Abstract
Background and Objective:
Clinical behavior of basal cell carcinoma (BCC) is known to be different according to histological growth pattern and basosquamous cell carcinomas (BSC) are known with their aggressive behavior and metastatic capacity. In this study, we evaluated bcl-2 and cyclin D1 expressions in BCC and BSC cases comparatively, to explore their predictive value on the aggressive behavior of these tumors.
Methods:
One hundred tumors belong to 92 patients diagnosed as basal cell carcinoma and basosquamous carcinoma were studied. Basal cell carcinomas were classified as aggressive and non-aggressive types according to growth pattern. Number of Cyclin D1 and bcl-2 positive cells in immunohistochemically stained serial sections were scored as low (0-1 +) and high (2 and 3+) in all tumors.
Results:
A statistically significant difference was found between non-aggressive (nodular type) and aggressive types (micronodular, infiltrative types and BSC) for these markers ( P <0.005). Cyclin D1 was higher in the aggressive group, while bcl-2 was lower in the aggressive group compared to the non-aggressive group.
Conclusion:
Higher Cyclin D1 and lower bcl-2 scores was correlated with aggressive tumor types and these results could be used as markers to predict aggressive behavior in BCC and BSCs.
Key Words: Basosquamous Carcinoma, Basal Cell Carcinoma, Cyclin D1, Bcl-2 Gene, Aggression
Introduction
Basal cell carcinoma (BCC) usually has a slow clinical course, minimal soft tissue invasion and a high cure rate (1, 2). It constitutes approximately 70% of all malignant tumors of the skin and is the most common cutaneous tumor (3). Exposure to intermittent and intense UV light and having a skin sensitive to sunlight are strong risk factors (4, 5). BCCs can rarely act aggressively and cause deep invasion, recurrence, and regional or distant metastases (3).
The relationship between the behavior and the growth pattern of BCC has been revealed and a classification system has been recommended accordingly (6). Accordingly, infiltrative, morpheaform and basosquamous subtypes tend to be more aggressive than nodular types. Long-term lesions and aggressive tumors often extend deep into the dermis. Deep extension may be diffuse or through the adnexae.
Basosquamous cell carcinoma was first described in 1910 and 1912 by MacCormac and Korlb (7). It is a morphologically intermediate type between basal cell carcinoma and squamous cell carcinoma (SCC) (2, 8). This tumor is focal Ber EP4 positive in some areas as opposed to the SCC. While BCC does not show EMA positivity, focal positivity is observed in basosquamous carcinomas (9). Similar to SCC, it is more local invasive than other forms of BCC. It is more prone to metastasis and local recurrence (8, 10).
A large gene family that regulates apoptosis has been identified. The first identified and best known anti-apoptotic gene is bcl-2. Bcl-2 is protein of 24 kDa localized at the 18th chromosome. Endoplasmic reticulum is expressed from nuclear and mitochondrial membranes. It extends the life of the cell without stimulating cell proliferation. While bcl-2 and bcl-xl play an inhibiting role on apoptosis, bax, bad, bcl-xs from the same family play an activating role (11). There are studies indicating that bcl-2 positive tumors have a slower course and better prognosis.
Transition from the G1 phase to the S phase in the cell cycle is regulated with the interaction of several groups of proteins with each other. Cyclin D1 (prad-1, bcl-1) is localized at the 11th chromosome. Cyclin D1 and the cyclin-dependent kinase complex phosphorylate the retinoblastoma (Rb) protein, making the cell pass on to other phases of cell division (12-14). The T (11;13) (q13;q32) translocation leads to excessive secretion of cyclin D1 (14) and its presence has been demonstrated in several malignant tumors.
The aim of this study was to investigate the relationship of the overexpression of cyclin D1, a cell cycle regulator, and bcl-2, an anti-apoptotic gene with aggressive behavior, in basal cell carcinoma and to reveal how these two immunohistochemical markers can be used in daily practice to foresee the course of BCCs and determine treatment and follow-up.
