Abstract
AIM
To evaluate the safety and efficacy of dexamethasone implant in patients with non-infectious posterior uveitis with cystoid macular edema (CME).
METHODS
Retrospective analysis of patients reports with CME secondary to non-infectious uveitis treated with dexamethasone implant. Data included type of posterior uveitis, any systemic immunosuppressive therapy, Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA), central macular thickness (CMT) on optical coherence tomography (OCT) and signs of intraocular inflammation at baseline and then at 2wk postoperatively and monthly thereafter. Follow-up is up to 10mo. Any per-operative and post-operative complications were recorded.
RESULTS
Six eyes of 4 patients with CME due to non-infectious posterior uveitis treated with dexamethasone implant. Diagnosis included idiopathic panuveitis, birdshot chorioretinopathy and idiopathic intermediate uveitis. At baseline mean ETDRS BCVA was 63 letters and mean CMT 556 µm at 2wk postoperatively mean ETDRS BCVA improved to 70 letters and mean CMT decreased to 329 µm. All eyes showed clinical evidence of decreased inflammation. The duration of effect of the implant was 5 to 6mo and retreatment was required in 2 eyes. Two patients required antiglaucoma therapy for increased intraocular pressures.
CONCLUSION
In patients with non-infectious posterior uveitis dexamethasone implant can be a short-term effective treatment option for controlling intraocular inflammation.
Keywords: Ozurdex®, cystoids macular oedema, central macular thickness, posterior uveitis
INTRODUCTION
Ozurdex® has been approved by the Food and Drug Administration (FDA) for the treatment of macular oedema and noninfectious posterior uveitis. The implant is manufactured by Allergan which is based in California. National Institute for Health and Care Excellence (NICE) has also recently recommended the dexamethasone implant in the treatment of macula oedema secondary to retinal vein occlusions. It has been reported that the annual incidence of uveitis is approximately 17-52 cases per 100 000 and the prevalence is 38-714 per 100 000. Macular oedema can cause visual impairment in one third of these cases[1].
Corticosteroids are potent anti-inflammatory agents that reduce macula edema by preventing leukocyte migration, reduce fibrin deposition, stabilize endothelial cell tight junctions, and inhibit synthesis of vascular endothelial growth factor, prostaglandins, and proinflammatory cytokines. There are various routes of steroid administration which included topical, local and systemic. Local and systemic corticosteroids have been used for the treatment of intermediate and posterior uveitis. However due to inadequate drug penetration to the posterior segment of the eye, posterior uveitis is often unresponsive to topical administration of steroids. Periocular and intraocular steroid administration have been used while long-term systemic steroid therapy is avoided due to potential side effects[2],[3].
Intravitreal corticosteroid such as dexamethasone implant can lead to high local drug concentration in the retina as it bypasses the blood-retinal barrier. Compare to anti- vascular endothelial growth factor (VEGF) treatments, dexamethasone implant has the advantages of being longer lasting not needing the repeated administration of the monthly regimen and cost effective. The drug is slowly released over a period of six months with peak levels occurring at about two months[4],[5]. The efficacy of Ozurdex® in the treatment of non-infectious posterior uveitis was also reported in a multicentre study known as the HURON (cHronic Uveitis evaluation of the intRavitreal dexamethasone implant)[6].
The dexamethasone implant provides a localised targeted therapy avoiding systemic complications but is associated with local ocular side effects such as cataract, glaucoma, risk of endopthalmitis and retinal detachment.
We present our cases consisting of 6 eyes in 4 uveitis patients with macula oedema all of whom responded to the dexamethasone implant proven by improvement in Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) and decreased in central macular thickness (CMT). Informed consents were obtained from all the patients. The study was approved by the institution review board and it followed the principles outlined in the Declaration of Helsinki.
