.38 for the LRI group (P<0.01; Table 4).
Table 4. Refractive astigmatism.
| Groups | Preoperative refractive cylinder (D) ±SD |
Postoperative at 6-mo refractive cylinder (D) ±SD |
1P | ||
| Sphere (D) | Cylinder (D) | Sphere | Cylinder (D) | ||
| Toric IOL | -1.95±1.37 | 1.59±0.52 | - 0.35±0.95 | 0.4±0.20 | P<0.01 |
| LRI | -1.80±1.42 | 1.91±0.63 | - 0.43±0.44 | 1.1±0.38 | P<0.01 |
| 2P | n.s. | n.s. | n.s. | p <0.01 | |
IOL: Intraocular lens; LRI: Limbal relaxing incisions; 1Wilcoxon Test; 2Mann-Whitney U tes.
Toric Intraocular Lens Misalignment
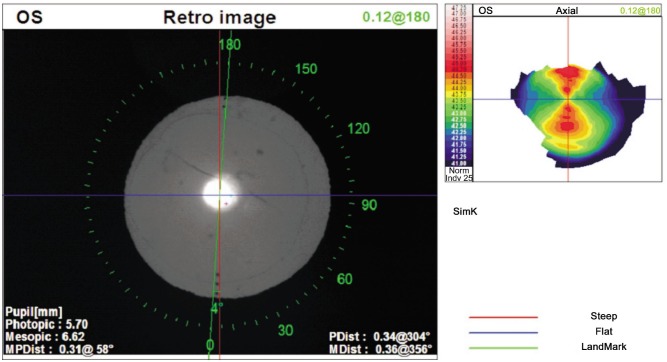
The toric IOL alignment was evaluated with the OPD Scan III[27]–[29]. The mean toric IOL misalignment was 6.8 ± 2.9 degrees (range 0 to 10 degrees; Figure 1).
Figure 1. “Toric IOL Rotation Summary” software of OPD Scan III (Nidek Co, Japan) to evaluate the postoperative alignmnet. In this case the misalignment is 4 degrees (implantation axis in green) from the target axis (red one).
Endothelial Cell Count
The average loss of endothelial cells in both groups considered was not statically different during the follow-up as reported in Table 5.
Table 5. Endothelial cells count.
| Groups | Baseline | Postoperative follow-up |
1P | ||
| 1mo | 3mo | 6mo | |||
| Toric IOL | 2486 | 2436 | 2392 | 2372 | P<0.01 |
| LRI | 2314 | 2299 | 2290 | 2270 | P<0.01 |
| 2P | n.s. | n.s. | n.s. | n.s. | |
IOL: Intraocular lens; LRI: Limbal relaxing incisions; 1Wilcoxon Test; 2Mann-Whitney U test.
cell/mm2
DISCUSSION
Astigmatism is one of the main ocular refractive defects which requires optical correction. Cataract surgery represents an opportunity to reduce this visual defect in order to grant a better quality of vision.
Recently, different surgical techniques have been developed to manage astigmatism during cataract surgery[6]–[13].
The LRI have been considered for many years to be one of the safer and better techniques with reduced effects of intra-operative and post-operative risks[8],[10],[11]. Complications of this incisional technique have been read in records for example post-operatory keratitis and often epithelial problems. In our study during the six month follow-up we did not find any intra or post-operations complications.
After the introduction on the market of the first intra-ocular sphero-cilindric lens in 1988, new models of IOL have been developed and powered. The toric IOL currently represents an alternative method to the incisional technique for the correction of slight and moderate astigmatism during cataract surgery[1],[14],[15]. The implantation of the toric IOL aims to overcome the complications linked to incisional technique granting more accurately reaching emmetropia and a greater stability and predictability of the refractive results[1],[15]–[19].
In our study we compared the two techniques evaluating the refractive and visual results; the variations of the parameters and corneal endothelial cell counts were reported and recorded.
In both groups, a significantly statistical increase in the BCVA was registered, while in the group treated with the toric IOL, a greater improvement of the UCVA was recorded. This result is in line with the recent literature published[6].
The residual refractive cylinder after six months is about 0.4 D±0.20 for the group implanted with the toric IOL and 1.1 D±0.38 for the LRI group. In a study by Mingo-Botin et al[6] the residual corneal astigmatism turns out to be about 0.61 D±0.41 and 1.32 D±0.60 over a period of three months after surgery for the IOL toric patients and the LRI group respectively.