Materials and Methods
One hundred tumors obtained from 92 patients diagnosed with BCC and basosquamous cell carcinoma were evaluated in this study. Twelve patients had a recurrent tumor. The primary and recurrent tumor could be accessed in 7 cases. However, the primary tumor of five patients could not be accessed as the diagnosis had been made at an external center. Information was obtained by accessing the clinic or the patient in recurrent tumors. Each pattern was examined separately in mixed types with nodular and infiltrative areas and nodular and micronodular areas.
The information related to the cases such as age, gender and tumor location was obtained from the biopsy reports.
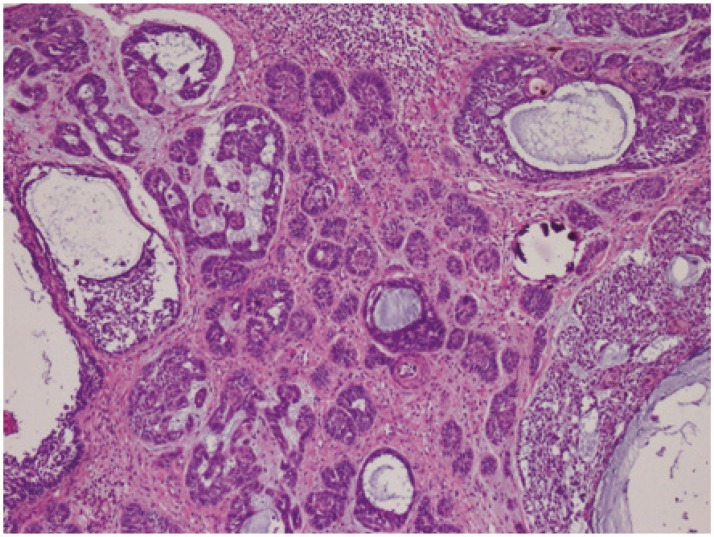
Four 4 micron thick sections were made from each paraffin block of the 100 tumors obtained from 92 patients. One of every 4 profiles was stained with hematoxylin-eosin and evaluated under the light microscope. Tumors were histopathologically divided into 3 different groups as aggressive (infiltrating BCC, micronodular BCC (Fig.1), noduloinfiltrative BCC, recurrent cases, and basosquamous carcinoma) and non-aggressive (nodular). The presence of desmoplasia was also evaluated.
Fig.1.
Micronodular BCC (Basal Cell Carcinoma) (H&E ×40)
When evaluating recurrence, surgical boundaries of the primary tumors were revaluated in cases whose primary and recurrence material were accessed and tumors with negative surgical boundaries were included in the study.
In order to evaluate cyclin D1 and bcl-2 expression with IHC method, 4 micron-thick sections from the tissued fixated with 10% buffered formalin and embedded in paraffin were taken to slides with polylysine. They were boiled under pressure with EDTA at a Ph value of 8 for 2 min and the antigen was exposed. Primary antibodies were placed as drops for cyclin D1 (DAKO, clone: SP4, RTU) and bcl-2 (DAKO, clone: 124, RTU), and the staining procedures were performed in accordance with the manufacturer's instructions.