CASE 1
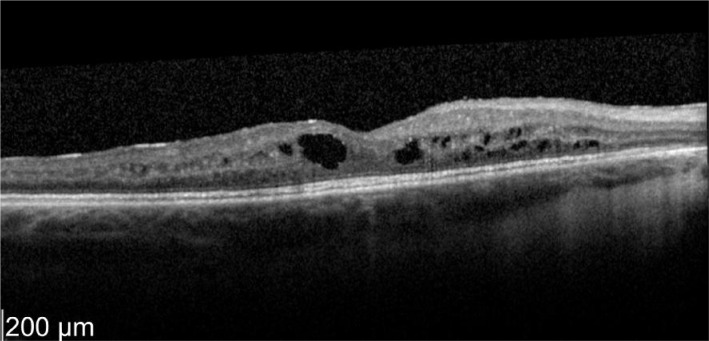
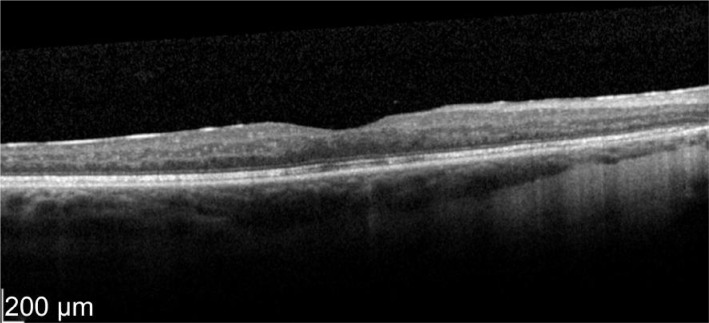
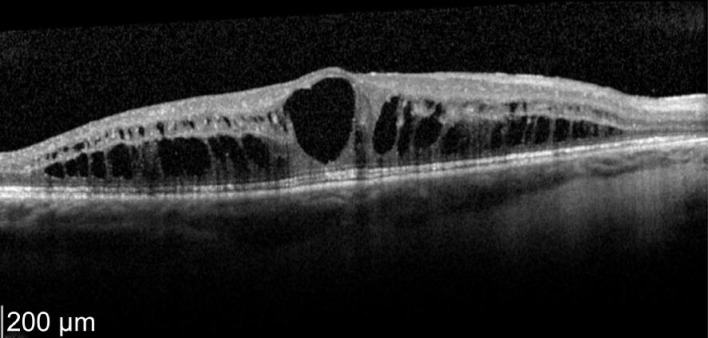
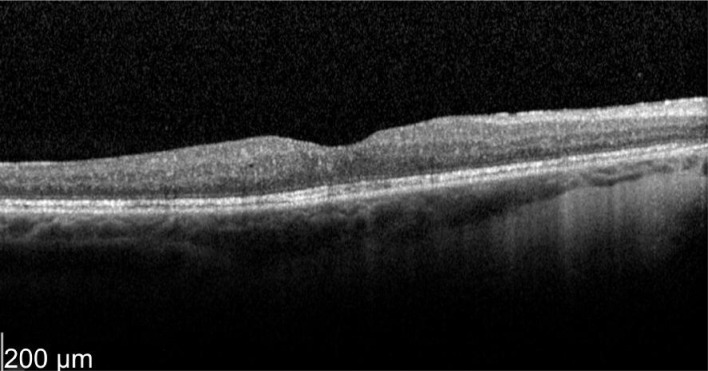
A 52-year-old female who has a history of bilateral panuveitis for 9y presented with left eye pain and redness for 10d waking her up at night. She was on oral Azathioprine but stopped the medications 18mo ago due to elevated liver enzymes. She also had 2 intravitreal triamcinolone injections to her left eye in the past for cystoid macular edema (CME) which responded to treatment but improvement were short acting lasting for 5wk. Decision was made for her to have longer acting agent such as intravitreal steroid implant. Left intravitreal dexamethasone was implanted on 11/1/2012. Two weeks later her left eye vision improved from 63 letters to 76 letters (+13 letters) with decreased in macular thickness (Figures 1, 2).
Figure 1. Pre-treatment OS vision 63 letters optical coherence tomographyshowed macular thickness of 696 µm.
