Abstract
Background and Objective:
Breast cancer is the commonest cancer of Indian women. Estrogen and Progesterone expression is seen in benign breast lesions and in breast carcinoma associated with good prognostic parameters and it correlates well with response to hormone therapy. Although a lot of studies have been conducted in the past on hormone receptor expression in breast cancer and few have correlated them with other prognostic parameters of breast cancer, the present study was intended to document the prevalence of hormone receptor positive breast carcinomas in our population; their importance in benign breast diseases; to document a reliable scoring system of hormone receptors expression by Quick scoring; to correlate them with most of the proven prognostic parameters of breast carcinoma.
Methods:
Tissue specimens from 25 patients with benign breast disease and 50 patients with breast carcinoma were assayed for estrogen (ER) and progesterone (PR) receptors using Quick scoring. ER/PR expression in breast carcinomas was correlated with various prognostic parameters including patients’ age, menopausal status, tumor size, type, MBR grade, NPI, lymphatic vessel invasion, lymph node stage, lymphomononuclear invasion, elastosis and HER2/neu status.
Result:
Scoring of steroid receptors paralleled intensity of hyperplasia in benign breast diseases but in breast carcinoma, it was inversely correlated with grade of tumor, NPI, HER2/neu status, tumor necrosis, lymphomononuclear infiltrate and elastosis. We found no relationship with tumor size, lymph node status or age.
Conclusion:
Assessment of hormone receptors for clinical management of breast cancer patients is strongly advocated to provide prognostic information and best therapeutic options.
Key Words: Estrogen Receptors, Progesterone Receptors, erbB-2 Receptor, Breast, Tumor
Introduction
Breast carcinoma is the most common malignant tumor and leading cause of death in women with more than 1,000,000 cases occurring worldwide annually. Although the incidence is low in most Asian and African countries, it is now the commonest cancer of urban Indian women and the second commonest in the rural women (1).
A crucial development in the evaluation of breast carcinoma has been the realization that the presence of hormone, estrogen and progesterone receptors (ER/PR) in the tumor tissue correlates well with response to hormone therapy and chemotherapy (2). These hormone receptors play a role in the development and progression of breast cancer and identify patients with a lower risk of relapse and better overall survival (3).
In benign breast disease, the intensity of steroid hormones expression parallels the degree of hyperplasia. A progression is noted from fibrocystic disease (35%) to fibrosing adenosis (50%) to lobular and ductal hyperplasia (85%) (4). A positive relationship is seen between PR presence and fibroblastic or epithelial proliferation (5). Fibroadenomas contain progesterone receptors almost universally, and estrogen receptors in approximately one fourth of the cases (6). PR expression is also seen in benign phyllodes tumors at a very high level, almost as high as in malignant tumors (5).
The determination of ER and PR on biopsies of invasive carcinomas prior to therapeutic manipulations has become a standard practice in the management of breast cancers because it provides information on response to hormonal therapy and prognosis. Tumors that contain estrogen receptors have a better prognosis and are more likely to respond to hormonal therapy than tumors that lack them. The presence of progesterone receptor in addition to ER increases the likelihood of response to hormonal therapy because its presence implies a functioning ER pathway (7, 8).
There is no worldwide accepted general agreement on a scoring system for the immunohistochemical evaluation of hormone receptor status. The two main features evaluated are the proportion of stained cells and the intensity of staining. Different methods used to evaluate these include: Quick score, H score and Allred score (9).
Although a lot of studies have been conducted in the past on hormone receptor expression in breast cancer and few have correlated them with some of the other proven prognostic parameters of breast cancer, the present study was intended not only to document the prevalence of hormone receptor positive breast carcinomas in our population but also their importance in benign breast diseases; to document a reliable scoring system of hormone receptors expression by Quick scoring method; and to correlate them with most of the other proven prognostic parameters of breast carcinoma.
Material and Methods
A prospective study from 2009 to 2010 was conducted in the department of pathology at PGIMS, Rohtak, Haryana, India.
The study group comprised 25 benign breast lesions and 50 cases of breast carcinoma (mastectomy specimens of breast cancers other than carcinoma along with all the cases which had received chemotherapy prior to mastectomy were excluded, as chemotherapy leads to significant histological changes making it difficult to evaluate MRB grade as well as NPI). Histopathological diagnosis was established on routine 4 micron thick hematoxylin and eosin stained sections. To study elastosis, Verhoff’s Van-Gieson’s was applied on tumor sections. Histologic grade was calculated by Modified Bloom-Richardson System (MBR). Lymph node stage was defined as 1 for node negative disease, stage 2 for upto three axillary nodes, and stage 3 for four or more positive nodes. Using MBR histologic grade and lymph node stage, Nottingham prognostic index (NPI) was calculated (10).