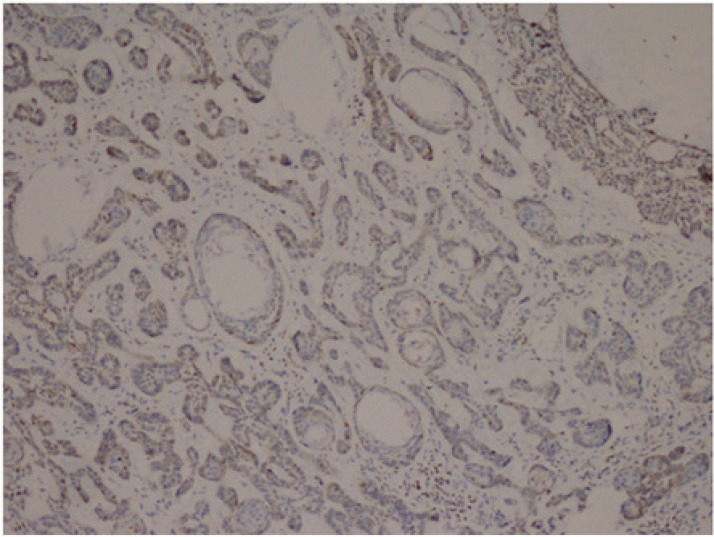
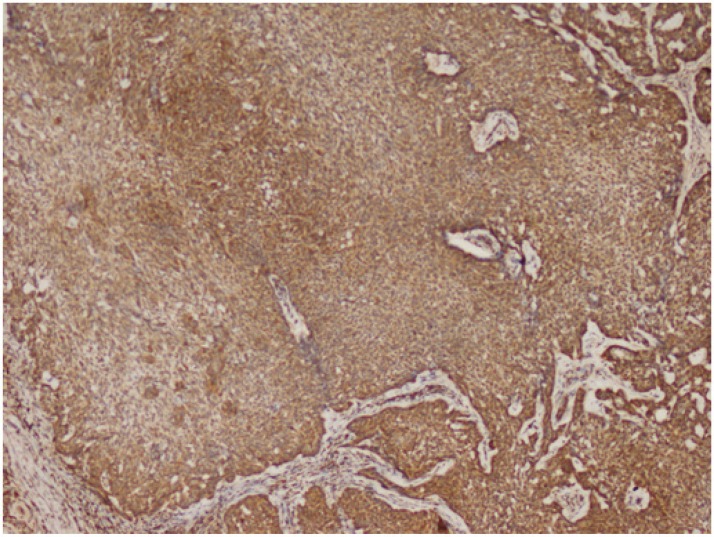
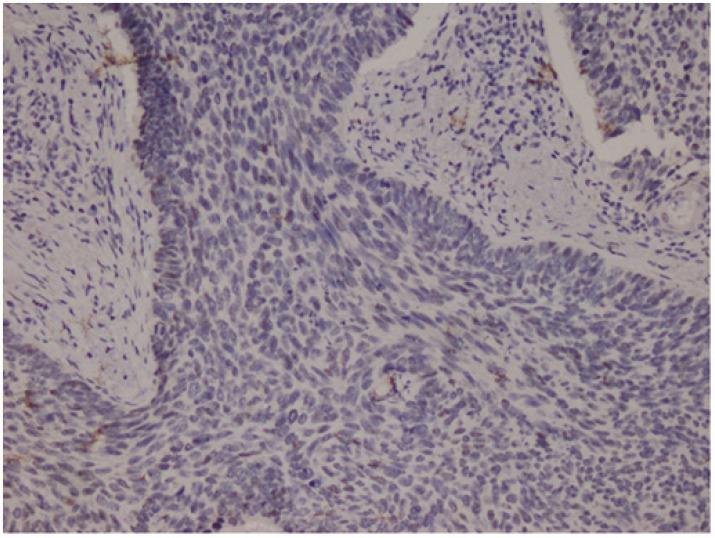
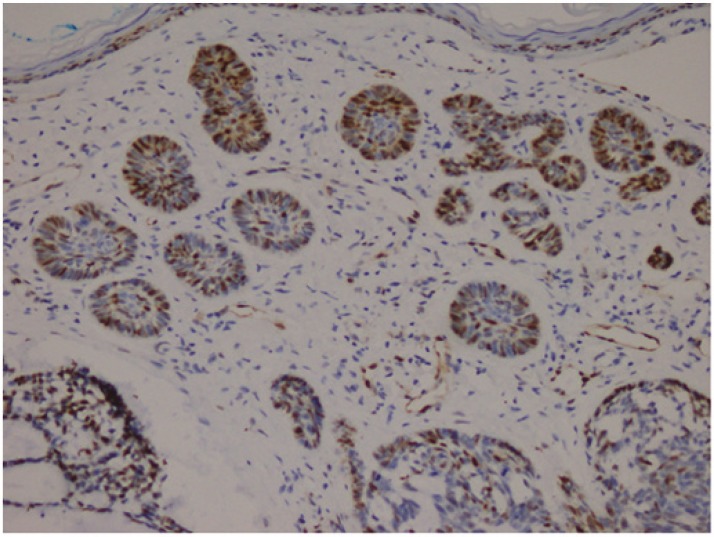
Cytoplasmic staining was considered positive for Bcl-2 evaluation. All preparations were evaluated in comparison with each other and a minimum of 1000 cells was counted and positivity rates measured. The grading used was 1+: Between 0 and 25% (Fig.2), 2+: between 26 and 50%, 3+: between 51 and 75%, and 4+ : >75% (Fig.3) cell positivity. Cyclin D1 positivity was graded as 1+: between 0 and 5% (Fig.4), 2+: between 5 and 25%, 3+: between 26 and 50%, and 4+: >50% (Fig.5) cell staining. 1 or 2 positive staining was considered "low" and 3 or 4 positive staining "high" for both markers.
Fig.2.
Micronodular BCC (Basal Cell Carcinoma), Bcl-2, 1+ staining (Bcl-2 ×40)
Fig.3.
Nodular BCC(Basel Cell Carcinoma), Bcl-2, 4+ staining (Bcl-2 ×100)
Fig.4.
Nodular BCC, Cyclin D1, 1+ staining (Cyclin D1, ×100)
Fig.5.
Micronodular BCC(Basel Cell Carcinoma), Cyclin D1, 4+ staining (Cyclin D1, ×100)
Statistics
The relationship between age and histological development patterns was evaluated with Anova analysis and post-hoc tests in statistical analyses. The relationship between cyclin D1 and bcl-2 and the histological development patterns was investigated with Pearson (χ2) chi-square and Fisher Exact test as required.
Results
