Abstract
Calcifying fibrous tumor (CFT) is a rare mesenchymal tumor, affecting children and young adults with a predilection for the soft tissue and the abdominal cavity. CFT of the tubular gastrointestinal tract is very rare with less than 20 cases of gastric CFTs reported in English literature. This benign hypocellular fibrosclerotic calcifying lesion can resemble other spindle cell tumors particularly sclerosing gastrointestinal stromal tumor of the stomach. Differentiating between these lesions is particularly important for prognostic and therapeutic purposes. Herein a case of gastric calcifying fibrous tumor incidentally detected during bariatric surgery in a 27-year-old woman is described, with a discussion on its clinicopathological features and differential diagnoses.
Key Words: Solitary Fibrous Tumor, Gastrointestinal Stromal Tumor, Stomach
Introduction
Calcifying fibrous tumor (CFT) is a rare benign soft tissue tumor originally described by Rosenthal and Abdul-Karim as childhood fibrous tumor with psammoma bodies (1). Calcifying fibrous pseudotumor was initially thought to represent a reactive process resulting from abnormal tissue healing but later studies suggested a true tumor with a potential for local recurrence of approximately 10% and was renamed as CFT in the current WHO classification. Majority of the CFTs occur in the abdominal cavity and the peritoneum. CFT of the gastrointestinal tract is quite rare. Literature search showed fewer than 20 cases of gastric and 3 small intestinal CFTs reported in English language (2-9). To our knowledge, this is probably the first case report from the Middle East. Gastric CFTs are rare benign lesions which are mostly incidentally detected .This hypocellular calcifying fibrosclerosing lesion should be differentiated from other spindle lesions of the stomach. Herein we discuss a case of incidentally detected gastric CFT, which showed histomorphological resemblance to sclerosing /burnt-out gastrointestinal stromal tumor (GIST).
Case Report
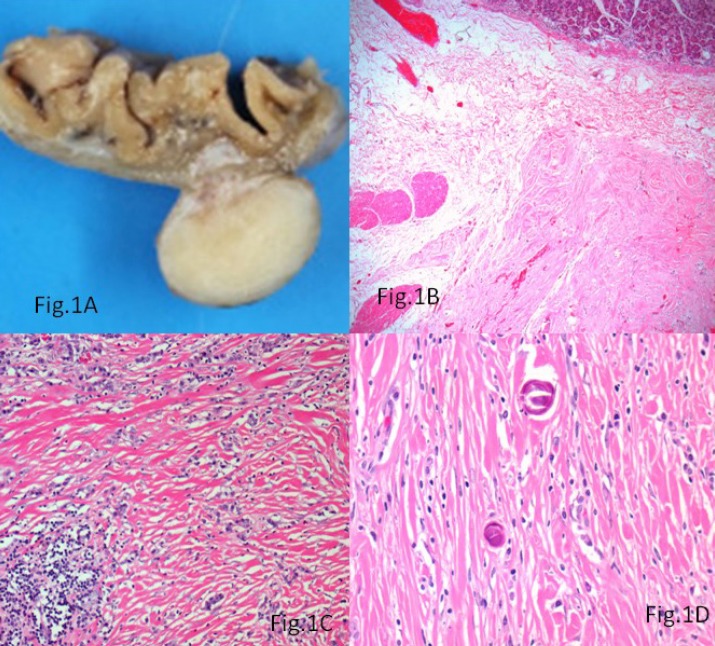
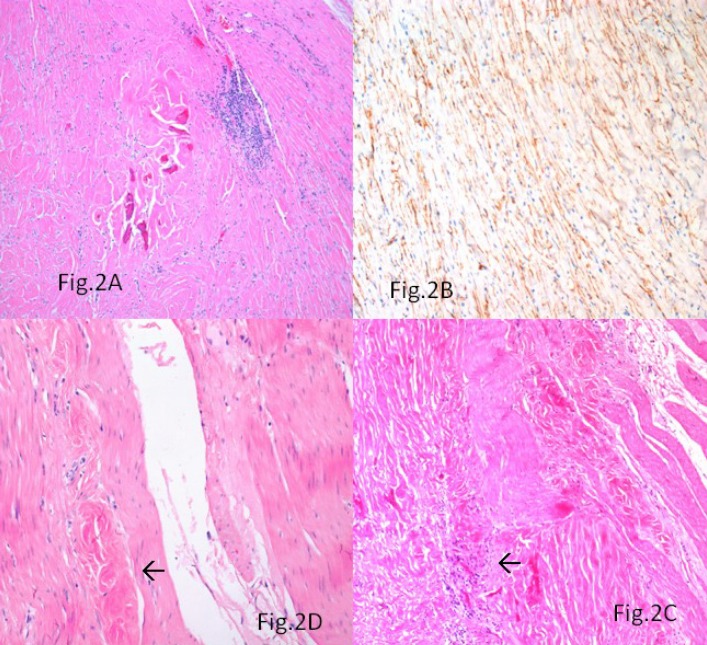
A morbidly obese (BMI-38.8) 27-year-old woman was admitted to the General Surgical Department for elective sleeve gastrectomy. Physical examination and laboratory findings were within normal limits. The patient underwent an upper gastrointestinal endoscopy, which showed mild antral Helicobacter pylori negative chronic gastritis, confirmed at biopsy. Her past medical history did not reveal any significant information. Intraoperatively, a nodular lesion was identified on the serosal aspect of the fundus of the stomach. A gastric sleeve of 21 cm in length along with the serosal nodular lesion was resected. Macroscopically, a 1.5 × 1 × 0.5 cm grey-white firm nodular mass, covered by intact mucosa was identified in the outer muscle coat and subserosa of the gastric sleeve (Fig.1A). Histologically, the mass was involving the subserosa and the outer muscularis propria with focal extension into the submucosa (Fig.1B). The lesion was uniformly hypocellular with hyalinised collagenous tissue containing lymphoplasmacytic infiltrate (Fig. 1C) and foci of psammomatous (Fig.1D) and dystrophic calcifications (Fig.2A). Sparse spindle cells with ovoid vesicular nuclei and fine chromatin were seen dispersed within the collagen bundles. Lymphoid follicles were noted at the periphery of the tumor. The tumor did not show any features of malignancy. Immunohistochemically, the spindle cells were vimentin positive and showed focal CD34 positivity (Fig. 2B). They were negative for SMA, CD117, Desmin, DOG-1, Calretinin and ALK-1.Immunostaining for IgG4 did not show any positivity.
