Abstract
Background and Objectives:
Sputum smear staining for acid-fast bacilli is initial approach to the diagnosis of pulmonary tuberculosis (PTB) but more than 50% of cases are sputum smear-negative. This study was aimed to investigate the diagnostic value of fiberoptic bronchoscopy (FOB) guided bronchoalveolar lavage (BAL) in patients suspected to have tuberculosis.
Methods:
This prospective cross-sectional study was carried out on 290 sputum smear-negative patients who were clinically suspicious for PTB in 2006-12. All patients were subjected to FOB andBAL, then BAL specimens stained and cultured.
Results:
Of the 290 patients, 173 cases (59.7%) were men and 117 cases (40.3%) were women with the age of 52.6±19.1 years (ranged 20-76 years). Of the total 290 BAL specimens, 110 specimens (38%) were positive for acid-fast bacilli. Sensitivity, specificity, PPV and NPV was 60%, 91%, 89% and 64%, respectively. Also, LR + and LR - was 64.6% and 0.44%, respectively.
Conclusion:
FOB guided BAL is a reliable, rapid and useful method for establishing the diagnosis of smear negative PTB with minimal complications.
Key Words: Pulmonary Tuberculosis, Bronchoalveolar Lavage, Sputum
Introduction
Tuberculosis continues to be a major health problem worldwide with variable clinical presentations (1, 2). In 2011, 8.7 million new cases of tuberculosis were reported and the highest cases were from Asia (3). Based on WHO report, in 2011, Iran had 16000 estimated number of tuberculosis cases (21 per 105population) (4). Pulmonary tuberculosis (PTB) is the most common form and prompt diagnosis is critical (2, 5, 6). According to WHO guidelines for control of tuberculosis, the initial approach is the detection of AFB in respiratory specimens (sputum) with bacteriological methods (7). Although mycobacterial culture is the gold standard and most specific test for the diagnosis, it requires 3-8 weeks to grow. Therefore, the culture is not available to guide the initial therapy (1, 2).
Sputum examination for acid-fast bacilli (AFB) is simple and inexpensive, but it is positive in 44% of cases and even less in children (1, 2, 8, 9).
Approximately more than 50% of the pulmonary tuberculosis is sputum smear-negative (10, 11).The diagnosis and treatment of these patients relies on clinical symptoms, but 20% are asymptomatic. The initiation of empirical therapy or employing other techniques can be challenging and time consuming because a physician is seeking to prove tuberculosis (2). Clinical and radiological based diagnosis can lead to either over- or underdiagnosis of tuberculosis. Mortality of sputum smear-negative, culture-positivetuberculosis cases are about 14.1%, insisting on the importance of diagnosis of sputum smear-negative PTB (12). On the other hand, unnecessary antituberculosis treatment may cause drug resistance and economic burden. Fiberoptic bronchoscopy(FOB) can provide an early confirmative diagnosis in such patients. Despite the fact that it is expensive and aggressive, it is generally accepted as an important technique in the diagnosis of PTB and provides useful material for diagnosis. Among the bronchoscopic materials, bronchoalveolar lavage (BAL) is the best diagnostic material for the diagnosis of PTB (2,5,13,14). Diagnosis rate is up to 86.6%with less complication in experienced hands (11).
The objective of this study was to assess the significance of BAL obtained by FOB in sputum smear-negative patients suspected to have PTB.
Methods
This cross-sectional prospective study was conducted in Shahid Beheshti Hospital, Babol, northern Iran from April 2006 to December 2012. The study involved 290 patients who had clinical and radiological findings suggestive for PTB. All patients were over 20 years of age and had 3 early morning sputum smears negative for AFB by Ziehl-Neelsen (ZN) stain. They had no response to 2 weeks antibiotics used for lower respiratory tract infection. All sputum specimens were cultured on Lowenstein-Jensen (LJ).
The patients informed about the procedure as well as the safety measures and consents were obtained. The study was approved by Babol University of Medical Sciences Ethics Committee. The patients with contraindications to bronchoscopy (uremia, coagulation disorders, thrombocytopenia, severe pulmonary hypertension and single lung) and moderate to massive pleural effusion were excluded.
The patients underwent bronchoscopy by flexible fiberopticbronchoscope through transnasal route in supine position and under intravenous sedation. All the patients underwent continuous monitoring of electrocardiogram, blood pressure and pulse oximetry. After inspection of the bronchial tree, BAL was done with 100 ml of normal saline at the end of bronchoscopy in the region suspected for lesion based on chest radiography. Thepatients were observed in the recovery room. BAL samples were sent for ZN and gram staining, fungal smear, cytopathology and mycobacterial culture. Proper disinfection of the bronchoscope in between use was mandatory.