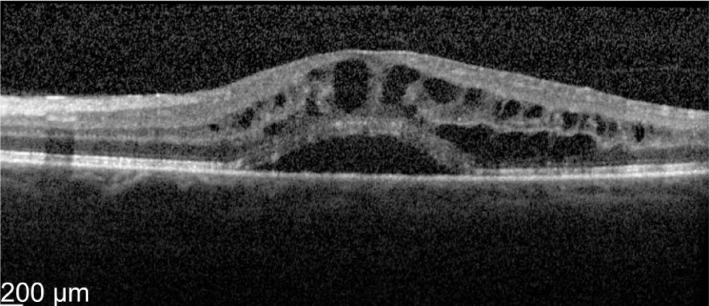
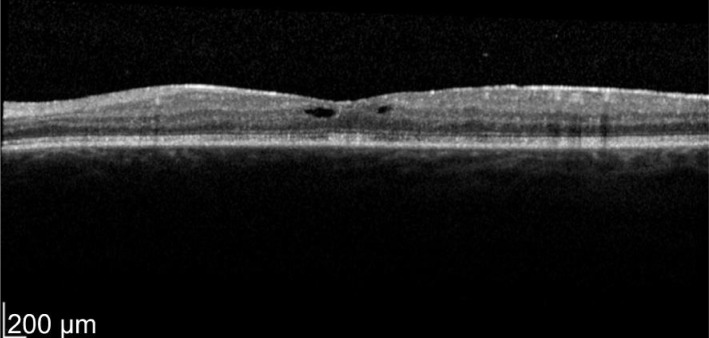
Figure 2. Two weeks post-treatment.
OS vision 76 letters with thickness of 365 µm. Even the neurosensory detachment persists.
CASE 2
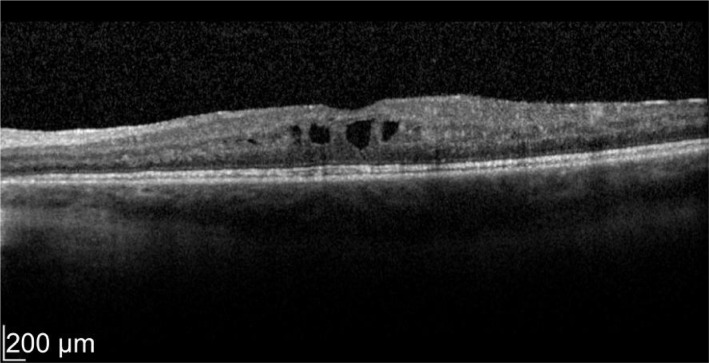
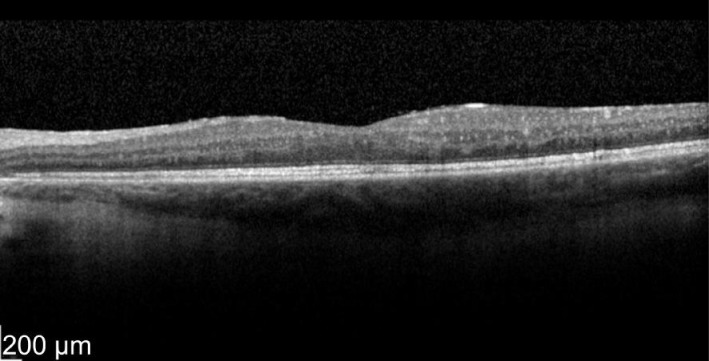
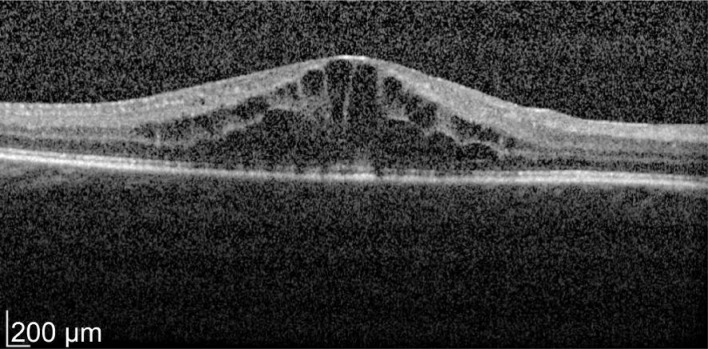
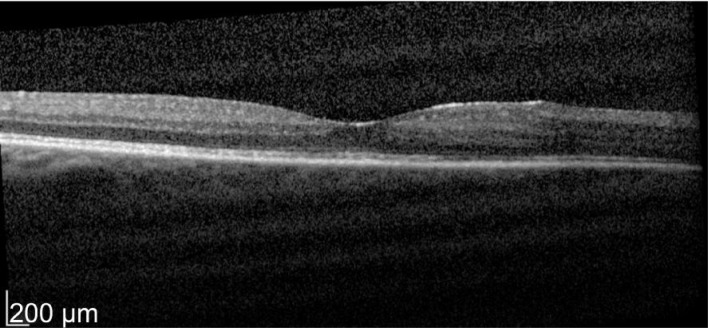
A 49-year-old male patient with a history of birdshot chorioretinopathy with bilateral CME. He complained of visual blurring in the right eye for 3wk. Intravitreal dexamethasone was implanted into the right eye on 29/3/2012. One month later his vision improved 78 to 83 letters with decreased in macular thickness from 621 to 319 µm as shown in Figures 3 and 4. He was a steroid responder with increased in intraocular pressure needing treatment with latanoprost/timolol maleate (Xalacom®) eye drops. He was also found to have increased in left macular oedema and was listed for left intravitreal dexamethasone implantation. With 2wk post intravitreal dexamethasone treatment his left eye vision improved from 72 to 82 letters and macular thickness decreased from 463 to 349 µm as shown in Figures 5 and 6. Five months after the right dexamethasone implantation his right eye vision decreased with recurrence of macular oedema which responded to a second right intravitreal treatment as shown in Figures 7 and 8.
Figure 3. Pre-treatment OD vision 78 letters.
Macular thickness of 621 µm.
Figure 4. Post-treatment at 4wk OD vision 83 letters with macular thickness of 319 µm.