Another data considered in the evaluation of the two techniques was the change of refractive corneal keratometric and topographic values. Between the two techniques a statistically significant variation was recorded because only in the group treated with LRI was a variation of keratometric and topographic indices to six-month follow-up documented. In the data analyzed the posterior corneal astigmatism did not demonstrate significant changes for both surgical techniques. As a baseline, we included in the study only those patients who presented with-the-rule posterior astigmatism according to the anterior corneal surface. This may support the reduced postoperative refractive astigmatism in the toric IOL group probably because the residual astigmatisms from the IOL calculator software were compensated by the IOL toric refractive overcorrection as suggested in the studies of Koch et al[31].
In conclusion, despite the documented efficacy for both surgery techniques in the aim of reducing the pre-operatory astigmatism, the toric IOL implantation was more effective and predictable compared to the LRI.
Toric IOL implantation does not need the use of specific surgical instruments allowing for the implantation without any increase in risk. The limit of this technique is the lack of alignment accuracy of the IOL.
Although both techniques reduced preoperative refractive astigmatism and guaranteed not statistically different results in BCVA, toric IOL implantation was more effective for UCVA than peripheral corneal relaxing incisions. Implantation of a toric IOL does not require special surgical skills or instrumentation and does not increase surgical risks. Our study of eyes with low (1.0-2.0 D) astigmatism found that toric IOL implantation resulted in better refractive and visual outcomes, and thus might be linked with greater spectacle freedom for distance vision than relaxing incisions. Further studies are needed to note the differences between the two surgical techniques. Moreover, they could address wider ranges of astigmatism, corneal aberration examination and other nomograms, and larger samples and longer follow-ups would be desirable to confirm these results.
Acknowledgments
The paper was presented at the XXXII Congress of the ESCRS on 13-17 Sepetember 2014 in London, UK.
Conflicts of Interest: Leon P, None; Pastore MR, None; Zanei A, None; Umari I, None; Messai M, None; Negro C, None; Tognetto D, None.
REFERENCES
- 1.Synek S. The latest generation of intraocular lenses, the problem of the eye refraction after cataract surgery. Coll Antropol. 2013;1:217–221. [PubMed] [Google Scholar]
- 2.Yu JG, Zhao YE, Shi JL, Ye T, Jin N, Wang QM, Feng YF. Biaxial microincision cataract surgery versus conventional coaxial cataract surgery: metaanalysis of randomized controlled trials. J Cataract Refract surg. 2012;38(5):894–901. doi: 10.1016/j.jcrs.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Visual outcome of cataract surgery; study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 2013;39(5):673–679. doi: 10.1016/j.jcrs.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Amesbury EC, Miller KM. Correction of astigmatism at the time of cataract surgery. Curr Opin Ophthalmol. 2009;20(1):19–24. doi: 10.1097/ICU.0b013e328319c27a. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer-Blasco T, Montès-Micò R, Peixoto-Matos SC, Gonzàlez-Mèijome JM, Cervino A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. doi: 10.1016/j.jcrs.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Mingo-Botìn D, Munoz-Negrete FJ, Won Kim HR, Morcillo-Laiz R, Rebolleda G, Oblanca N. Comparison of toric intraocular lenses and peripheral corneal relaxing incision to treat astigmatism during cataract surgery. J Cataract Surg. 2010;36(10):1700–1708. doi: 10.1016/j.jcrs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Rubestein JB, Raciti M. Approaches to corneal astigmatism in cataract surgery. Curr Opin Ophthalmol. 2013;24(1):30–34. doi: 10.1097/ICU.0b013e32835ac853. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M, Queen Elizabeth Astigmatism Study Group Limbal relaxing incision versus on-axis incision to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31(12):2261–2265. doi: 10.1016/j.jcrs.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Poll JT, Wang L, Koch DD, Weikert MP. Correction of astigmatism during cataract surgery: toric intraocular lens compared to peripheral corneal relaxing incisions. J Refract Surg. 2011;27(3):165–171. doi: 10.3928/1081597X-20100526-01. [DOI] [PubMed] [Google Scholar]
- 10.Muller-Jensen K, Fischer P, Siepe U. Limbal relaxing incision to correct astigmatism in clear corneal cataract surgery. J Refract Surg. 1999;15(5):586–589. doi: 10.3928/1081-597X-19990901-12. [DOI] [PubMed] [Google Scholar]
