Abstract
Background & Objectives:
Colon cancer is currently of high incidence and mortality rate. Identifying the factors influencing its prognosis can be very beneficial to its clinical treatment. Recent studies have shown that lymph nodes ratio can be considered as an important prognostic factor. The aim of the present study is to investigate the effect of this factor on the prognosis of the patients presenting with stage III colon cancer and to compare the result with the effect of lymph node stage on their prognosis.
Materials:
This cross-sectional study was carried out on 66 patients of stage III colon cancer, who met the study inclusion criteria. Patients were categorized into four groups based on Kaplan-Meier plots: LNR1 0-12%, LNR2 13-40%, LNR3 41-84% and LNR4 85-100%. Survival was estimated by Kaplan-Meier method, and differences analyzed by Log-rank test. A Cox proportional hazards model was used for multivariate analysis.
Results:
Lymph nodes ratio was a significantly variable both in overall survival ( P <0.0001) and in disease-free survival ( P =0.009). Lymph node stage was significant in overall survival ( P =0.008) but not in disease-free survival ( P =0.05). Multivariable analysis of overall survival showed lymph nodes ratio as the only independent prognostic factor.
Conclusion:
Lymph node ratio is a more accurate prognostic factor than lymph node stage in overall survival and, in particular, in disease-free survival in patients with stage III colon cancer.
Key Words: Colon cancers, Prognosis, Lymph Nodes
Introduction
Colorectal cancer, which is fatal and quite common (1) is ranked as the fourth malignant tumor in the world (2). In Iran, 5000 new cases of colorectal cancer (7 out of 100000) are reported yearly. The standardized age rate of this cancer in Iran in 2003, 2004 and 2005 was 9.27, 9.64 and 9.90 out of 100000 Iranian males and 9.12, 9.47 and 9.13 out of 100000 Iranian females, respectively (3-5). Considering the prevalence of this cancer, knowing the factors influencing the survival rate of the patients is of great importance. One of these factors is the involvement of lymph nodes, which can decrease the survival rate (2). Right now tumor node metastasis (TNM) staging system is applied to determine the stage of cancer and subsequently its prognosis. This staging system uses some variables such as tumor invasion, local lymph nodes involvement and distant metastasis. Detecting lymph nodes metastasis can reduce 5-year survival rate from 80% in patients at stage II to 50% in patients at stage III, i.e. metastasis to lymph nodes (6, 7). In this respect, many studies have been done to clarify the number of examined optimal lymph nodes for determining the stage of nodes involvement (8-10); however, the obtained results varied and there is no certainty about the minimum number of examined lymph nodes to determine the stage of cancer (11).
Lymph nodes ratio (LNR) which means the ratio of involved to the total resected lymph nodes, is an important prognostic factor in malignancies of colon (11), rectum (12), stomach (13-15), breast (16), bladder (17), and pancreas (18). Studies have suggested that LNR is more precise than positive number of lymph nodes to predict the survival rate (19-21). Therefore, using LNR along with TNM system may help us more to predict the relapse and survival of the cancer (22, 23). LNR can be easily affected by the evaluation of lymph nodes number and their surgical resection; thus, its real value to determine the prognosis remains vague (24).
The preliminary aim of this study was to discover the effect of LNR on the prognosis of the patients presenting with stage III colon cancer and to compare the result with the effect of lymph node stage on their prognosis. Whereas there are limited studies about real impact of the LNR in patients with low lymph nodes, we decided to investigate the effects of LNR and the total number of lymph nodes on overall survival and disease-free survival in these patients, too.
Materials and Methods
In this cross-sectional study, 66 consecutive patients that underwent curative resection for stage III adenocarcinoma of the colon, in two centers (Hazrat Rasoul-e-Akram and Firouzgar hospitals), Tehran, were identified from a departmental database Iran between 2003 and 2008. Patients who had undergone neoadjuvant treatment, colon cancer other than adenocarcinoma NOS type, such as mucinous (10 cases), endocrine (1 case), and signet-ring cell (6 cases), those with marginal involvement (no case), and those who had died due to other causes (1 case due to stomach cancer) were excluded. Special microscopic types of adenocarcinoma such as mucinous carcinoma and signet ring adenocarcinoma have a worse prognosis than the ordinary type of adenocarcinoma. So we excluded these types of cancer to eliminate their confounding effect on the prognosis of patients. Besides, subjects presenting with stage I, II, or IV colon cancer and those with distant metastases were excluded.
