Abstract
Background and Objectives:
Angiogenesis is essential for growth and metastasis of solid malignancies. Tumor vessel count and expression of vascular endothelial growth factor (VEGF), a potent angiogenic factor, have been associated with prognosis. This study was designed to assess vessels density by using CD31 and CD105 (Endoglin) and their correlation with expression of VEGF and proliferative index (Ki67) in Glioblastoma multiforme (GBM).
Methods:
We examined these parameters in GBM specimens from 50 adult patients; referred to Al-Zahra hospital Pathology Lab between 2001 to 2006.These patients did not receive pre-operative therapy. Paraffin-embedded tumor specimens were immunohistochemically stained for CD31, CD105 (Endoglin), VEGF and Ki67 (proliferation index) monoclonal antibodies. Microvessel density (MVD) was evaluated by immunostaining for CD31 and CD105.Then the results were compared between the two and also with VEGF receptors and Ki67 index.
Results:
CD105-MVD was significantly higher in Glioblastoma compared with peritumoral normal (14.28 vs. 6.68: P=0.012). We did not find such difference for CD31. The mean of CD105-MVD was significantly higher than CD31-MVD in Glioblastoma tissue (P<0.001) although there was a significant positive relationship between them (Pearson's r=0.630 P<0.001).The VEGF scoring for tumoral tissue was 12 % (score:1), 46% (score:2) and 42% (score:3).For peritumoral normal tissue were 92% (score:1) and 8% (score:2) . So they reach to statistical significance (Chi Square, P = 0001). Both MVD of CD105 and CD31 have significant relationship with VEGF (P<0.001).
Conclusion:
We suggest that Endoglin can be used as a specific and sensitive marker for evaluation of angiogenesis in Glioblastoma.
Key Words: CD105, CD31 Antigens, Vascular Endothelial Growth Factor, Angiogenesis, Glioblastoma Multiforme
Introduction
Glioblastoma multiforme (GBM) is the most frequent and malignant primary tumor of the central nervous system in adults. Despite the availability of aggressive therapies, median survival rates from the time of diagnosis range between 12 and 15 months, and fewer than 3% of patients survive more than 5 years (1). Among solid tumors, this tumor displays the most angiogenic features and the highest degree of vascular proliferation and endothelial cell hyperplasia. Angiogenesis is a key pathologic event in high grade glioma and necessary for the progression of a localised neoplasm to a highly aggressive tumor (2). So microvessel density (MVD) has been reported to be an independent prognostic factor for adult gliomas (3, 4).
CD105 (cluster of differentiation molecule 105) is a transmembrane homodimer protein of 180 kDa which is characteristically found on endothelial (blood vessel) walls (2). It is a key component of the transforming growth factor b (TGFb) receptor signaling pathway, involved with both receptors 1 and 2 (2). CD105 fulfills key roles in angiogenesis and vasculogenesis during development (possibly through preventing apoptosis in hypoxic endothelial cells (2). As a marker for blood vessels CD105 is associated with immature vessels, and some studies have suggested it could be a marker that preferentially stains for novel angiogenic vessels. It has also been proposed that CD105 is a marker for mesenchymal stem cells (5). In some tumor types it has been correlated negatively with prognosis, but its role in high grade gliomas (particularly in childhood) is not yet clears (6-8, 2). Because of its apparent specificity for tumor-associated blood vessels, CD105 is also of interest as a therapeutic target, with monoclonal antibody therapy in early stage clinical trials (9-11).The measurement of angiogenesis is complicated by the fact that it is a dynamic process. Most studies have been focused on the product of angiogenesis, microvascular density (MVD), which is analyzed at a special point of time (12). Conventional immunohistochemical measurement of microvessel density as a proxy marker for angiogenic activity has utilized markers of mature endothelial cells such as CD31, CD34 or factor VIII. Variable results have been achieved, both in high grade gliomas and other tumor types (2) with some groups finding strong correlations with prognosis, but other centers finding no link to outcome (7).
This study was designed to assess vessels density by using CD31 and CD105 (Endoglin) and their correlation with expression of VEGF and proliferative index (Ki67) in Glioblastoma multiforme (GBM).