One hundred tumors obtained from 92 patients were evaluated. There were 53 (58%) males and 39 (42%) females. The ages changed between 31 and 93 yr. The mean ages for the females and males were quite similar. The distribution of the lesions by subtype is presented in Table 1. There were 23 non-aggressive tumors (nodular type), and 77 aggressive tumors (infiltrative BCC, BSC, and mixed types). In the aggressive group, there were 44 BCC, 22 BSC, and 9 mixed type lesions.
Table 1.
The distribution of Cyclin D1 in aggressive and non-aggressive groups
| Histological Type | Cyclin D1 |
P value | |||
|---|---|---|---|---|---|
| 1+, 2+ (LOW) |
3+, 4+ (HIGH) |
||||
| Number of Patients (N) | % | Number of Patients (N) | % | ||
| BSC + I + MN | 43 | 55.9 | 34 | 44.2 | <0.005 |
| N | 26 | 81.3 | 6 | 18.7 | |
| TOTAL | 69 | 63.3 | 40 | 36.7 | |
I: Infiltrative BCC, N: Nodular BCC, MN: Micronodular BCC, BSC: Basosquamous carcinoma
Recurrence was present in 12 of the 92 patients. These recurrences consisted of 3 BSC, 9 infiltrative and 2 nodular type cases. Primary tumors of 4 cases could not be accessed. One of the recurrent tumors that could not be accessed was nodular in appearance. Besides, the primary of one tumor was infiltrative type while the recurrence had basosquamous cell carcinoma histology. Desmoplasia was seen in infiltrative BCC and BSC cases.
When cyclin D1 and bcl2 values were evaluated as low (0-1+) and high (2-3+) groups, there was a statistically significant difference between the non-aggressive (nodular type) group and aggressive types (BSC, infiltrative, micronodular) for both markers (Table 1 and 2).
Table 2.