Demographic information, lab examination results, biopsy reports, and information about survival and relapse were gathered. While going over the patients’ medical files, we noticed age, sex, tumor location, tumor histologic grade, the total number of lymph nodes, total number of positive lymph nodes, and the ratio of positive lymph nodes to total lymph nodes (LNR), and signs of relapse like increasing serum level of CEA or liver metastasis.
For evaluation of overall survival and disease-free survival, subjects were called, and their consent was obtained for participation in the study, too.
Patients were categorized into four groups based on Kaplan-Meier plots as follow, i.e.: LNR1 0-12%, LNR2 13-40%, LNR3 41-84%, and LNR4 85-100%.
Finally, SPSS 18.0 was used to analyze the data. Survival was estimated by Kaplan-Meier method, and differences analyzed by Log-rank test. The patients who survived or had no evidence of disease recurrence were regarded as censored events. A Cox proportional hazards model was used for multivariate analysis. P value <0.05 was considered significant.
This study was approved by the Ethics Committee of the Iran University of Medical Science.
Results
Demographic and pathologic features of all 66 cases have been shown briefly in Table 1. Of 66 cases, 41 were male and 25 were female. Their ages ranged from 28 to 77. Thirty four of the cases were under 60 and 32 aged 60 and above. The tumor was mostly located in rectosigmoid. The commonest histologic grade was moderate. The total number of lymph nodes ranged from 1 to 45, and the number of metastatic lymph nodes ranged from 1 to 40. The total number of lymph nodes was 12 and above in 28 cases (42.42%) and less than 12 in 38 cases (57.57%). The lymph node stage was categorized into 3 levels according to TNM system of cancer staging (25): N1, 1-3 metastatic lymph nodes; N2a, 4-6 metastatic lymph nodes; N2b, 7 and above metastatic lymph nodes. In this respect, N1 included 38 cases, N2a 12 cases, and N2b 16 cases. LNR percentage ranged from 3.57% to 100%. In our study we determined the cut-off points by using the LNR to draw the Kaplan-Meier survival curve. The average follow-up period was 72.5 months. Overall survival ranged from 6 to 108 months, and disease-free survival ranged from 2 to 102 months.
