Abstract
We describe a case of fetal parvovirus B19 infection resulting in preterm birth and leading to hydrops fetalis requiring multiple in utero transfusions. The infant developed chronic postnatal anemia responsive to intravenous immunoglobulin therapy. Serum viral load decreased after immunoglobulin treatment but remained detectable for over 1 year.
Keywords: congenital, fetal, hydrops, parvovirus
CASE REPORT
A female neonate was born at 26 weeks, 6 days (26 + 6) gestation to a 30-year-old gravida 5, para 2 mother with blood type O+, negative Coombs test, nonreactive rapid plasma reagin, negative human immunodeficiency virus antibody screen, negative hepatitis B surface antigen, and unknown group B streptococcal colonization status. The prenatal course was notable for the diagnosis of nonimmune fetal hydrops at 22 + 4 weeks gestation, when ultrasound demonstrated placentomegaly, cardiomegaly, pericardial effusion, echogenic bowel, abdominal ascites, and elevated middle cerebral artery (MCA) velocities. Maternal serologic testing at that time revealed a negative parvovirus B19 (B19) immunoglobulin (Ig)M and a positive IgG. Amniotic fluid analysis was positive for B19 by polymerase chain reaction (PCR) performed at a commercial laboratory (Viracor, Lee's Summit, MO); amniotic fluid viral load was 1.8 × 109 IU/mL. Here and below, we use the terms viremia/viral load to refer to B19 DNAemia. Testing of amniotic fluid for cytomegalovirus and Toxoplasma gondii by PCR was negative. The mother reported no known parvovirus exposures and no history of fever, rash, or other symptoms of acute parvovirus infection during pregnancy.
Due to the degree of hydrops and suspected severe fetal anemia, percutaneous umbilical cord blood sampling (PUBS) and intrauterine transfusion of packed red blood cells (PRBC) were performed on 3 occasions, at 22 + 4, 23 + 0, and 24 + 3 weeks gestation. The preprocedure fetal hematocrit (Hct) levels for the 3 procedures were 8.3%, 16.8%, and 10.4% , respectively, and the MCA Doppler peak systolic velocities were >1.5 multiples of the median in each case. After each of the PUBS procedures, MCA peak systolic velocities were noted to have normalized. Fetal magnetic resonance imaging at 23 + 3 revealed placentomegaly and normal central nervous system architecture.
The patient's mother subsequently presented with premature rupture of membranes and signs of preterm labor at 26 + 3, and the 932 gram neonate was delivered via cesarean delivery at 26 + 6. Apgar scores were 2, 2, and 7 at 1, 5, and 10 minutes of life, respectively. The newborn was emergently intubated because of respiratory depression and received chest compressions for 15 seconds. Initial physical examination was significant for hydrops with ecchymoses and petechiae across the torso (present prior to chest compressions) and blood in the endotracheal tube. There was no hepatosplenomegaly. Pathologic examination of the placenta was notable for acute chorioamnionitis with funisitis and vasculitis of chorionic plate vessels and intervillous and subchorionic thrombus formation.
Initial postnatal studies demonstrated white blood cell count of 23 200/mm3, hemoglobin (Hgb) of 12.8 g/dL, Hct of 39.9%, and reticulocytes of 12.0%. The platelet count was 41 000/mm3. On the first day of life, the neonate received a platelet transfusion for thrombocytopenia and episodic bleeding from central line sites and into her endotracheal tube. She also underwent exchange transfusion for hyperbilirubinemia (maximum total bilirubin on day of life 1 was 14.8 mg/dL). By the second day of life, reticulocytes were noted to have dropped to 3%, with Hct stable at 35.5%. The neonate clinically stabilized over the following week, was extubated, and had no further evidence of bleeding. Over the following 2 months, however, her Hgb dropped to 6.8 g/dL, Hct decreased to 20.7%, and reticulocytes decreased to 0.6% (Figure 1). She required PRBC transfusions on days of life 18, 33, 49, and 67.
Figure 1.