The distribution of Bcl-2 in aggressive and non-aggressive groups
| Histological Type | Bcl-2 |
P value | |||
|---|---|---|---|---|---|
| 1+, 2+ (LOW) |
3+, 4+ (HIGH) |
||||
| Number of Patients (N) | % | Number of Patients (N) | % | ||
| BSC + I + MN | 63 | 81.8 | 14 | 18.,2 | <0.005 |
| N | 9 | 28.1 | 23 | 71.9 | |
| Total | 72 | 66.1 | 37 | 33.9 | |
I: Infiltrative BCC, N: Nodular BCC, MN: Micronodular BCC, BSC: Basosquamous carcinoma
When we evaluated the aggressive types of BSC and infiltrative BCC separately, there was no significant difference between them in terms of bcl-2 and cyclin D-1.
No statistically significant difference was found with the statistical comparisons performed between gender, age, tumor location and the cyclin D1, bcl-2 expression and tumor subtypes.
Discussion
Basal cell carcinoma is the most common type of skin cancer. Although the risk of metastasis and tumor-related death is quite low, locally aggressive types can progress to deeper tissues, especially in the head and neck region, and lead to recurrence and morbidity (2, 15-18).
The development pattern plays an important role among the parameters used to determine the prognosis of basal cell carcinomas and accordingly tumors are divided into two groups as aggressive and non-aggressive (1,2,18). Nodular type tumors constitute the non-aggressive group which can easily be excised with negative and well-defined boundaries while the infiltrative, micronodular and morfeiform types constitute the aggressive group. Difficulty in clinically determining the boundaries in aggressive tumors makes the excision with a safe boundary difficult and a higher rate of recurrence is encountered (19). A combination of more than one development pattern can be found together in some tumors and the classification is made according to the predominant type in this case (20).
Changes occurring during the developmental process in the tumor cells are reflected in the character of the stroma, leading to myofibroblastic proliferation and desmoplasia in infiltrative types (2, 21). A parallelism has been shown between aggressive behavior and desmoplasia, which is evaluated with smooth muscle actin positivity in the tumor stroma (22).
Desmoplasia was seen in patients with infiltrative BCC and BSC in our study. It was not seen in the micronodular and nodular types.
Basosquamous carcinoma is a skin cancer with known aggressive behavior and metastatic capacity (7, 23-25). They can be identified as lesions containing the characteristics of both basal and squamous cell carcinomas, and their transitional features between these two types is emphasized (9). The distinction of these tumors with an aggressive development pattern and desmoplastic stroma from other aggressive types can create problems. Immunohistochemical studies have shown that positivity for Ber EP4 in the BCC-like and for EMA in the SCC-like areas helps with the diagnosis (26). On the other hand, a positive result was obtained with bcl-2 in the typical BCC areas but the result was negative in transition areas and SCC-like areas (27).
Ramdial et al. graded bcl-2 positivity from one to four in 50 non-aggressive and 25 aggressive BCC cases and evaluated it in comparison with the development pattern. Although bcl-2 positivity was present in all BCC cases, a significant relationship was found between low-grade (1-2+) positivity and aggressive behavior. High positivity (3-4+) was found in the nodular and superficial types. There was 2-3+ staining in micronodular BCCs (28). Similarly, bcl-2 expression was higher in non-aggressive types compared to aggressive cases in our study. Our findings were statistically significant (P=0.01).
The role of cyclin D1 in BCC biological behavior was investigated by Staibano et al. (12) for the first time and it was emphasized that this behavior can be used as an independent parameter to differentiate aggressive and non-aggressive tumors. Saldanha et al. focused on cyclin D1 and aneuploidy as aggressive phenotype and poor prognosis signs (1). When we evaluated cyclin D1 values as low (1-2 +) and high (3-4 +) groups, the cyclin D1 expression in the non-aggressive (nodular type) group was higher than in the aggressive group. Our results were statistically significant (P=0.040).