Figure 5. Pre-treatment OS vision72 letters with macular thickness 463 µm.
Figure 6. Two weeks post-treatment.
OS vision 82 letters with macular thickness of 349 µm.
Figure 7. Five months post-treatment.
OD vision 65 letters.
Figure 8. Two weeks later.
OD vision 79 letters with macular thickness of 266 µm. Right eye with macular thickness of 620 µm.
CASE 3
A 51-year-old female patient with history of CME secondary to idiopathic intermediate uveitis presented with decreased in vision both eyes for 2mo despite taking oral prednisolone 30 mg. She was found to have increased in macular thickness both eyes. In view of the need for treatment, she was listed for intravitreal dexamethasone implantation to both eyes. With 2wk post intravitreal dexamethasone treatment, his right eye vision improved with decreased in macular thickness as shown in Figures 9 and 10. Improvement in the left eye are shown in Figures 11 and 12. Despite having a history of being a steroid responder, her intraocular pressure remained stable with topical antiglaucoma treatment not needing surgical intervention.
Figure 9. OD vision 43 letters and macular thickness of 675 µm.
Figure 10. Two weeks post treatment OD vision 49 letters with decreased in macular thickness to 224 µm.
Figure 11. OS vision 63 letters with CMT of 446 µm despite 2mo on oral prednisolone.
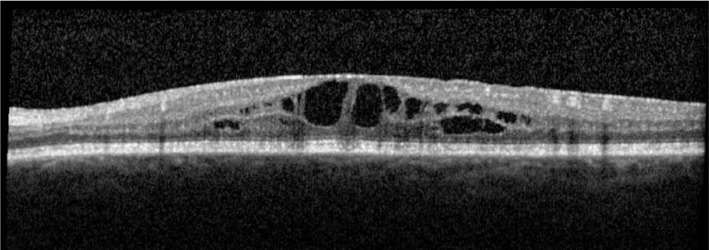
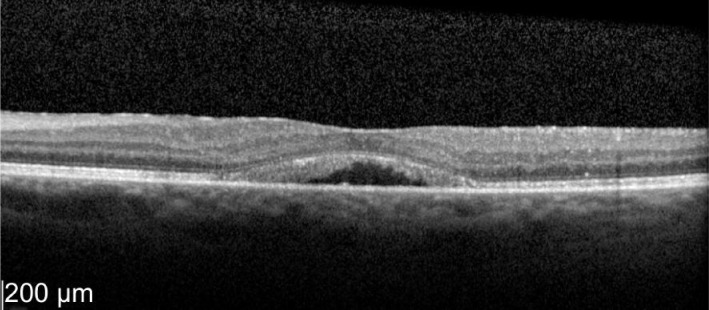
Figure 12. Two weeks post-treatment OS vision improved to 78 letters with macular thickness of 333 µm.