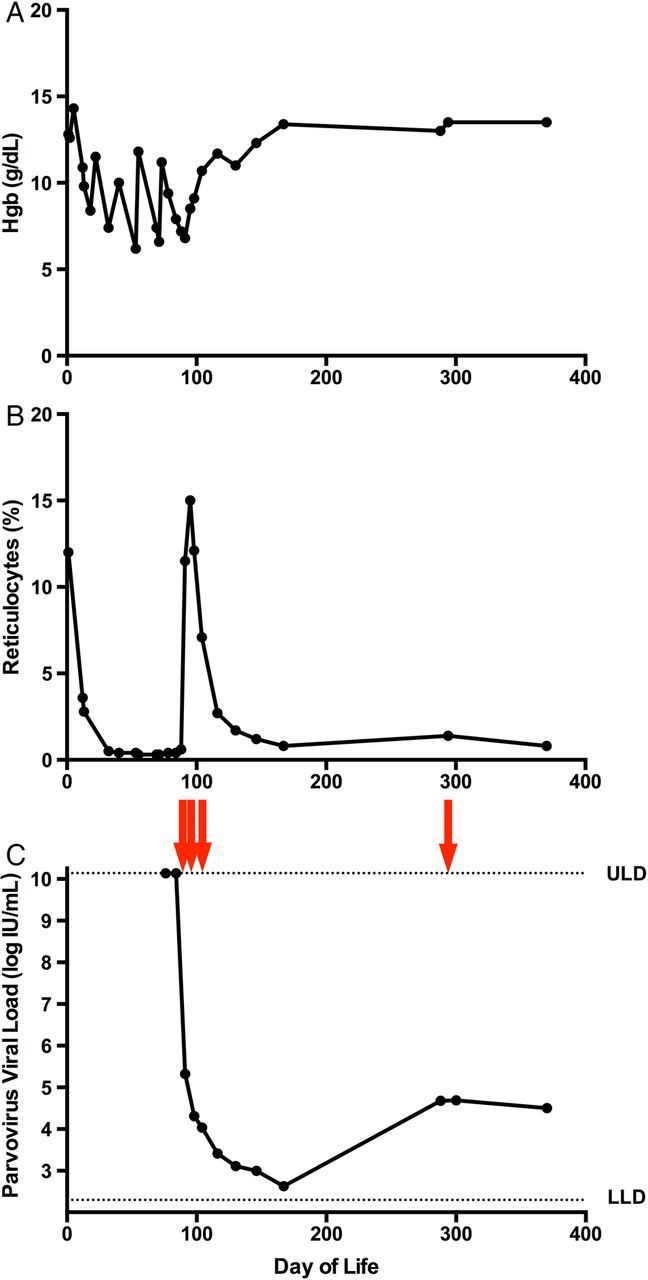
Response to treatment with intravenous immunoglobulin (IGIV) on parvovirus B19 viral load and reticulocyte count. The course of (A) hemoglobin (Hgb), (B) reticulocyte count, and (C) parvoviral load are shown over the first 400 days of life. Arrows indicate doses of IGIV. Abbreviations: ULD, upper limit of detection; LLD, lower limit of detection.
Due to persistent anemia and low reticulocyte count, serum B19 PCR was obtained on day of life 76, showing a viral load of >1.38 × 1010 IU/mL (equivalent to >1 × 1010 viral copies/mL)—exceeding the threshold of detection of the assay. Serum parvoviral load was repeated on day of life 85 and remained greater than the limit of detection; concomitant Hgb was 7.9 g/dL, Hct 25%, and reticulocytes 0.4%. On day of life 89, the patient received intravenous Ig (IGIV) at a dose of 500 mg/kg for the diagnosis of pure red cell aplasia (PRCA) due to congenital B19 infection.
Three days later, her parvoviral load had decreased to 210 000 IU/mL. Concomitant Hgb was 7.2 g/dL, Hct 21%, and reticulocytes 0.6%. Subsequently, 2 doses of IGIV at a higher dose of 1 g/kg were given on days of life 93 and 96. On day of life 99, the infant's parvoviral load had decreased further to 20 400 IU/mL, and her Hgb and reticulocytes increased to 9.1% and 15%, respectively. She was discharged.
The 2 weeks after discharge saw a further improvement in her Hgb and Hct; reticulocytes were 7.1%. Her parvoviral load continued to decrease to a nadir of 468 IU/mL on day of life 168. On day of life 289, her viral load was noted to have increased to 47 800 IU/mL despite a stable clinical course and normal hematologic parameters. A repeat dose of IGIV was administered on day of life 297 in response to the increase in viral load, but her viral load remained essentially unchanged (less than 2-fold decrease) over the next 3 months. Serum parvovirus serologies on day of life 370 were notable for a positive IgG and negative IgM. Her medical course has been complicated with issues with speech and swallowing secondary to a paralyzed vocal cord, but she has been advancing with respect to age-adjusted developmental milestones and has had no further episodes of anemia.