Materials and Methods
Patients and specimens
Paraffin embedded tissue blocks and H&E stained slides of glioblastoma cases were retrieved from pathology archive of Alzahra Hospital, Medical University of Isfahan, between 2001 and 2010. Among 520 slides which were screened to ascertain their appropriate diagnoses. Among these, 50 appropriate paraffin embedded tissue blocks were selected. The mean age of the studied patients was 48.45 years and sex distribution was 35 males and 15 females.
All specimens were again served for staining with routine Hematoxylin and Eosin sections and evaluated by two pathologists according to WHO Classification of Brain Tumors. Only permanent samples were used for staining and immunohistochemistry. Three slides with 3 μm thicknesses were prepared from each sample and served for staining by CD31, CD105, VEGF and Ki67 monoclonal antibodies.
Immunohistochemical Method
Slides deparaffinized with xylol and then rehydrated with alcohol series. Immunohistochemistry was performed by using the avidin-biotin-peroxidase complex (ABC) method. Endogenous peroxidase activity was blocked by immersing the slides into 0.3% H2O2 for 5-10 min. The sections were then individually covered with specific antibodies. The primary antibody used was a monoclonal antibody (clone SN6H, Dako) at 1:25 dilution for Endoglin, a monoclonal antibody (mAb, clone JC70A, Dako) at 1:50 dilution for CD31, and a monoclonal antibody MIB-1 (Dako) at 1:50 dilution for Ki67 cDNA fragment. In each specimen, the secondary antibody was applied by peroxidase-conjugated streptavidin (Dako) for 15 min and 3,3-diaminobenzidine tetrachloride for 7-10 min as chromogen. For Endoglin immunostaining, specimens were pre-treated with proteinase K enzyme (DAKO) at room temperature for 10 minutes. Prior to staining for CD31 and MIB-1(13), the sections were treated by four washes of 10 mmol/L citrate buffer in a microwave for 5 min. Negative controls were obtained by substituting primary antibodies with non-immune serum.
Slides Evaluation
Microvessel density was evaluated by immunostaining for CD31 and CD105 according to the procedure described by Weidner et al. (14). The four most vascularized areas (hot-spots) in the tumor were located at low magnification (×40) and then counted at (×400) magnification in each of these areas. Each positive endothelial cell or cluster of endothelial cells with or without a lumen in contact with a spot was counted as an individual vessel. The mean of vessels in four fields was used as CD105 microvessel density or CD31 microvessel density. For statistical analysis, we alter the MVD value into the mean number of microvessels/mm2.VEGF stained cells were counted in 1000 cells as a percentage; then scored as 1( less than 25%) , 2 (25-50% ) and 3 ( more than 50%).
The Ki67 proliferating index was calculated in percentage by counting 1000 nuclei of tumor cells. All degrees of nuclear staining intensity were considered as positive.
Statistical Analysis
Data were analyzed by using the SPSS software version 16.0. The mean and SD of microvessel density in tumoral and peritumoral normal tissues were determined. Statistical analysis included one-way analysis of variance, Chi-square, Wilcoxson, Kruskal-Wallis and t- student. A linear regression analysis (Pearson’s r) was used to identify the relationship between two variables. According to odds ratio and 95% confidence interval the probability (P) value less than 0.05 was considered as significant.