Fig. 1.
A) A circumscribed grey white tumor involving the subserosa and outer muscle coat of the stomach; B) The tumor showing focal submucosal extension (Hematoxylin &Eosin, × 200); C) The fibrosclerotic tissue containing lymphoplasmacytic infiltrate (Hematoxylin & Eosin, ×400); D) Psammomatous calcifications within the lesion (Hematoxylin & Eosin, ×400)
Fig. 2.
A) Dystrophic calcifications within the stroma and lymphoid follicles at the periphery of the lesion (Hematoxylin &Eosin, ×200). (B) Focal CD34 immunopositivity within the spindly cells (avidin biotin peroxidase, ×400). (C) Lymphocytic aggregates at the tumor-muscle interface highlighted by arrow (Hematoxylin &Eosin, ×200). (D) Minute CFT-like hyalinised foci seen in the muscularis propria highlighted by arrow (Hematoxylin & Eosin, ×400
Based on the above histomorphology and immunohistochemical findings, a diagnosis of gastric calcifying fibrous tumour was rendered. The patient is currently asymptomatic, 8 months after surgery.
Discussion
Although around 75% of the CFTs originated in the abdominal cavity and the peritoneum, CFT of the tubular GI tract is quite rare (10). Reported cases of gastric CFTs were mostly incidentally detected as in this case, with a mean age of 53 yr, no sexual predominance, and predominantly involved the body and posterior wall. However, gastric CFT presenting as ulcer has been reported (7). Contrary to the cases reported, the age of our patient was towards the lower limit similar to the experience of Elpek et al. (8). Histologically, the intramurally located paucicellular calcified and hyalinised lesion raised the suspicion of a sclerosing or burnt-out GIST, a more common tumor in this site. However, the presence of psammomatous calcifications and the mononuclear inflammatory infiltrate combined with the lack of immunopositivity for CD117 and DOG-1 in the residual spindle cells favored a diagnosis of CFT. The tumor did not show any histological or immunohistological evidence of inflammatory fibroid polyp (IFP), schwannoma, leiomyoma, Inflammatory myofibroblastic tumour (IMT) or other specific lesion. The focal CD34 positivity demonstrated in the spindle cells has been previously reported (2). The occurrence of calcified bodies with minute lumina and longitudinal smooth- contoured calcified bodies suggesting calcified vascular channels in the tumor has been described which however was absent in our case (2). The presence of lymphocytic aggregates at the tumor-muscle interface (Fig.2C) and minute CFT-like hyalinised foci within the muscularis propria adjacent to the tumor (Fig. 2D) suggest a slowly growing tumefactive process resulting from immune- mediated or other-type tissue injury (2).
The pathogenesis of CFTs remains unclear .They are thought to represent a localized inflammatory fibrosclerosis in response to immune-mediated or other-type tissue injury affecting the muscularis propria. No prior history of any surgeries or tissue injuries in the form of gastric ulcers was elicited in this case. Case reports of CFTs arising in inflammatory myofibroblastic tumor (IMT) and Castleman disease are now considered to represent a peculiar pattern of tissue reaction or repair after minor injuries or focal circumscribed regressive change within the tumor (11). The significance of the presence of scattered IgG4+plasma cells in some of the cases were linked to IgG4 sclerosing lesions but still remains to be explored. Our case was negative for IgG4+ plasma cells.