DISCUSSION
Parvovirus B19 is a small, nonenveloped single-stranded DNA virus. It is a common infectious human pathogen, with up to 50% of young adults and over 90% of elderly individuals being seropositive [1]. The virus causes a variety of clinical manifestations, from Fifth disease and polyarthropathy to myocarditis and neurologic manifestations including meningoencephalitis and stroke [2–4]. Transplacental transmission of B19 after maternal viremia can result in fetal hydrops or death [5, 6]. More rarely, congenital infection may be followed by persistent viremia in the neonate [7–10].
The cellular tropism of B19 is mediated by its recognition of the globoside (erythrocyte P antigen) receptor, and humans lacking this receptor are not susceptible to B19 infection [11]. During B19 viremia, reticulocyte levels decrease due to destruction of erythroid progenitors. Chronic infection with B19 can lead to PRCA in neonates, immunocompromised children, and adults [12, 13]. As noted in this case, B19 infection may induce thrombocytopenia, presumably through inhibition of megakaryocytopoesis [14]. Bony lesions have been described in congenital B19 infection [15], but these were not noted on radiographs in the current case.
Diagnosis of B19 infection relies on either detection of specific antibodies or viral nucleic acids [16, 17]. Measurement of maternal B19-specific IgM is commonly used for assessment of maternal infection, although performance may vary by assay and may not be positive even in confirmed congenital B19 infection [18], as demonstrated in the case presented herein. Detection of low-avidity anti-B19 IgG may suggest recent infection [19], but this modality demonstrates lower sensitivity than PCR and is not currently in widespread clinical use. Polymerase chain reaction-based detection of B19 DNA in amniotic fluid is considered the optimal study for diagnosis of congenital infection [1], and PCR may also be performed on infant serum or bone marrow. Maternal and fetal B19 viral loads are positively correlated, but fetal loads may exceed maternal ones by several orders of magnitude [20]. No viral inclusions were noted on histopathological examination of the placenta, and placental parvovirus PCR was not performed [21].
Congenital B19 infection may lead to fetal anemia and hydrops [2, 6]. Successful intrauterine transfusion for B19-mediated hydrops has been reported in several case series [5, 7, 22]. One report of favorable fetal response to intrauterine therapy with B19 hyperimmune serum has been presented, but this strategy remains experimental [23].
CONCLUSIONS
Only very limited data are available to guide treatment of postnatal PRCA due to in utero B19 infection. A small number of cases of neonates with anemia and documented persistence of B19 infection has been reported [8–10, 24, 25]. The infant in this case may have been at particular risk, because extreme prematurity would have limited transplacental transfer of maternal Ig. In general, close monitoring of serum Hgb, degree of reticulocyte response, and, in some cases, parvoviral load, in conjunction with transfusions in symptomatic neonates, have been performed [9, 10, 24, 25]. Treatment of B19-induced PRCA with IGIV therapy has been reported primarily in immunocompromised adults [13, 26]; however, case reports exist of Ig treatment of neonates and infants with persistent anemia [7–10, 24]. In several cases, stabilization of Hgb and/or decrease in parvoviral load were noted in neonates with PRCA after Ig therapy [7–10, 24]. A standard Ig-dosing scheme for B19 treatment in neonates has not been established. Our patient responded well to 3 doses of 500–1000 mg/kg, leading to a significant decrease in viral load and increase in reticulocyte count. This case of long-term persistence of viremia with stable hematologic markers illustrates that ongoing positive serum parvoviral PCRs should be interpreted with caution in asymptomatic individuals with normal hematologic parameters. Parvovirus B19 load may be useful in distinguishing PRCA from the physiologic Hgb nadir and in assessing response to therapy in the neonatal period. However, the value of longitudinal monitoring of viral load in B19-infected infants in the setting of normal hematologic parameters is less clear and merits further investigation.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grants R01 AI092743 and R21 AI098654 [to A. J. R.] and T32AI007531 [to S. S. N.]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Report of the Committee on Infectious Diseases of the American Academy of Pediatrics (AAP Red Book), 2012.