Active PTB was confirmed were Mycobacterium tuberculosiswas cultured.
Results
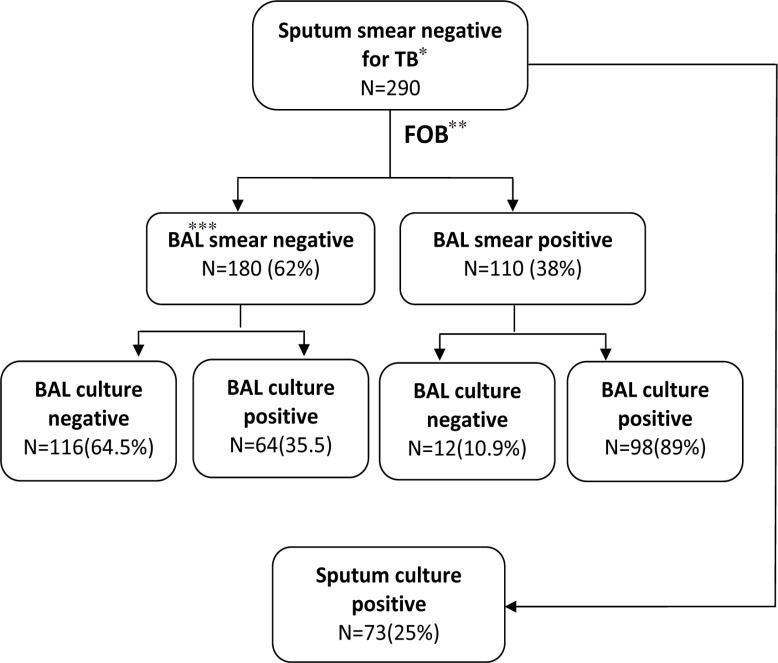
FBO was performed on 290 sputum smear-negative patients, 173 men (59.7%) and 117 women (40.3%), the mean age was 52.6±19.1 years with range of 20 to 76 years. The patients aged 50 to 60 years (17.9%) were the ages most frequently affected. ZN staining of BAL samples was positive in 110 (38%) patients and in 98 cases, BAL samples showed growth of M. tuberculosis on LJ. Of 180 patients (62%) with negative ZN staining of BAL samples, 64 samples showed growth of M. tuberculosison LJ medium (Fig.1). Therefore, the sensitivity, specificity, PPV and NPV for BAL was 60%, 91%,89% and 64%, respectively. Moreover, LR+ and LR- was 64.6% and 0.44%,respectively (Table 1). Seventy three out of the 290 sputum specimens (25%) were positive culture for M. tuberculosis. Apart from some specific entities, cytopathologic examination of all BAL specimens revealed no significant difference between sputum smear-negative and smear-positive. Findings of cytopathologic examination were shown in (Table 2).
Fig.1.
Study flowchart
*: TuberCulosis **: Fiberopticbronchoscopy ***: Bronchoalveolar Lavage
Table 1.
Diagnostic validity of sputum smear-negative TB for BAL examination
| BAL Examination (%) | Confidence Intervals (95%) | |||
|---|---|---|---|---|
| Sensitivity | 60 | 53 to 68 | ||
| Specificity | 91 | 86 to 96 | ||
| Positive Predictive Value(PPV) | 89 | 83 to 95 | ||
| Negative Predictive Value(NPV) | 64 | 57 to 71 | ||
| LR+ | 64.6 | 3.71 to 11.22 | ||
| LR- | 0.44 | 0.36 to 0.53 |
Table 2.
Cytopathologic findings of all BAL specimens
| Cytology Findings | N | % |
|---|---|---|
| Strongyloidosis | 1 | 0.3 |
| Aspergilosis | 1 | 0.3 |
| Adenocarcinoma | 4 | 1.3 |
| Squamous cell carcinoma | 7 | 2.4 |
| Dysplasia | 4 | 1.3 |
| Inflammation | 273 | 94.1 |
| Total | 290 | 100 |
Discussion
In the regions with high PTB prevalence, when the clinical diagnosis of PTB is likely, but sputum smear is negative for acid-fast bacilli or patient is unable to produce sputum, especially when there is no time for further investigations, empirical treatment and closely monitoring of the patient is highly recommended. Although, sputum smear ZN staining is rapid and inexpensive for the detection of PTB, it cannot reveal acid-fast bacilli in all patients. Poor quality sputum sample, technical errors in preparation and staining of the sputum smear and deficient microscopic examination can contribute to the smear negative results. On the other hand, early diagnosis and treatment is the best option for the control of TB. Therefore, FOB and BAL is used to establish the diagnosis of PTB with low morbidity and avoiding delay in the treatment (2,5,15).