Table 1.
Demographic and pathologic features of cases
| Variables | Total Number of cases: 66 | |
|---|---|---|
| Gender | Male | 41(62%) |
| Female | 25(38%) | |
| Age Average In Terms Of Year(Sd*) | 56.89(13.21) | |
| Tumor Location |
Right | 18(27%) |
| Left | 4(6%) | |
| Rectosigmoid | 44(67%) | |
| Tumor Differentiation Grade |
High | 19(29%) |
| Moderate | 32(48%) | |
| Low | 15(23%) | |
| Average No. Of Total Lymph Nodes(SD) | 12.32(7.85) | |
| Average No. Of Metastatic Lymph Nodes(SD) | 5.20(6.53) | |
| Average Percentage Of LNR(SD) | 45.81%(34.54) | |
| Number Of Cases In LNR1 | 19(28%) | |
| Number Of Cases In LNR2 | 14(21%) | |
| Number Of Cases In LNR3 | 17(26%) | |
| Number Of Cases In LNR4 | 16(25%) | |
| Average Overall Survival In Terms Of Months(SD) | 50.71(28.39) | |
| Average Disease-free Survival In Terms Of Months(SD) | 43.08(30.33) | |
Standard Deviation
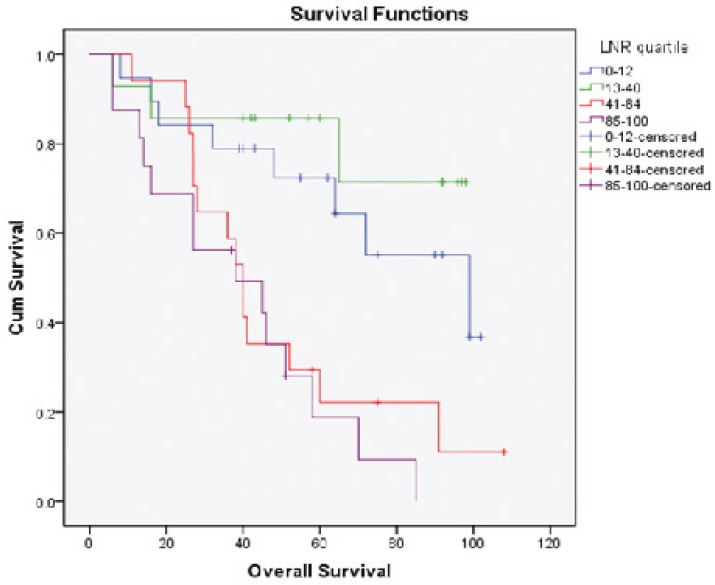
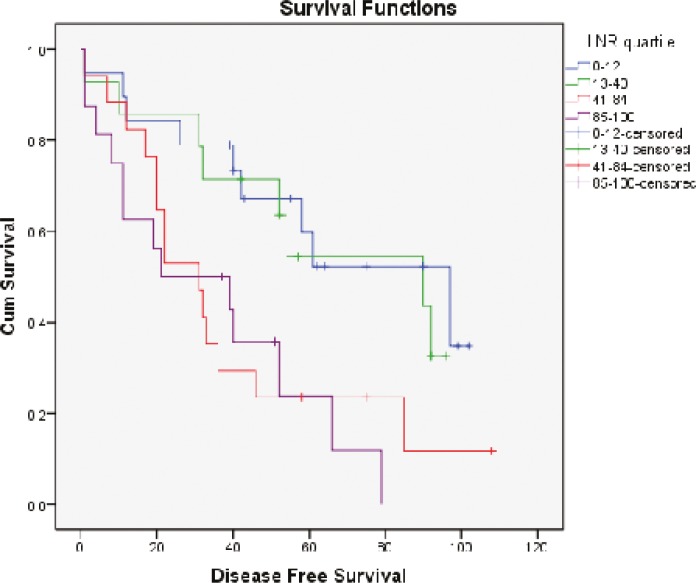
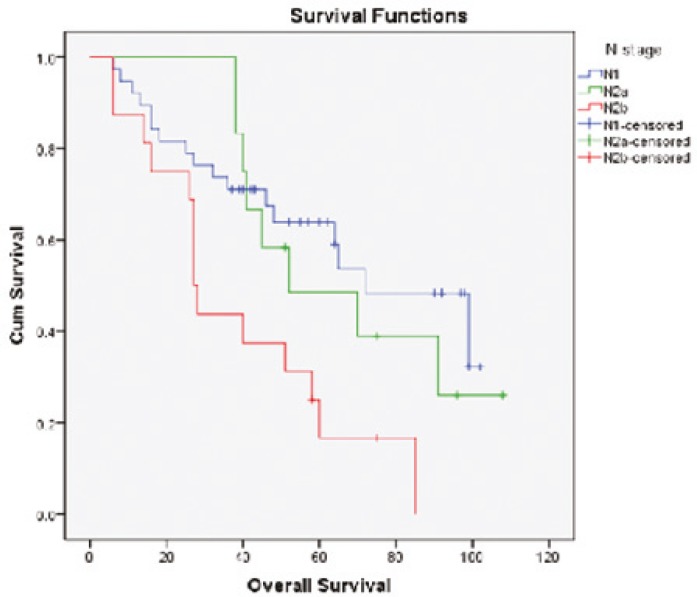
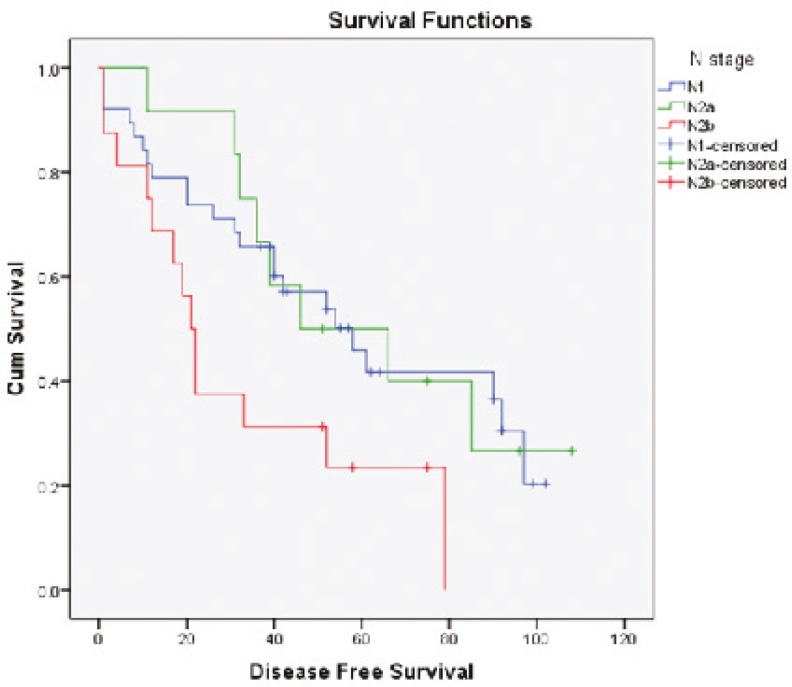
Survival diagrams were drawn twice: once based on LNR and once based on lymph node stage. Overall survival rate for the four subgroups of LNR was 57.9%, 78.6%, 17.6% and 12.5%, respectively. The overall survival rate in LNR1 is lower than that in LNR2, but then along with the increase in LNR, survival rate falls (P<0.0001) (Fig. 1). Disease-free survival rate for the four subgroups of LNR was 52.6%, 42.9%, 17.6% and 18.8% respectively. Along with the increase in LNR, disease-free survival rate declines except for LNR4 which is not significantly different from LNR3 (P=0.009) (Fig. 2). Overall survival determined for each lymph node stage is shown by the third diagram: N1 55.3%, N2a 33.3% and N2b 12.5%. As it is expected, along with the increase in lymph node stage, survival rate decreases and the difference was statistically significant (P=0.008) (Fig. 3). Disease-free survival rate was calculated for each lymph node stage, too: N1 (39.5%), N2a (33.3%) and N2b (18.8%). The difference between N1 and N2a is not statistically significant (P=0.050) (Fig. 4).
Fig.1.
Overall survival curves of patients according to the LNR
Fig.2.
Disease-free survival curves of patients according to the LNR
Fig.3.