Patients were divided into three groups using 3.4 and 5.4 as cut off points with NPI of < 3.4 regarded as good prognostic category, 3.4 - 5.4 as moderate and > 5.4 as poor (7). Sections from benign tumors were stained immunohistochemically by peroxidase - antiperoxidase method for ER and PR, whereas sections from malignant tumor were stained for ER, PR and HER2/neu (11). Positive and negative controls were run with each batch of IHC staining. Tumor s that showed strong complete membranous staining in > 10% cells were taken as positive for HER2/neu (2). Nuclear staining was taken as positive for estrogen and progesterone receptors. ER/PR expressions were assessed by Quick scoring which is based on assessment of proportion score and intensity score. A score for the proportion of stained cells (0 = no nucleus stained, 1 = <1% nuclei stained, 2 = 1-10% nuclei stained, 3 = 11-33% nuclei stained, 4 = 34- 66% nuclei stained and 5 = 67-100% nuclei stained) and the intensity of staining (0 = no staining, 1 = weak staining, 2 = moderate staining and 3 = strong staining) was assigned to each tumor . The scores were summed to give a maximum of 8. Patients with tumors scoring 2 or less were regarded as ER/PR negative (12). On the basis of ER and PR expression, patients were divided into four groups: ER+ve/PR+ve, ER+ve/PR-ve, ER-ve/PR+ve and ER-ve/PR-ve.
Original H and E sections were reviewed in conjunction with the IHC stained sections. Hormone receptor expression in breast carcinoma was correlated with various prognostic parameters independent. The results obtained were interpreted and correlated statistically. Correlation between intensity score, proportion score and total Quick score for ER and PR and prognostic parameters like MBR grade, NPI, lymph node stage and HER2/neu was calculated by Pearson’s coefficient of correlation. When the data was qualitative, such as in menopausal status, tumor necrosis, lymphomononuclear infiltration, lymphatic vessel invasion and elastosis, a chi square test was used to assess the association between these parameters and ER and PR expression taken as positive or negative (Quick scores of 0 and 2 were taken as negative and rest of the scores as positive). A p-value of <0.05 was taken as significant.
This study was conducted after obtaining the ethical approval from the Ethical Review Committee of our institution and our institution took care of the entire financial burden for the completion of this prospective research study.
Results
Our study included 25 cases of benign breast diseases and 50 cases of breast carcinoma. Benign lesions included 4 non-proliferative and 21 proliferative lesions. Average age at diagnosis was 29 ± 9.4 yr. Fibroadenoma was the most common benign proliferative breast lesion (64%). All cases of non-proliferative lesions were of fibrocystic disease.
Positive hormone receptor expression for both ER and PR was noted in 24 out of 25 cases (96%) of benign breast lesions. A progression in Quick score of ER and PR was observed from fibrocystic disease to proliferative lesions. ER quick score was 2 and 3 in fibrocystic disease and 4, 5, 6 and 7 in hyperplastic lesions. PR score was 4 in 75% cases of fibrocystic disease and 6 or more in hyperplastic lesions. None of the cases showed a score of 8 for ER while 57% cases had a score of 8 for PR (Table 1).
Table 1.
Quick score for ER and PR seen in various benign breast lesions (n=25) and cases of carcinoma breast (n=50)
| QUICK SCORE |
ER
*
|
PR
**
|
||||
|---|---|---|---|---|---|---|
| Non-proliferative | Proliferative | Malignant | Non- proliferative | Proliferative | Malignant | |
| 0 | 0 | 0 | 10(20) | 0 | 0 | 10(20) |
| 2 | 1(25) | 0 | 24(48) | 0 | 0 | 19(38) |
| 3 | 3(75) | 4(19) | 1(2) | 0 | 0 | 1(2) |
| 4 | 0 | 7(33.3) | 2(4) | 3(75) | 0 | 2(4) |
| 5 | 0 | 5(23.8) | 1(2) | 0 | 0 | 2(4) |
| 6 | 0 | 4(19) | 3(6) | 1(25) | 2(9.5) | 5(10) |
| 7 | 0 | 1(4.7) | 4(8) | 0 | 7(33.3) | 5(10) |
| 8 | 0 | 0 | 5(10) | 0 | 12(57.2) | 6(12) |
| Total | 4(16) | 21(84) | 50(100) | 4(16) | 21(84) | 50(100) |
: Estrogen receptor
: Rrogesteron Receptor
Among the 50 patients of breast carcinoma, 74% were more than 40 years of age and 52% were premenopausal. The most common histological type of breast carcinoma was infiltrating ductal carcinoma – not otherwise specified (IDC-NOS) constituting 36 (72%) cases. Medullary carcinoma comprised only 4 (8%) cases and metaplastic type constituted 3 (6%) cases. Each lobular, papillary and mixed ductal and lobular type constituted only 2 (4%) cases. Mucinous carcinoma constituted just one (2%) case.