Several studies have already shown the advantages of bronchoscopy in the diagnosis of PTB in different patients. Worodria et al. revealed that bronchoscopy with BAL increases the speed and sensitivity of PTB diagnosis in HIV patients (16). Moreover, Kumar et al. concluded that FOB is useful to detect PTB and allows initiating appropriate treatment (17). Menon et al. revealed that FOB and BAL for detecting of AFB was better than gastric aspiration in children suspected to have PTB (18).
In this study, FOB was performed for 290 sputum smear-negative patients who were clinically suspected for PTB, with 60%, 91%, 89% and 64% sensitivity, specificity, PPV and NPV, respectively.
Altefet al. performed a study on 75 sputum smear-negative suspected patients for PTB and revealed that the total yield of FOB was 83.33% that was in line with our study (14). In the study conducted on 56 sputum smear-negative patients, Yuksekol et al. found that BAL smears and culture for M. tuberculosiswere positive in 13(23%) and 28(50%) patients, respectively. In comparison to our study, the result of ZN positive BAL smears were lower, however, their positive cultures were more than ours. They concluded that FOB is useful and mandatory in the selected patients (9). Previous studies have reported that FOB and BAL play significant roles in diagnosis of PTB with a sensitivity of 80-93% and a specificity of 70-95% (2,5,12). Shin et al. obtained 75.9%, 97.2%, 95.3% and 84.3% sensitivity, specificity, PPV and NPV of BAL, respectively. All their results are higher than our study and they concluded FOB is a useful procedure in the rapid diagnosis of PTB (2).
Furthermore, Jacomelli et al. reported 60% sensitivity and 100% specificity of BAL. Although the sensitivity is lower than our result, they obtained higher specificity and showed that bronchoscopy was a reliable technique (6).
Kamal et al. found that FOB and BAL are useful methods not only in the diagnosis of PTB but also to confirm other pulmonary pathologies (8). Some authors showed that performing methods such aspostbronchoscopic sputum culture and transbronchial biopsy adding to BAL examination raised the sensitivity and specificity (6,10). Tamura et al. showed the value of FOB in patients whose prebronchoscopic sputum specimens were negative for acid-fast bacilli and PCR. They concluded that FOB provides rapid and definitive diagnosis of PTB (13).FOB is considered as invasive and expensive procedure with the risk of transmission of tuberculosis or other infections (12).
It is worth noting that FOB has low complication rate (pneumothorax and bleeding) and mostly there is no need for postbronchoscopic hospitalization (6). In our study, no one showed any complications.
Although sputum smear positive PTB was more infectious than sputum smear-negative cases, the patients who were sputum negative serve as an important source of tuberculosis spread in the community. On the other hand, empirical antituberculosis therapy before to prior finding of any bacteriologic evidence culture result may cause drug resistance in community and economic strife.
Conclusion
Taking all together, FOB and BAL are safe and effective methods with good sensitivity, specificity, PPV and NVP for the rapid diagnosis of PTB in sputum smear-negative patients. However, further studies for cost-effectiveness of FOB for sputum smear-negative patients are recommended, especially in resource-limited countries.
Acknowledgments
No fund was received to conduct this study. The authors wish to thank Mrs. Shirkhani for the statistical analysis.The authors declare that there is no conflict of interests.
References
- 1.Lange Ch, Mori T. Advances in the diagnosis of tuberculosis. Respirology. 2010;15(2):220–40. doi: 10.1111/j.1440-1843.2009.01692.x. [DOI] [PubMed] [Google Scholar]
- 2.Shin JA, Chang YS, Kim TH, Kim HJ, Ahn CM, Byun MK. Fiberoptic bronchoscopy for the rapid diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis. 2012;12 doi: 10.1186/1471-2334-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumla A, Raviglione M, Hafner R, Von Reyn CF. Tuberculosis. N Engl J Med. 2013;368:754–55. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) estimates of tuberculosis incidence by rate. 2011. Available in: http://www.who.int/gho/publications/world_health_statistics/EN_WHS2013_Full.pdf.