CASE 4
A 30-year-old female patient with a history of recurrent panuveitis presented to the eye clinic with left visual blurring for 3wk. She also has a history of autoimmune polyendocrinopathy with posterior non-granulomatous uveitis affecting both eyes for the past 2y. Her uveitis episodes frequently flared up when she tapered topical steroids. Due to the recurrent uveitis and secondary complication of cystoid macular oedema resulting in worsening visual morbidity, she underwent intravitreal dexamethasone implantation to her left eye. Two weeks later her left eye vision improved with decreased in macular thickness as shown in Figures 13 and 14.
Figure 13. Pre-treatment OS vision 61 letters with central macula thickness of 496 µm.
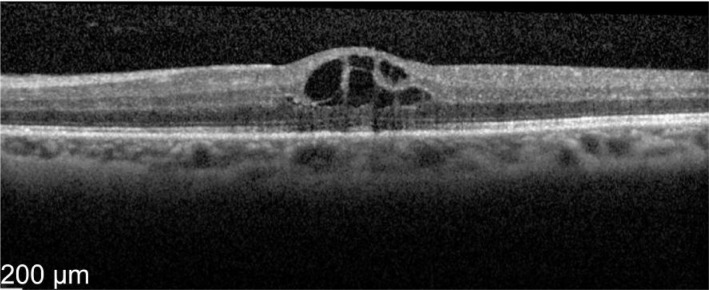
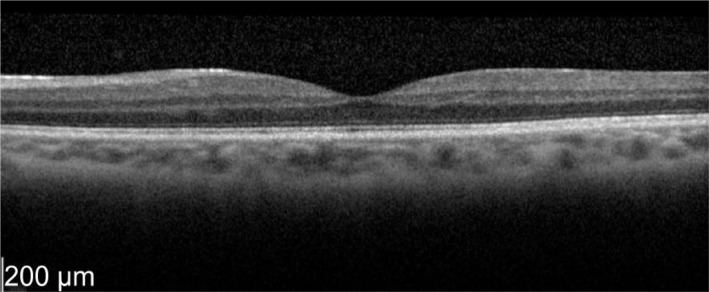
Figure 14. Two weeks later OS vision improved to 72 letters with CMT of 230 µm.
DISCUSSION
In our study 2 of the 6 eyes needed antiglaucoma treatment and 1 of the 6 eyes needed cataract surgery within 1y. Results in Table 1 summarised the reduction in retinal thickness and increased in letters read with intravitreal dexamethasone treatment in our group of patients. The complication rates for glaucoma and cataract are slightly higher compared to previous studies. Intravitreal treatment with dexamethasone implant also meant that the systemic complications of immunusuppressants or anti-tumour necrosis factor (TNF) administrations can be prevented. Limitation of our study included it being a retrospective analysis and small patient group of 6 eyes in 4 patients.