Twenty three cases (46%) were grade II while 15 cases (30%) belonged to grade III and 12 cases (24%) to grade I. Twenty eight cases (56%) had lymph node involvement, out of which half (28%), were in stage II and half (28%) were in stage III i.e., had metastasis in more than 3 lymph nodes. Majority of the cases in our population had intermediate (44%) and poor (38%) prognostic index.
Tumor necrosis was seen in 27 out of 50 cases (54%), 34 cases (68%) had an associated lymphomononuclear cell infiltrate around the tumor and 30 cases (60%) had lymphatic vessel invasion by the tumor cells. The elastosis was recorded in only 11 cases (22%).
Thirty two out of fifty cases (64%) showed the presence of HER2/neu staining on immunohistochemistry (grade 3+). Whereas 18 cases (36%) were negative.
Unlike benign lesions, carcinomas showed a lower quick score for both ER and PR. Out of 50, thirty four (68%) and twenty nine (58%) had a score of either 0 or 2 for ER and PR respectively. Only 12 cases (24%) had a Quick score of 6/7/8 for ER. Similarly, only 16 (32%) tumors had a score of 6/7/8 for PR (Table 1). Fifteen out of 50 cases (30%) were ER+/PR+; 6 cases (12%) were ER-ve/PR+ve; 1 case (2%) was ER+ve/PR-ve and 28 cases (56%) were ER-ve/PR-ve. Of the various histological types, all cases of lobular, papillary and mucinous carcinomas were positive for both ER and PR whereas all cases of metaplastic, most of the medullary and 50% of mixed carcinomas were negative for both (Table 2).
Table 2.
Correlation of various prognostic parameters with ER & PR expression in breast carcinoma (n=50)
|
ER+ve
*
/PR+ve
**
|
ER+ve/PR-ve
|
ER-ve/PR+ve
|
ER-ve/PR-ve
|
||
|---|---|---|---|---|---|
| No.(%) | No.(%) | No.(%) | No.(%) | ||
| Menopausal status | Pre-menopausal | 6(23) | 0 | 6(23) | 14(54) |
| Post-menopausal | 9(37.5) | 1(4.1) | 0 | 14(58.3) | |
| Patients age | <40yrs | 3(23) | 1(7.7) | 4(30.8) | 5(38.5) |
| >40yrs | 12(32.4) | 0 | 2(5.4) | 23(62.2) | |
| Tumor type | Ductal (IDC) | 9(25) | 0 | 6(17) | 21(58) |
| Lobular (ILC) | 2(100) | 0 | 0 | 0 | |
| Medullary | 0 | 1(25) | 0 | 3(75) | |
| Metaplastic | 0 | 0 | 0 | 3(100) | |
| Mucinous | 1(100) | 0 | 0 | 0 | |
| Papillary | 2(100) | 0 | 0 | 0 | |
| Mixed | 1(50) | 0 | 0 | 1(50) | |
| MBR *** grade | Grade I | 10(83) | 0 | 0 | 2(17) |
| Grade II | 5(22) | 1(4) | 5(22) | 12(52) | |
| Grade III | 0 | 0 | 1(7) | 14(93) | |
| Lymph node stage | Stage I | 9(41) | 0 | 2(9) | 11(50) |
| Stage II | 2(14) | 0 | 3(22) | 9(64) | |
| Stage III | 4(28.5) | 1(7) | 1(7) | 8(57.5) | |
| NPI **** | Good | 8(89) | 0 | 0 | 1(11) |
| Intermediate | 4(18) | 0 | 5(22.7) | 13(59) | |
| Poor | 3(15.8) | 1(5.2) | 1(5.2) | 14(73.8) | |
| Tumor Necrosis | Present | 2(7.4) | 0 | 3(11.1) | 22(81.5) |
| Absent | 13(56.5) | 1(4.5) | 3(13) | 6(26) | |
| Lymphatic Vessel Invasion | Present | 7(23.3) | 1(3.3) | 4(13.3) | 18(60) |
| Absent | 8(40) | 0 | 2(10) | 10(50) | |
| Lymphomononuclear Infiltrate | Present | 0 | 1(3) | 6(17.6) | 27(79.4) |
| Absent | 15(93.7) | 0 | 0 | 1(6.3) | |
| Elastosis | Present | 9(82) | 0 | 0 | 2(18) |
| Absent | 6(15.4) | 1(2.6) | 6(15.4) | 26(66.6) | |
| HER2 ***** / Neu | Positive | 3(9.4) | 1(3.1) | 6(18.75) | 22(68.75) |
| Negative | 12(66.6) | 0 | 0 | 6(33.4) | |
Estrogen Receptor
Progesterone Receptor
Modified Bloom-Richardson System
Nottingham prognostic index
A significant correlation (P-value < 0.05) was observed with tubule formation, nuclear pleomorphism, mitotic count, MBR grade, NPI, tumor necrosis, lymphomononuclear infiltrate, elastosis and HER2/neu status. Quick score for ER and PR was not associated with patients’ age, menopausal status, tumor size, lymph node stage and lymphatic vessel invasion (P-value > 0.05) (Table 3).