Overall survival curves of patients according to the lymph node stage
Fig.4.
Disease-free survival curves of patients according to the lymph node stage
Moreover, we divided LNR into equal ranges of 25% i.e. 0-25%, 26-50%, 51-75% and 76-100%, according to study by Wang et al. (23) and analyzed it. Overall survival rate for LNR1, LNR2, LNR3 and LNR4 was as follows respectively: 61.5%, 46.7%, 28.6% and 11.1% (P=0.003), and disease-free survival rate was as follows respectively: 46.2%, 33.3%, 28.6% and 16.7% (P=0.044).
Considering the above, LNR effect on survival, particularly disease-free survival, is more significant than lymph node stage. The probable prognostic factors in overall and disease-free survival such as age, gender, tumor location, tumor grade, lymph node stage and LNR group were introduced to Cox regression analysis to determine significant variables. Table 2 shows that the LNR and age were the only independent prognostic factors for overall survival and disease-free survival, respectively.
Table 2.
Results of Cox regression analysis to identify the significant variable in overall survival and disease-free survival
| Variables | P Value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Overall Survival | Age | 0.94 | 1.028 | 1.062-0.995 |
| Gender | 0.470 | 1.340 | 2.970-0.605 | |
| Tumor Location(Left) | 0.295 | 3.117 | 15.131-0.642 | |
| Tumor Grade(Moderate) | 0.037 | 0.463 | 1.291-0.166 | |
| Lymph Node Stage (N2a) | 0.234 | 1.695 | 5.929-0.484 | |
| LNR | 0.004 | 1.205 | 2.804-0.518 | |
| Disease –Free Survival | Age | 0.044 | 1.030 | 1.061-1.001 |
| Gender | 0.266 | 1.558 | 3.404-0.713 | |
| Tumor Location(Rectosigmoid) | 0.452 | 2.350 | 9.900-0.558 | |
| Tumor Grade(Low) | 0.008 | 0.316 | 0.826-0.121 | |
| Lymph Node Stage (N2b) | 0.319 | 1.240 | 3.855-0.399 | |
| LNR | 0.055 | 1.600 | 3.887-0.658 |
Discussion
The lymph node ratio, defined as the ratio of involved to the total resected lymph nodes, has gained increasing attention. LNR is more precise than positive number of lymph nodes to predict the survival rate in patients presenting with colorctal cancer (19-21), even after neoadjuvant therapy that absolute number of harvested lymph nodes decreased (26). Therefore, using LNR along with TNM system may help us more to predict the relapse and survival of the cancer (22-23).