We found no significant difference between the infiltrative BCC and BSC cases in the aggressive group in terms of cyclin-1 and bcl-2 expression.
Conclusions
Immunohistochemically detected cyclin D1 and bcl-2 are markers that can be used to predict aggressive behavior in BCC and BSCs, and the infiltrative and micronodular growth pattern seen in the mixed types should also be noted in the report besides the infiltrative and micronodular types in the aggressive group.
Acknowledgements
This work was supported (financial and material) by the Ege University, İzmir, TURKEY.
Disclosure summary.
Conflict of interest
All authors declare that there is no conflict of interests.
References
- 1.Saldanha G, Fletcher A, Slater DN. Basal cell carcinoma. A dermatopathological and moleküler biological update. Br J Dermatol. 2003;148(2):195–202. doi: 10.1046/j.1365-2133.2003.05151.x. [DOI] [PubMed] [Google Scholar]
- 2.Walling HW, Fosko SW, Geraminejad PA, Whitaker DC, Arpey CJ. Aggressive basal cell carcinoma: Presentation, Pathogenesis, and Management. Cancer Metastasis Rev. 2004;23(3-4):389–402. doi: 10.1023/B:CANC.0000031775.04618.30. [DOI] [PubMed] [Google Scholar]
- 3.Staibano S, Lomuzio L, Mezza E, Argenziano G. Prognostic value of apoptotic index in cutaneous basal cell carcinomas of head and neck. Oral Oncol. 1999;35(6):541–7. doi: 10.1016/s1368-8375(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 4.Kricker A, Armstrong BK, Heenan PJ. A dose-response curve for sun exposure and basal cell carcinoma. Int J Cancer. 1995 Feb;60(4):482–8. doi: 10.1002/ijc.2910600410. [DOI] [PubMed] [Google Scholar]
- 5.Lock-Andersen J, Drzewiecki KT, Wulf HC. Eye and hair color, skin type and constitutive skin pigmentation as risk factors for BCC and cutaneous malignant melanoma: A Danish case-control study. Acta Derm Venereol. 1999;79(1):74–80. doi: 10.1080/000155599750011778. [DOI] [PubMed] [Google Scholar]
- 6.El-Bahrawy M, El-Masry N, Alison M, Poulsom R, Fallowfield M. Expression of -catenin in basal cell carcinoma. Br J Dermatol. 2003 May;148(5):964–70. doi: 10.1046/j.1365-2133.2003.05240.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowman PH, Ratz JL, Knoepp TG, Barnes CJ, Finley EM. Basosquamous carcinoma. Dermatol Surg. 2003;29(8):830–3. doi: 10.1046/j.1524-4725.2003.29217.x. [DOI] [PubMed] [Google Scholar]
- 8.Lopes de Faria J, Nunes PHF. Basosquamous cell carcinoma of the skin with metastases. Histopathology. 1988;12(1):85–94. doi: 10.1111/j.1365-2559.1988.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin RC 2nd, Edwards MJ, Cawte TG, Sewell CL, McMasters KM. Basosquamous carcinoma. Cancer. 2000;88(6):1365–9. doi: 10.1002/(sici)1097-0142(20000315)88:6<1365::aid-cncr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Khan ZM. Basal cell carcinoma with thickened basement membrane. Am J Dermatopathol. 1999;21(1):111–2. doi: 10.1097/00000372-199902000-00092. [DOI] [PubMed] [Google Scholar]
- 11.Staibano S, Lo Muzio L, Pannone G, Scalvenzi M, Salvatore G, Errico ME, et al. Interaction between bcl-2 and p53 in neoplastic progression of basal cell carcinoma of head and neck. Anticancer Res. 2001;21(6A):3757–64. [PubMed] [Google Scholar]
- 12.Staibano S, Lo Muzio L, Pannone G, Mezza E, Argenziano G, Vetrani A, et al. DNA ploidy and cyclin D1 expression in Basal cell carcinoma of the Head and Neck. Am J Clin Pathol. 2001;115(6):805–13. doi: 10.1309/GGE7-WL7J-VRWD-R4VG. [DOI] [PubMed] [Google Scholar]