- 5.Boonsarngsuk V, SuwannaphongS , Laohavich CH. Combination of adenosine deaminase activity and polymerase chain in bronchoalveolar lavage fluid in the diagnosis of smear-negative active pulmonary tuberculosis. Int Infect Dis. 2012;16(9):663–8. doi: 10.1016/j.ijid.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Jacomelli M, Silva PRAA, Rodrigues AJ, Demarzo SE, Seicento M, Figueiredo VR. Bronchoscopy for the diagnosis of pulmonary tuberculosis in patients with negative sputum smear microscopy results. J Bras Pneumol. 2012;38(2):167–73. doi: 10.1590/s1806-37132012000200004. [DOI] [PubMed] [Google Scholar]
- 7.Quaiser S, Agarwal A, Khan R, Haque Sh F. Fiberoptic bronchoscopy, as a valuable diagnostic option in sputum negative pulmonary tuberculosis: A prospective study. Int Appl Basic Med Res. 2012;2(2):123–7. doi: 10.4103/2229-516X.106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamal R, Sharma R, Sahasrabuddhe T, Dash SK, Showkat M, Gaikwad N. A prospective study to evaluate the utility of bronchoalveolar lavage by fiberoptic bronchoscopy in sputum smear negative patients with high suspicion of pulmonary tuberculosis. Med J Dr.D.Y.Patil Univ. 2012;5(1):43–6. [Google Scholar]
- 9.Yuksekol I, Bal S, Ozkan M, Bedrihan I, Tozkoparan E, Demiric N, et al. The value of fiberoptic bronchoscopy in the diagnosis of smear negative pulmonary tuberculosis. Tuberk Toraks. 2003;51(4):405–9. [PubMed] [Google Scholar]
- 10.Pipatvech K, Kiatboonsri S, Boonsarngsuk V, Hongmanee P, Choothakan S. The value of post-bronchoscopy sputum examination in the diagnosis of smear-negative pulmonary tuberculosis. Thai J Tuberculosis Chest Dis Crit Care . 2007:45–50. [Google Scholar]
- 11.Kalawat U, Sharma KK, Reddy PNR, Kumar AG. Study of bronchoalveolar lavage in clinically and radiologically suspected cases of pulmonary tuberculosis. Lung India. 2010;27(3):122–4. doi: 10.4103/0970-2113.68307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohan A, Sharma SK. Fiberoptic bronchoscopy in the diagnosis of sputum smear-negative pulmonary tuberculosis: current status. Indian J Chest Dis Allied Sci. 2008;50(1):67–78. [PubMed] [Google Scholar]
- 13.Tamura A, Shimada M, Matsui Y, Kawashima M, Suzuki J, Ariga H, et al. The value of fiberoptic broncoscopy in culture-positive pulmonary tuberculosispatients whose pre-bronchoscopic sputum specimens were negative both for smear and PCR analyses. Inter Med. 2010;49:95–102. doi: 10.2169/internalmedicine.49.2686. [DOI] [PubMed] [Google Scholar]
- 14.Altaf Bachh A, Gupta R, Hag I, Varudkar HG. Diagnosing sputum/smear-negative pulmonary tuberculosis: Does fibre-optic bronchoscopy play a significant role? Lung India. 2010;27(2):58–62. doi: 10.4103/0970-2113.63607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra TJ, Dash S, Srinivas G, Prabhakara PV. A study on rapid confirmation of pulmonary tuberculosis in smear-negative acid fast bacilli cases by using fiberoptic bronchoscopy, done through a trans oropharyngeal spacer. J Family Community Med. 2012;19(1):43–6. doi: 10.4103/2230-8229.94014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worodria W, Davis JL, Cattamanchi A, Andama A, Den Boon S, Yoo SD et al. Bronchoscopy is useful for diagnosing smear-negative tuberculosis in HIV-infected patients. Eur Respir J. 2010;36:446–56. doi: 10.1183/09031936.00010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Kumar Gupta A, Haroon Khan M. Role of flexible fiberoptic bronchoscopy in suspected sputum smear negatine pulmonary tuberculosis cases microscopy center under RNTCP. Int J Med Sci public Health. 2014;3(1) [Google Scholar]
- 18.Menon PR, Lodha R, Singh U, Kabra SK. A prospective assessment of the role of bronchoscopy and bronchoalveolar lavage in evaluation of children with pulmonary tuberculosis. J Trop Pediatr . 2010 doi: 10.1093/tropej/fmq105. doi:10.1093/tropej. [DOI] [PubMed] [Google Scholar]