Results
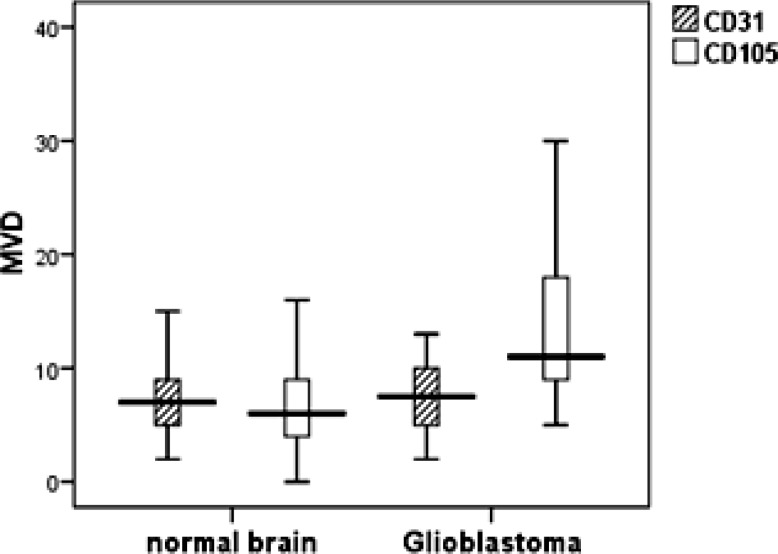
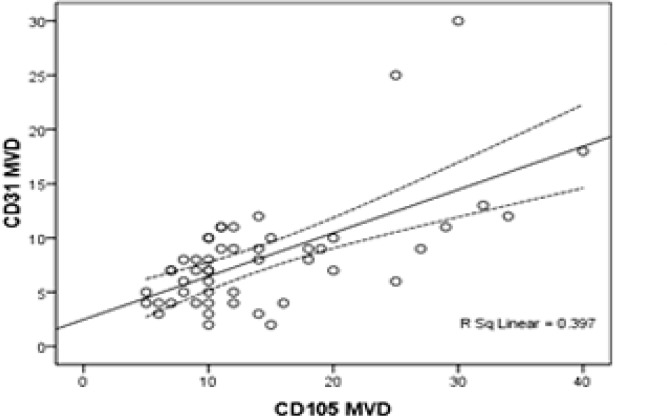
In the 50 Glioblastoma specimens, the mean of CD31-MVD was 8.18 ± 5.15 microvessels/field (range, 2-30) and in 25 peritumoral non-neoplastic tissue, it was 7.88 ± 4.91 microvessels /field (range, 2-25). CD31-MVD did not significantly differ between non-neoplastic and neoplastic areas (P=0.807) (Fig.1). In contrast, CD105 was seldom expressed in the blood vessels of normal brain tissue and only stained the neoplastic blood vessels. The mean microvessel density using CD105 in neoplastic areas was 14.28 ± 8.13 (range, 5-40) compared with 6.68 ± 4.20 (range, 0-16) in non-neoplastic areas. We observed a significant difference between them (P=0.012).The correlation between CD105 –MVD and CD31-MVD is shown in Table 1 and Fig. 2. We introduced a cutoff point of ≥10 for MVD based on visual inspection of individual histograms. In this cutoff point there was a significant difference between CD105-MVD in neoplastic and normal brain tissue (P<0.001). CD105 specificity and sensitivity for diagnosis of neoplastic angiogenesis were respectively 80% and 74% further more odds ratio of CD105 for neoplastic and peritumoral normal tissues was 11:1. We did not detect any significant difference between them for CD31-MVD (P=0.71) even by changing its cutoff point. The mean CD31-MVD compared with the mean CD105-MVD in neoplastic areas was lower (P<0.001). In the case-by-case analysis, CD31-MVD in 10 patients was equal to CD105-MVD and in the other, it was lower than CD105-MVD (Wilcoxson test P<0.001). We observed a statistically positive correlation between them (Pearson's r=0.630 P<0.001, Adjusted r Square=0.385).
Fig.1.
Mean CD105 - MVD and CD31-MVD in glioblastoma and peritumoral non neoplastic brain tissues. ( P=0.807 for CD31-MVD and P=0.012 for CD105-MVD)
Table 1.
Correlation Between Angiogenesis Markers with Neoplastic and Non-Neoplastic Tissues
|
Positive
(≥10 MVD/f) N (%) |
Negative
(<10 MVD/f) N (%) |
P value | |||
|---|---|---|---|---|---|
| Glioblastoma | CD105 | 37(74 ) | 13(34 ) | <0.001 | |
| CD31 | 14(28 ) | 36(72 ) | 0.712 | ||
| Normal Brain | CD105 | 5(20 ) | 20(80 ) | ||
| CD31 | 6(24 ) | 19(76 ) | |||
CD105 odds ratio 11:1 95% Confidence Interval: 3.54-36.53
Fig.2.