Table 1. Results of non-infectious posterior uveitis following two weeks of dexamethasone intravitreal administration. Change in VA (ETDRS letters) and foveal thickness (CMT µm).
| Patient | Eyes | Age | Sex | Diagnosis | Systemic immuno suppresion prior to Ozurdex® | Baseline VA | Post treatment VA | Baseline CMT (µm) | Post treatment CMT (µm) | Frequency of Ozurdex® treatment | Duration of follow up (mo) |
| No.1 | OS | 52 | F | Bilateral panuveitis | Y | 63 | 76 | 696 | 365 | Once | 18 |
| No.2 | OD | 49 | M | Birdshot chorioretinopathy | N | 78 | 83 | 621 | 319 | Twice | 16 |
| No.2 | OS | 49 | M | Birdshot chorioretinopathy | N | 72 | 82 | 463 | 349 | Once | 16 |
| No.3 | OD | 51 | F | Idiopathic Intermediate uveitis | Y | 43 | 49 | 675 | 224 | Once | 14 |
| No.3 | OS | 51 | F | Idiopathic Intermediate uveitis | Y | 63 | 78 | 446 | 333 | Once | 14 |
| No.4 | OS | 30 | F | Panuveitis | N | 61 | 72 | 496 | 230 | Once | 12 |
VA: Visual acuity; CMT: Central macular thickness.
In bilateral noninfectious uveitis, the corticosteroids tend to be administered orally rather than locally (bilateral panuveitis, birdshot chorioretinopathy) starting at a high dose followed by a slow taper. Ozurdex® is reported to be effective in short term results, but the side effect profile of multiple implants is not yet clear and further data are needed. Complications of treatment such as cataracts and ocular hypertension increases much as the number of injections.
In the Huron study, 229 patients with intermediate and posterior uveitis were randomised into 3 groups: a single administration with dexamethasone implant 0.7 mg (n=77) or dexamethasone implant 0.35 mg (n=76) or a sham procedure (n=76). Results showed clear benefits of intravitreal dexamethasone in controlling the inflammation, improving the vision and decreasing macular thickness. The proportion of patients with a vitreous haze score of zero at 8th week was 47% in patients with dexamethasone implant 0.7 mg and 36% in the 0.35 mg dexamethasone when compared to the sham group.
The proportion of patients with BCVA gain of 15 letter was significantly greater in both active treatment groups compared with sham. There was also a greater decrease in mean CMT at 8th week in the treatment group compared with sham. Twenty-three percent of patients with dexamethasone treatment required IOP-lowering medications and the incidence of cataracts were 15% in the 0.7 mg dexamethasone implant patients, 12% in patients with 0.35 mg and 7% in the sham treatment [6]. Myung et al[7] reported good outcomes of dexamethasone implantation to control intraocular inflammation on non-infectious posterior uveitis patients with effect lasting 3-4mo.
In conclusion, from the results analysed with improvement in vision and decreased in macular thickness we advocate the use of dexamethasone implant in patients with non-infectious posterior uveitis. It is an effective treatment for controlling intraocular inflammation especially in resistant uveitis macular oedema.
Acknowledgments
Conflicts of Interest: Yap YC, None; Papathomas T, None; Kamal A, None.
REFERENCES
- 1.Karim R, Sykakis E, Lightman S, Fraser-Bell S. Interventions for the treatment of uveitic macular edema: a systematic review and Meta-analysis. Clin Ophthalmol. 2013;7:1109–1144. doi: 10.2147/OPTH.S40268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson MR, Whitcup SM. Pharmacologic and clinical profile of dexamethasone intravitreal implant. Expert Rev Clin Pharmacol. 2012;5(6):629–647. doi: 10.1586/ecp.12.55. [DOI] [PubMed] [Google Scholar]
- 3.De Smet MD, Julian K. The role of steroids in the management of uveitic macular edema. Eur J Ophthalmol. 2011;21(Suppl. 6):S51–55. doi: 10.5301/EJO.2010.6056. [DOI] [PubMed] [Google Scholar]
- 4.Hunter RS, Lobo AM. Dexamethasone intravitreal implant for the treatment of noninfectious uveitis. Clin Ophthalmol. 2011;5:1613–1621. doi: 10.2147/OPTH.S17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 6.Lowder C, Belfort R, Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, Li XY, Cui H, Whitcup SM, Ozurdex HURON Study Group Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 7.Myung JS, Aaker GD, Kiss S. Treatment of noninfectious posterior uveitis with dexamethasone intravitreal implant. Clin Ophthalmol. 2010;4:1423–1426. doi: 10.2147/OPTH.S15696. [DOI] [PMC free article] [PubMed] [Google Scholar]