Despite their morphologic similarity with the soft tissue and peritoneal counterparts, gastric CFTs are unique in that they have smaller size, higher mean age of presentation and no tendency for local recurrence compared to soft tissue CFTs. Meanwhile peritoneal CFTs show a female predominance, young age of presentation and multifocal occurrence (10).
Gastric CFTs should be differentiated from other spindle cell lesions of the stomach particularly sclerosing calcified GIST as it can have prognostic and therapeutic implications. Sclerosing GISTs show lack of psammomatous calcifications and lymphoplasmacytic infiltrates, and the residual spindle cells are immunoreactive for CD117 and CD34. Gastric CFTs do not show KIT/PDGFRA mutations.
The other differential diagnoses to be considered are inflammatory fibroid polyp (IFP), schwannoma, sclerosing leiomyoma, IMT and plexiform fibromyxoma. IFP commonly occurs in the antrum involving the submucosa and is characterized by high cellularity, onion skin pattern and eosinophil rich stroma, features that are absent in CFTs. Reactive nodular fibrous pseudotumor are usually of bigger size, associated with peritoneal surface, express cytokeratin and SMA and are usually associated with history of trauma or surgery. Gastric schwannoma and sclerosing leiomyoma are mainly differentiated by their immunoprofile. Another recently described tumour plexiform fibromyxoma is a uniformly larger tumor of the antrum with prominent capillary network, fibromyxoid stroma and exhibit higher cellularity towards the external border of the tumor with frequent expression of SMA.
Gastric CFTs has a benign course with no tendency for local recurrence. Surgical resection is the main mode of treatment with endoscopic submucosal dissection and resection playing a role in the submucosally located tumors, as reported by Ogasawara et al. (4).
Conclusion
Gastric CFTs are very rare and underdiagnosed. Awareness about their distinct clinicopathological features would help histopathologists to differentiate them from other gastric spindle cell lesions, particularly GIST.
Acknowledgements
The authors declare that there is no conflict of interests.
References
- 1.Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies. Clinicopathologic features in two cases. Arch Pathol Lab Med. 1988;112:798–800. [PubMed] [Google Scholar]
- 2.Agaimy A, Bihl MP, Tornillo L, Wunsch PH, Hartmann A, Michal M. Calcifying fibrous tumor of the stomach: Clinicopathologic and molecular study of seven cases with literature review and reappraisal of histiogenesis. Am J Surg Pathol. 2010;34(2):271–8. doi: 10.1097/PAS.0b013e3181ccb172. [DOI] [PubMed] [Google Scholar]
- 3.Attila T, Chen D, Gardiner GW, Ptak TW, Marcon NE. Gastric calcifying fibrous tumor. Can J Gastroenterol. 2006;20:487–9. doi: 10.1155/2006/378532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogasawara N, Izawa S, Mizuno M, Tanabe A, Ozeki T, Noda H. Gastric calcifying fibrous tumor removed by endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5(9):457–60. doi: 10.4253/wjge.v5.i9.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang KY, Park HS, Moon WS, Lee H, Kim CY. Calcifying fibrous tumor of the stomach: a case report. J Korean Surg Soc. 2012;83(1):56–9. doi: 10.4174/jkss.2012.83.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasilakaki T, Skafida E, Tsavari A, Arkoumani E, Koulia K, Myoteri D et al. Gastric calcifying fibrous tumor: A very rare case report. Case Rep Oncol. 2012;5(2):455–8. doi: 10.1159/000342137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan SF, Yang H, Li Z, Teng GJ. Gastric Calcifying fibrous pseudotumor associated with ulcer: report of one case with a literature review. Br J Radiol. 2010;83(993):e188–e191. doi: 10.1259/bjr/26883993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elpek GO, Kupesiz GY, Ogus M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol Int. 2006;56(4):227–31. doi: 10.1111/j.1440-1827.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- 9.Emanuel P, Qin L, Harpaz N. Calcifying fibrous tumor of small intestine. Ann Diagn Pathol. 2008;12(2):138–41. doi: 10.1016/j.anndiagpath.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Chen KT. Familial peritoneal multifocal calcifying fibrous tumor. Am J Clin Pathol. 2003;119(6):811–5. doi: 10.1309/MXC6-TWEL-UUH4-20W0. [DOI] [PubMed] [Google Scholar]
- 11.Azam M, Husen YA, Pervez S. Calcifying fibrous pseudotumor in association with hyaline vascular type Castleman’s disease. Indian J Pathol Microbiol. 2009;52:527–529. doi: 10.4103/0377-4929.56151. [DOI] [PubMed] [Google Scholar]