Correlation Between CD105 Microvessel Density (MVD) and CD31-MVD in Glioblastomas (Pearson's r=0.630 P<0.001)
The VEGF scoring for tumoral tissue was 12%( score:1), 46%(score:2) and 42% (score:3). In peritumoral normal tissue were 92% (score: 1) and 8% (score: 2). So they reach to statistical significance (P = 0001). Both MVD of CD105 and CD31 have significant positive relationship with VEGF (P<0.001).
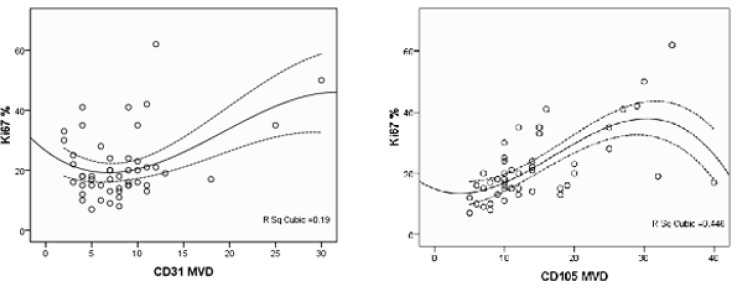
The mean proliferation index (Ki 67) of tumoral cells was 21.44 ±11.258 (range, 7-62). Both CD105 and CD31- MVD was correlated with proliferation index (Pearson's r=0.611 P<0.001, Adjusted r Square=0.360 and Pearson's r=0.360 P=0.01, Adjusted r Square=0.111 respectively); but the association was stronger for CD105 than CD31. A significantly higher Ki67 value was evidenced in cases displaying MVD≥10% in both CD31 and CD105 (P<0.001 and P=0.013 respectively). Using a cubic regression model to survey the relationship between Ki67 with CD31 and CD105-MVD, we observed a stronger correlation between them than linear model (r=0.668 Adjust r square=0.41 P<0.001 and r=0.436 Adjust r square=0.137 P=0.02 respectively) (Fig. 3).
Fig. 3.
Correlation of CD105 and CD31 MVD with Proliferation Index (Ki 67) of Glioblastoma Cells. (R=0.668 P<0.001 for CD105-MVD and R=0.436 P=0.02 for CD31-MVD)
Discussion
Tumor angiogenesis and its clinical significance have been studied in a variety of cancers. In this study, we compared anti-CD31 mAb with anti-CD105 mAb to introduce a better marker in evaluation of Glioblastoma angiogenesis. We revealed a difference in CD105 expression in endothelial cells between non-neoplastic and neoplastic areas in glioblastoma.
In contrast to Endoglin, CD31 a pan-endothelial marker was expressed in endothelial cells of both normal and neoplastic tissues. It means that CD105 is a specific marker for recognizing the angiogenesis in neoplastic tissue. This is in line with previous studies on meningiomas (15) and pediatric brain tumors (7). In the study conducted by Minhajat et al. (16), this subject was revealed on several tumors including: brain, lung, breast, stomach, and colon cancers. But in their study, there were only nine Glioblastoma specimens. To our knowledge it was the first time that this comparison was performed on Glioblastoma and normal brain tissue with 50 samples. In this study we showed that CD105 expression in neoplastic endothelial cells was more than CD31 expression. Thus, it can be said that CD105 is a sensitive angiogenesis marker in neoplastic tissues. This subject was previously shown by several studies on Glioblastoma, breast, prostate cancer, colorectal and endometrial carcinomas and tongue SCC which showed that CD105 was more expressed in the neoplastic vessels compared with CD31. However, some studies on meningiomas, oligodendrogliomas, and craniopharyngiomas showed that CD34 (another pan-endothelial marker) is expressed more than CD105 in the neoplastic endothelial cells (6, 15, 17). Yao et al. (18) did not show any difference between CD105-MVD and CD31-MVD in Glioblastoma and anaplastic astrocytomas (two high grade Gliomas) and also showed that, in low grade astrocytoma CD31 expression was higher than CD105. The hypothesis that explains this difference between various studies is entrapment of preexisting vessels, in low grade tumors which is different from neovascularization in high grade tumors (12, 18). Furthermore CD34 is not a specific marker and can stain some mesenchymal cells and may be difficult to interpret (19). We also showed a positive relationship between CD105 and CD31-MVD in Glioblastoma. This association between CD105 and pan-endothelial markers was in line with previous studies on Glioblastoma and other tumors (8, 15).