This study investigated the role of LNR in the prognosis of 66 patients with stage III colorectal cancer. Among our cases, 28 (42.42%) had 12 and above resected lymph nodes and 38 (57.57%) had less than 12. American Joint Committee on Cancer recommends the assessment of 12 or more nodes for accurate staging (19). However, even in the standardized practices, number of dissected lymph nodes is 12 or fewer in about 50% of patients with colon cancer (27). In the studies by Shimomura et al. (24) and Chin et al (19), only above 12 lymph nodes was considered significant. Also in the study by Berger et al. (2), lymph node ratio was a prognostic factor in patients with 10 or more dissected lymph nodes, but not for patients with less than 10 examined lymph nodes. In contrast, Vaccaro et al. (21), Wang et al. (23) and Chen et al. (28) showed that the prognostic value of the LNR is independent from the total number of resected lymph nodes. The LNR and preoperative CEA level were independent prognostic factors for overall and disease-free survival in patients with fewer than 12 resected lymph nodes, in a study by Huh and colleague, too (29).
Whereas, few studies have evaluated real impact of the LNR in survival of patients with low lymph nodes, we decided to perform the present study without excluding the patients with less than 12 lymph nodes.
The difference in the number of resected lymph nodes from colectomy specimen can be related to the extent of surgical lymph node dissection, surgeon's experience, the thoroughness of the pathologists, and the actual number of regional lymph nodes (29). Insufficient lymphadenectomy in our patients can be attributed to tumor location. Generally, right side of the colon is associated with higher number of resected lymph nodes, because of larger piece of mesenteric lymphatic chains that can be excised during right colectomy than during left colectomy (27). In addition, since ascending colon is thick, right side tumors become symptomatic later than descending colon and rectum. Thus, greater delay in diagnosis lead to more advanced stages. In our study, only 27% of the patients had their tumors in the right colon, and the rest had their tumors in rectosigmoid (67%) and the left colon (6%).
Although we did not exclude the cases with less than 12 lymph nodes, LNR was still a more prognostic factor than lymph node stage both in overall survival and in disease-free survival.
In our study, we determined the cut-off points by using the LNR to draw the Kaplan-Meier survival curve, which is similar with studies by Lee et al. (11), Peschaud et al. (20) and Vaccaro et al. (21).
Our analysis revealed that overall survival rate obtained in LNR1 (57.9%) was lower than that in LNR2 (78.6%), while it was expected to be higher. In disease-free survival, the same difference but slighter is seen between LNR3 (17.6%) and LNR4 (18.8%). The reason can be attributed to the small number of patients in each stage. Significant differences in overall survival rates also were observed by grouping patients according to quartiles of 25 (P=0.003).
A multivariate analysis revealed that the LNR and age were the only independent prognostic factors for overall survival and disease-free survival, respectively.
As the result shows LNR is a significant prognostic factor both in overall survival and in disease-free survival, and low LNR patients were shown to display significant better survival rates than high LNR patients are.
Results of our study are consistent with those of previous studies. Chin and colleagues in 2009 showed that the significance of LNR in predicting of 5-year disease-free survival in stage III colon cancer is better than N stage (19). Peschaud and coworkers in their study demonstrated that “LNR is the most significant prognostic factor for both overall and disease-free survival in patients with rectal cancer, even in patients with fewer than 12 lymph nodes examined” (20). Ng and colleagues in 2009 revealed the ratio of involved to the total resected lymph nodes in node-positive colorectal cancer had great importance (22). Huh et al. determined the prognostic importance of LNR and preoperative CEA level in recurrence rate and survival of colon cancer patients with less than 12 harvested lymph nodes (29). MICU et al. reported “lymph node ratio can be considered a more accurate and potent modality for prognosis in stage III colorectal cancer and may improve stratification in this heterogeneous group of patients” (30). Similarly, prognostic significance of LNR in colon cancer has been evaluated in others studies (11, 12, 21, 23, 28, 31- 33).