- 2.Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004; 350:586–97. [DOI] [PubMed] [Google Scholar]
- 3.Douvoyiannis M, Litman N, Goldman DL. Neurologic manifestations associated with parvovirus B19 infection. Clin Infect Dis 2009; 48:1713–23. [DOI] [PubMed] [Google Scholar]
- 4.Servant-Delmas A, Lefrère J, Morinet F, Pillet S. Advances in human B19 erythrovirus biology. Clin Virol 2010; 84:9658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fairley CK, Smoleniec JS, Caul OE, Miller E. Observational study of effect of intrauterine transfusions on outcome of fetal hydrops after parvovirus B19 infection. Lancet 1995; 346:1335–7. [DOI] [PubMed] [Google Scholar]
- 6.Miller E, Fairley CK, Cohen BJ, Seng C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br J Obstet Gynaecol 1998; 105:174–8. [DOI] [PubMed] [Google Scholar]
- 7.Rugolotto S, Padovani EM, Sanna A, et al. Intrauterine anemia due to parvovirus B19: successful treatment with intravenous immunoglobulins. Haematologica 1999; 84:668–9. [PubMed] [Google Scholar]
- 8.Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev 2002; 15:485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson AC, Montegudo AE, Steele RW. Congenital human parvovirus B19 infection with persistent viremia. Clin Pediatr (Phila) 2015; 54:409–413. [DOI] [PubMed] [Google Scholar]
- 10.Lejeune A, Cremer M, von Bernuth H, et al. Persistent pure red cell aplasia in dicygotic twins with persistent congenital parvovirus B19 infection-remission following high dose intravenous immunoglobulin. Eur J Pediatr 2014; 173:1723–6. [DOI] [PubMed] [Google Scholar]
- 11.Brown KE, Hibbs JR, Gallinella G, et al. Resistance to parvovirus B19 infection due to lack of virus receptor. N Engl J Med 1994; 330:1192–6. [DOI] [PubMed] [Google Scholar]
- 12.Adams ST, Schmidt KM, Cost KM, Marshall GS. Common variable immunodeficiency presenting with persistent parvovirus B19 infection. Pediatrics 2012; 130: e1711–5. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzman G, Meyers P, Cohen B, et al. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet 1988; 2:1159–62. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava A, Bruno E, Briddell R, et al. Parvovirus B19-induced perturbation of human megakaryocytopoiesis in vitro. Blood 1990; 76:1997–2004. [PubMed] [Google Scholar]
- 15.Cantey JB, Pritchard MA, Sánchez PJ. Bone lesions in an infant with congenital parvovirus B19 infection. Pediatrics 2013; 131:e1659–63. [DOI] [PubMed] [Google Scholar]
- 16.Buller RS, Storch G. Evaluation of a real-time PCR assay using the lightcycler system for detection of parvovirus B19 DNA. J Clin Microbiol 2004; 42:3326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol 2004; 53:459–75. [DOI] [PubMed] [Google Scholar]
- 18.Bonvicini F, Puccetti C, Salfi NC, et al. Gestational and fetal outcomes in B19 maternal infection: a problem of diagnosis. J Clin Microbiol 2011; 49:3514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enders M, Weidner A, Rosenthal T, et al. Improved diagnosis of gestational parvovirus B19 infection at the time of nonimmune fetal hydrops. J Infect Dis 2008; 197:58–62. [DOI] [PubMed] [Google Scholar]
- 20.de Haan TR, Beersma MF, Claas EC, et al. Parvovirus B19 infection in pregnancy studied by maternal viral load and immune responses. Fetal Diagn Ther 2007; 22:55–62. [DOI] [PubMed] [Google Scholar]
- 21.Rogers BB, Singer DB, Mak SK, et al. Detection of human parvovirus B19 in early spontaneous abortuses using serology, histology, electron microscopy, in situ hybridization, and the polymerase chain reaction. Obstet Gynecol 1993; 81:402–8. [PubMed] [Google Scholar]
- 22.Heegard ED, Hasle H, Skibsted L, et al. Congenital anemia caused by parvovirus B19 infection. Pediatr Inf Dis J 2000; 19:1216–8. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda H, Sakaguchi K, Shibasaki T, et al. Intrauterine therapy for parvovirus B19 infected symptomatic fetus using B19 IgG-rich high titer gammaglobulin. J Perinat Med 2005; 33:561–3. [DOI] [PubMed] [Google Scholar]
- 24.Donders GG, van Lierde S, Van Elsacker-Niele AM, et al. Survival after intrauterine parvovirus B19 infection with persistence in early infancy: a two-year follow-up. Pediatr Infect Dis J 1994; 13:234–6. [PubMed] [Google Scholar]
- 25.Brown KE, Green SW, Antunez de Mayolo J, et al. Congenital anaemia after transplacental B19 parvovirus infection. Lancet 1994; 343:895–6. [DOI] [PubMed] [Google Scholar]
- 26.Crabol Y, Terrier B, Rozenberg F, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus B19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis 2013; 56:968–77. [DOI] [PubMed] [Google Scholar]