We also determined a cutoff point for MVD in Glioblastoma which figures more than it showed abnormal angiogenesis. In this point, specificity and sensitivity of CD105 for diagnosis of neoplastic angiogenesis were 72% and 80% respectively; whereas there was no difference between CD31-MVD in Glioblastoma and normal brain tissue. We showed that CD105 odds ratio was 11:1 which means that abnormal angiogenesis (CD105-MVD≥10 microvacular/field) is 11 times more in Glioblastoma than normal brain tissue. Interestingly, Mineo et al. (20), in their study on non-small cell lung cancer demonstrated a cutoff point, much higher than our cutoff and by a multivariate analysis showed that only CD34 but not CD31 and CD105 were significant predictive factors for overall survival.
VEGF expression significantly correlated with high CD105 and CD31 expression. The same results have been obtained in previous studies on Glioblastoma, hepatocellular carcinoma, colon, prostate and lung cancers;but in renal cell carcinoma VEGF does not correlate with MVD(21-25). In this study, we have demonstrated that there was a positive relationship between Ki67 (proliferation index) with CD105-MVD and CD31-MVD, but this association was stronger with CD105-MVD. While 36% from deviation of Ki67 obtained by CD105-MVD variations, only 11% from it obtained by CD31-MVD variations. Prior study on Glioblastoma had shown this association only with CD105-MVD (8).This suggests that tumor angiogenesis could play a role in furthering cell proliferation, as in some other tumors (8).
Previous studies on brain tumors have shown that Ki67 increases with tumor malignancy grade (13, 26, 27). Ki67 also correlated with the possibility of recurrence and survival of brain tumors (13). Therefore, the association between Ki67 and MVD can show the significance of MVD in prognosis of brain tumors. Of course, this association was assessed in previous studies (4). Because the association between Ki67 with CD105-MVD was stronger, we assumed that CD105 is a better marker for evaluation the prognosis of brain tumor. We did not perform this survey but previous studies showed it in Glioblastoma and other cancers (8,18).
We performed a cubic regression model between Ki67 with CD31-MVD and CD105-MVD and observed that this model obtained their associations in a better way. By this plot, it appears that the cubic model follows the shape of the data in a better way. This model says that, after a certain point, the Ki67 decreases. Of course this should be studied more to be accepted. Further studies should be performed to elucidate this model in various tumors with various vascularities.
Our defects in this study were lower control samples and other demographic data was not determined.
Conclusion
We could say that endoglin is a specific and sensitive marker for evaluation of angiogenesis in Glioblastoma.
Acknowledgments
Authors wish to express their gratitude to all patients who participated in this study. We would also like to thank pathology personnel of Al-Zahra Hospital for their sincere technical assistance. The authors declare that there is no conflict of interests.
References
- 1.Galan-Moya EM, Le Guelte A, Lima Fernandes E, Thirant C, Dwyer J, Bidere N, et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011;12(5):470–6. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith SJ, Tilly H, Ward JH, Macarthur DC, Lowe J, Coyle B, et al. CD105 (Endoglin) exerts prognostic effects via its role in the microvascular niche of paediatric high grade glioma. Acta Neuropatho. 2012;124(1):99–10. doi: 10.1007/s00401-012-0952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumor vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 4.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 6.Netto GC, Bleil CB, Hilbig A, Coutinho LM. Immunohistochemical evaluation of the microvascular density through the expression of TGF-beta (CD 105/endoglin) and CD 34 receptors and expression of the vascular endothelial growth factor (VEGF) in oligodendrogliomas. Neuropathology. 2008;28(1):17–23. doi: 10.1111/j.1440-1789.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 7.Birlik B, Canda S, Ozer E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl Neurobiol. 2006;32(5):532–8. doi: 10.1111/j.1365-2990.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 8.Behrem S, Zarkovic K, Eskinja N, Jonjic N. Endoglin is a better marker than CD31 in evaluation of angiogenesis in glioblastoma. Croat Med J. 2005;46(3):417–22. [PubMed] [Google Scholar]