In addition, same studies have been conducted in Iran; Nadoshan et al. investigated the prognostic value of lymph node ratio in node positive patients presenting with rectal cancer and recieved neoadjuvant chemoradiotherapy. They have determined the prognostic impact of LNR on survival of these patients who had decreased number of resected lymph nodes (34). Ghahramani and colleagues concluded that “lower total lymph nodes and higher lymph node ratio were associated with poorer oncologic outcomes in patients with colorectal cancer. In addition, they disclosed tumor stage is a more significant prognostic factor than node stage in patients with inadequate lymph nodes evaluation and staging” (35) that is contrary to our findings.
Present study had some limitations such as small sample size and selection of patients from 2 different teaching hospitals and subsequently operation by various surgical teams. It is suggested that more conformation should be considered in future studies. On the other hand, acceptable cutoff value for LNR has not been defined, so extended studies need to be designed for determination of a valid cutoff point.
Conclusion
LNR is an accurate prognostic factor for both overall and disease-free survival rates in the patients with stage III colon cancer and can help to determine the prognosis of colorectal cancers when the accurate staging in patients with fewer than 12 lymph nodes is not possible.
Acknowledgements
The authors declare that there is no conflict of interests.
References
- 1.Ahnen DJ, Macrae FA. Colorectal cancer: Epidemiology, risk factors, and protective factors. http://www.uptodate.com/contents/colorectal-cancer-epidemiology-risk-factors-and-protective-factors.
- 2.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–12. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Registry Report 1986–1987. Tehran, Iran: Ministry of Health, Deputy for Health Directory, CDC Cancer Office; 2008-2009. (Persian) [Google Scholar]
- 4.National Cancer Registry Report 1986–1987. Tehran, Iran: Ministry of Health, Deputy for Health Directory, CDC Cancer Office; 2006-2007 . (Persian) [Google Scholar]
- 5.National Cancer Registry Report 1986–1987. Tehran, Iran: Ministry of Health, Deputy for Health Directory, CDC Cancer Office; 2007-2008. (Persian) [Google Scholar]
- 6.Hermanek P. pTNM and residual tumor classifications: problems of assessment and prognostic significance. World J Surg. 1995;19:184–90. doi: 10.1007/BF00308624. [DOI] [PubMed] [Google Scholar]
- 7.Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg. 1995;180:705–12. [PubMed] [Google Scholar]
- 8.Joseph NE, Sigurdson ER, Hanlon AL, Wang H, Mayer RJ, MacDonald JS, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol. 2003;10:208–13. doi: 10.1245/aso.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein NS, Sanford W, Coffey M, Layfield LJ. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma: trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol. 1996;106:209–16. doi: 10.1093/ajcp/106.2.209. [DOI] [PubMed] [Google Scholar]
- 10.Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol. 1999;17:2896–2900. doi: 10.1200/JCO.1999.17.9.2896. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14(5):1712–7. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 12.Sang-Min Lee, Jong-Seok Shin, Hong-Jo Choi, Ki-Jae Park, Young-Hoon Roh, Hyuk-Chan Kwon, et al. Prognostic implication of metastatic lymph node ratio in node-positive rectal cancer. J Korean Surg Soc. 2011;80:260–6. doi: 10.4174/jkss.2011.80.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakcak I, Yıldız BD, Avşar FM, Akturan S, Kilic K, Cosgun E, et al. Does N ratio affect survival in D1 and D2 lymph node dissection for gastric cancer? . 2011 World J Gastroenterol ;17(35):4007–12. doi: 10.3748/wjg.v17.i35.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–84. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 15.Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–85. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 16.Voordeckers M, Vinh-Hung V, Van de Steene J, Lamote J, Storme G. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol. 2004;70:225–30. doi: 10.1016/j.radonc.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169:943–5. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 18.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–40. [PubMed] [Google Scholar]