- 13.Mateoiu C, Pirici A, Bogdan F. Immunohistochemical nuclear staining for p53, PCNA, Ki-67 and bcl-2 in different histologic variants of basal cell carcinoma. Rom J Morphol Embryol. 2011;52(1 Suppl):315–9. [PubMed] [Google Scholar]
- 14.Ibrahim ZA, Narihan MZ, Ojep DN, Soosay AE, Pan KL. Cyclin D1 expression in acral melanoma: a case control study in Sarawak. Malays J Pathol. 2012;34(2):89–95. [PubMed] [Google Scholar]
- 15.Weedon D. Skin Pathology. 2nd Ed. Churchill and Livingstone; 2002. [Google Scholar]
- 16.Silverman MK, Kopf AW, Grin CM, Bart RS, Levenstein MJ. Recurrence of BCC Part I: Overview. J Dermatol Surg Oncol. 1991;17(9):713–8. doi: 10.1111/j.1524-4725.1991.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 17.Silverman MK, Kopf AW, Grin CM, Bart RS, Levenstein MJ. Recurrence rates of treated basal cell carcinomas. Part II: Currettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17(9):720–6. doi: 10.1111/j.1524-4725.1991.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 18.Leffell D, Headington JT, Wong DS, Swanson NA. Aggressive-growth basal cell carcinoma in young adults. Arch Dermatol. 1991;127(11):1663–7. [PubMed] [Google Scholar]
- 19.Randle H. Basal cell carcinoma: Identification and treatment of the high risk patient. Dermatol Surg. 1996;22(3):255–61. doi: 10.1111/j.1524-4725.1996.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 20.Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. J Am Acad Dermatol. 1990;23(6 pt1):1118–26. doi: 10.1016/0190-9622(90)70344-h. [DOI] [PubMed] [Google Scholar]
- 21.Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 1999;141(3):415–23. doi: 10.1046/j.1365-2133.1999.03033.x. [DOI] [PubMed] [Google Scholar]
- 22.Bozdoğan O, Erkek E, Atasoy P, Kocak M. bcl-2 related proteins, alpha-smooth muscle actin and amyloid deposits in aggressive and non-aggressive basal cell carcinomas. Acta Derm Venereol. 2002;82(6):423–7. doi: 10.1080/000155502762064548. [DOI] [PubMed] [Google Scholar]
- 23.Borel DM. Cutaneous basosquamous carcinoma. Review of the literature and report of 35 cases. Arch. Pathol. 1973;95(5):293–7. [PubMed] [Google Scholar]
- 24.Martin RC, Edwards MJ, Cawte TG, Sewell CL, McMasters KM. Basosquamous carcinoma. Analysis of prognostic factors influencing recurrence. . Cancer. 2000;88(6):1365–9. doi: 10.1002/(sici)1097-0142(20000315)88:6<1365::aid-cncr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.de Faria J. Basal cell carcinoma of the skin with areas of squamous cell carcinoma: A basosquamous cell carcinoma. J Clin Pathol. 1985;38(11):1273–7. doi: 10.1136/jcp.38.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beer TW, Shepherd P, Theaker JM. BerEP4 and EMA aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37(3):218–23. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 27.Delehedde M, Cho SH, Sarkiss M, Brisbay S, Davies M, El-Naggar AK, et al. Altered Expression of bcl-2 Family member proteins in nonmelanoma skin cancer. Cancer. 1999;85(7):1514–22. [PubMed] [Google Scholar]
- 28.Ramdial PK, Madaree A, Reddy R, Cetty R. Bcl-2 protein expression in aggressive and non-aggressive basal cell carcinomas. J Cutan Pathol. 2000;27(6):283–91. doi: 10.1034/j.1600-0560.2000.027006283.x. [DOI] [PubMed] [Google Scholar]