- 9.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–23. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman R, Smith S, Rahman C, Grundy R. Antiangiogenic therapy and mechanisms of tumor resistance in malignant glioma. J Oncol. 2010;2010(25):1231. doi: 10.1155/2010/251231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumors. Cardiovasc Res. 2010;86(1):12–9. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 12.Saad RS, El-Gohary Y, Memari E, Liu YL, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol. 2005;36(9):955–61. doi: 10.1016/j.humpath.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Kayaselçuk F, Zorludemir S, Gümürdühü D, Zeren H, Erman T. PCNA and Ki-67 in central nervous system tumors: correlation with the histological type and grade. J Neurooncol. 2002;57(2):115–21. doi: 10.1023/a:1015739130208. [DOI] [PubMed] [Google Scholar]
- 14.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 15.Barresi V, Cerasoli S, Vitarelli E, Tuccari G. Density of microvessels positive for CD105 (endoglin) is related to prognosis in meningiomas. Acta Neuropathol. 2007;114(2):147–56. doi: 10.1007/s00401-007-0251-4. [DOI] [PubMed] [Google Scholar]
- 16.Minhajat R, Mori D, Yamasaki F, Sugita Y, Satoh T, Tokunaga O. Organ-specific endoglin (CD105) expression in the angiogenesis of human cancers. Pathol Int. 2006;56(12):717–23. doi: 10.1111/j.1440-1827.2006.02037.x. [DOI] [PubMed] [Google Scholar]
- 17.Dallago CM, Oliveira MC, Barbosa-Coutinho LM, Ferreira NP. Angiogenesis in craniopharyngiomas: Microvascular density and tissue expression of the vascular endothelial growth factor (VEGF) and endostatin. Endocr Pathol. 2005;16(4):355–62. doi: 10.1385/ep:16:4:355. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25(3):201–6. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 19.Mikalsen LT, Dhakal HP, Bruland OS, Nesland JM, Olsen DR. Quantification of angiogenesis in breast cancer by automated vessel identification in CD34 immunohistochemical sections. Anticancer Res. 2011;31(12):4053–60. [PubMed] [Google Scholar]
- 20.Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, et al. Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7(11):3410–5. [PubMed] [Google Scholar]
- 21.Behrem S, Zarkovic K, Eskinja N, Jonjic N. Endoglin is a better marker than CD31 in evaluation of angiogenesis in glioblastoma. Croat Med J. 2005;46(3):417–22. [PubMed] [Google Scholar]
- 22.Yao Y, Pan Y, Chen J, Sun X, Qiu Y, Ding Y. Endoglin (CD105) expression in angiogenesis of primary hepatocellular carcinomas: analysis using tissue microarrays and comparisons with CD34 and VEGF. Ann Clin Lab Sci. 2007;37(1):39–48. [PubMed] [Google Scholar]
- 23.Minhajat R, Mori D, Yamasaki F, Sugita Y, Satoh T, Tokunaga O. Endoglin (CD105) expression in angiogenesis of colon cancer: analysis using tissue microarrays and comparison with other endothelial markers. Virchows Arch. 2006;448(2):127–34. doi: 10.1007/s00428-005-0062-8. [DOI] [PubMed] [Google Scholar]
- 24.Gohary YM, Silverman JF, Olson PR, Liu YL, Cohen JK, Miller R, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007;127(4):572–9. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 25.Raica M, Cimpean AM, Anghel A. Immunohistochemical expression of vascular endothelial growth factor (VEGF) does not correlate with microvessel density in renal cell carcinoma. Neoplasia. 2007;54(4):278–84. [PubMed] [Google Scholar]
- 26.Gupta K, Radotra BD, Banerjee AK, Nijhawan R. Quantitation of angiogenesis and its correlation with vascular endothelial growth factor expression in astrocytic tumors. Anal Quant Cytol Histol. 2004;26(4):223–9. [PubMed] [Google Scholar]
- 27.Lebelt A, Dziecioł J, Guzińska-Ustymowicz K, Lemancewicz D, Zimnoch L, Czykier E, et al. Angiogenesis in gliomas. Folia Histochem Cytobiol. 2008;46(1):69–72. doi: 10.2478/v10042-008-0009-4. [DOI] [PubMed] [Google Scholar]