- 19.Chin CC, Wang JY, Yeh CY, Kuo YH, Huang WS, Yeh CH. Metastatic lymph node ratio is a more precise predictor of prognosis than number of lymph node metastases in stage III colon cancer. Int J Colorectal Dis. 2009;24:1297–302. doi: 10.1007/s00384-009-0738-7. [DOI] [PubMed] [Google Scholar]
- 20.Peschaud F, Benoist S, Julié C, Beauchet A, Penna C, Rougier P, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–73. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 21.Vaccaro CA, Im V, Rossi GL, Quintana GO, Benati ML, Perez de Arenaza D, et al. Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon Rectum. 2009;52:1244–1250. doi: 10.1007/DCR.0b013e3181a65f0b. [DOI] [PubMed] [Google Scholar]
- 22.Ng M, Roy-Chowdhury S, Lum SS, Morgan JW, Wong JH. The impact of the ratio of positive to total lymph nodes examined and outcome in colorectal cancer. Am Surg. 2009;75:873–6. [PubMed] [Google Scholar]
- 23.Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–8. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 24.Shimomura M, Ikeda S, Takakura Y, Kawaguchi Y, Tokunaga M, Egi H, et al. Adequate lymph node examination is essential to ensure the prognostic value of the lymph node ratio in patients with stage III colorectal cancer. Surg Today. 2011;41:1370–9. doi: 10.1007/s00595-010-4446-2. [DOI] [PubMed] [Google Scholar]
- 25.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 7th ed. New York (NY): Springer; 2010. Colon and rectum. [Google Scholar]
- 26.Kang J, Hur H, Min BS, Lee KY, Kim NK. Prognostic impact of the lymph node ratio in rectal cancer patients who underwent preoperative chemoradiation. J Surg Oncol. 2011;104:53–8. doi: 10.1002/jso.21913. [DOI] [PubMed] [Google Scholar]
- 27.Prandi M, Lionetto R, Bini A, Francioni G, Accarpio G, Anfossi A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg. 2002;235:458–63. doi: 10.1097/00000658-200204000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg. 2011;253(1):82–7. doi: 10.1097/SLA.0b013e3181ffa780. [DOI] [PubMed] [Google Scholar]
- 29.Huh JW, Kim CH, Kim HR, Kim YJ. Factors predicting oncologic outcomes in patients with fewer than 12 lymph nodes retrieved after curative resection for colon cancer. J Surg Oncol. 2012;105(2):125–9. doi: 10.1002/jso.22072. [DOI] [PubMed] [Google Scholar]
- 30.Micu B, Micu C, Leucuta Dc, Crivii C, Constantea N. The role and prognostic impact of lymph node ratio on stage III colorectal cancer. Clujul Medical. 2013;86(3):245–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Lu YJ, Lin PC, Lin CC, Wang HS, Yang SH, Jiang JK, et al. The impact of the lymph node ratio is greater than traditional lymph node status in stage III colorectal cancer patients. World J Surg . 2013;37(8):1927–33. doi: 10.1007/s00268-013-2051-4. [DOI] [PubMed] [Google Scholar]
- 32.Galizia G, Orditura M, Ferraraccio F, Castellano P, Pinto M, Zamboli A, et al. The lymph node ratio is a powerful prognostic factor of node-positive colon cancers undergoing potentially curative surgery. World J Surg. 2009;33:2704–13. doi: 10.1007/s00268-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 33.Kim YS, Kim JH, Yoon SM, Choi EK, Ahn SD, Lee SW, et al. Lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total mesorectal excision followed by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(3):796–802. doi: 10.1016/j.ijrobp.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 34.Nadoshan JJ, Omranipour R, Beiki O, Zendedel K, Alibakhshi A, Mahmoodzadeh H. Prognostic value of lymph node ratios in node positive rectal cancer treated with preoperative chemoradiation. Asian Pac J Cancer Prev. 2013;14(6):3769–72. doi: 10.7314/apjcp.2013.14.6.3769. [DOI] [PubMed] [Google Scholar]
- 35.Ghahramani L, Moaddabshoar L, Razzaghi S, Hamedi SH, Pourahmad S, Mohammadianpanah M. Prognostic value of total lymph node identified and ratio of lymph nodes in resected colorectal cancer. Ann Colorectal Res. 2013;1(3):81–91. [Google Scholar]