- 11.Loncar VL, Vickovic IP, Ivekovic R, Mandic Z. Limbal relaxing incision during cataract surgery. Acta Clin Croat. 2012;51(2):289–292. [PubMed] [Google Scholar]
- 12.Lukenda A, Martinovic ZK, Kalauz M. Excimer laser correction of hyperopia, hyperopic and mixed astigmatism: past, present,and future. Acta Clin Croat. 2012;51(2):299–304. [PubMed] [Google Scholar]
- 13.Anderson I, Sanders DR, van Saarloos P, Ardrey WJ., 4th Treatment of irregular astigmatism with a 213 nm solid-state, diode-pumped neodymium:YAG ablative laser. J Cataract Refract Surg. 2004;30(10):2145–2151. doi: 10.1016/j.jcrs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Savini G, Hoffer KJ, Ducoli P. A new slant on toric intraocular lens power calculation. J Refract Surg. 2013;29(5):348–354. doi: 10.3928/1081597X-20130415-06. [DOI] [PubMed] [Google Scholar]
- 15.Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39(4):624–637. doi: 10.1016/j.jcrs.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Hirnschall N, Gangwani V, Crnej A, Koshy J, Maurino V, Findl O. Correction of moderate corneal astigmatism during cataract surgery: toric intraocular lens versus peripheral corneal relaxing incisions. J Cataract Refract Surg. 2014;40(3):354–361. doi: 10.1016/j.jcrs.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Agresta B, Knorz MC, Donatti C, Jackson D. Visual acuity improvements after implantation of toric intraocular lenses in cataract patients with astigmatism: a systematic review. BMC Ophthalmol. 2012;12:41. doi: 10.1186/1471-2415-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachernegg A, Ruckl T, Riha W, Grabner G, Dexl AK. Rotational stability and visual outcome after implantation of a new toric intraocular lens for the correction of corneal astigmatism during cataract surgery. J Cataract Refract Surg. 2013;39(9):1390–1398. doi: 10.1016/j.jcrs.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Toto L, Vecchiarino L, D'Ugo E, Cardone D, Mastropasqua A, Mastropasqua R, Di Nicola M. Astigmatism correction with toric IOL: analysis of visual performance, position, and wavefront error. J Refract Surg. 2013;29(7):476–483. doi: 10.3928/1081597X-20130617-06. [DOI] [PubMed] [Google Scholar]
- 20.Iovieno A, Yeung SN, Lichtinger A, Alangh M, Slomovic AR, Rootman DS. Cataract surgery with toric intraocular lens for correction of high corneal astigmatism. Can J Ophthalmol. 2013;48(4):246–250. doi: 10.1016/j.jcjo.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 22.Nichamin LD. Nomogram for limbal relaxing incisions. J Cataract Refract Surg. 2006;32(9):1408. doi: 10.1016/j.jcrs.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Nichamin LD. Modified astigmatism correction nomogram. J Refract Surg. 2008;24(6):562–563. doi: 10.3928/1081597X-20080601-02. [DOI] [PubMed] [Google Scholar]
- 24.Langerman DW. Architectural design of a self-sealing corneal tunnel, single-hinge incision. J Cataract refract surg. 1994;20(1):84–88. doi: 10.1016/s0886-3350(13)80052-1. [DOI] [PubMed] [Google Scholar]
- 25.Chang DF. Comparative rotational stability of single-piece open-loop acrylic and plate-haptic silicone toric intraocular lenses. J Cataract Refract Surg. 2008;34(11):1842–1847. doi: 10.1016/j.jcrs.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Weinand F, Jung A, Stein A, Pfutzner A, Beker R, Pavlovic S. Rotational stability of a single-piece hydrophobic acrylic intraocular lens: new method for high-precision rotation control. J Cataract Refract Surg. 2007;33(5):800–803. doi: 10.1016/j.jcrs.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Zuberbuhler B, Signer T, Gale R, Haefliger E. Rotational stability of the AcrySoft SA60TT toric intraocular lenses: a cohort study. BMC Opthalmol. 2008;8:8. doi: 10.1186/1471-2415-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira TB, Almeida A. Comparison of the visual outcomes and OPD-scan results of AMO Tecnis toric and Alcon Acrysoft IQ toric intraocular lenses. J Refract Surg. 2012;28(8):551–555. doi: 10.3928/1081597X-20120703-03. [DOI] [PubMed] [Google Scholar]
- 29.Solomon JD. Outcomes of corneal spherical aberration-guided cataract surgery measured by the OPD-scan. J Refract Surg. 2010;26(11):863–869. doi: 10.3928/1081597X-20100129-01. [DOI] [PubMed] [Google Scholar]
- 30.Gualdi L, Cappello V, Giordano C. The use of NIDEK OPD Scan II wavefront aberrometry in toric intraocular lens implantation. J Refract Surg. 2009;25(1 Suppl):S110–115. doi: 10.3928/1081597X-20090115-06. [DOI] [PubMed] [Google Scholar]
- 31.Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Surg. 2012;38(12):2080–2087. doi: 10.1016/j.jcrs.2012.08.036. [DOI] [PubMed] [Google Scholar]