Table 3.
Correlation of ER/PR status with the clinico-pathological parameters in breast carcinoma (n=50)
| Clinico-Pathological Parameter |
P-
VALUE
|
||||||
|---|---|---|---|---|---|---|---|
|
ER
|
PR
|
||||||
| IS * | PS ** | QS *** | IS | PS | QS | ||
| Age | > 0.10 | > 0.10 | > 0.10 | > 0.10 | > 0.10 | > 0.10 | NS **** |
| Menopausal Status | ─ | ─ | 0.159 | ─ | ─ | 0.535 | NS |
| Tumor Size | > 0.10 | > 0.10 | > 0.10 | > 0.10 | > 0.10 | > 0.10 | NS |
| Tubule formation | < 0.05 | < 0.02 | 0.02 | < 0.01 | < 0.01 | < 0.01 | S ***** /HS |
| Nuclear Pleomorphism | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | HS ****** |
| Mitotic Count | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | HS |
| MBR ******* grade | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | HS |
| NPI ******** | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | HS |
| Lymph Node Stage | > 0.10 | > 0.10 | > 0.10 | > 0.10 | > 0.10 | > 0.10 | NS |
| Tumor Necrosis | ─ | ─ | 0.001 | ─ | ─ | 0.001 | HS |
| Lymphatic Vessel Invasion | ─ | ─ | 0.322 | ─ | ─ | 0.349 | NS |
| Lympho-Mononuclear Infiltrate | ─ | ─ | 0.001 | ─ | ─ | 0.001 | HS |
| Elastosis | ─ | ─ | 0.001 | ─ | ─ | 0.002 | HS |
| HER2/neu | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | HS/S |
: intensity score
: proportion score
: total quick score
: not significant
: significant
: highly significant
:Modified Bloom-Richardson System
:Nottingham prognostic index
Discussion
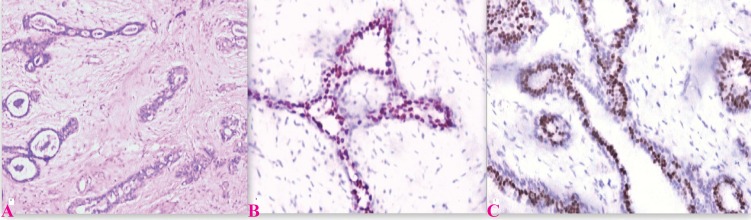
The data indicates that the incidence of steroid receptor parallels the intensity of hyperplasia in benign breast disease and the benign lesions universally express PR and that too with strong intensity (4, 5, 6, 13). These results reveal that in non-malignant breast diseases, the level of hormone expression parallels with increasing mammary hyperplasia and, as demonstrated by ER-PR concomitant presence, reveal an overall hypersensitivity to hormone once a certain proliferation step is reached by epithelial cells. The report of increasing ER/PR levels as epithelial cell proliferation rises provides circumstantial evidence for hormonal abnormalities in women with benign mastopathies and for the favourable response of this condition to therapy with various gonadal hormones and their synthetic analogs (Fig. 1).
Fig.1.
Expression of ER and PR in fibroadenoma; A) Fibroadenoma H & E (×100); B) ER expression in fibroadenoma (×200); showing nuclear staining with intensity score 3 and proportion score 5(Quick Score 8); C) PR expression in fibroadenoma (×400); showing nuclear staining with intensity score 3 and proportion score 5(Quick Score 8)
Although our sample is small, our results reveal that in comparison with white American women, the Indian females with breast cancer have lower hormone receptor positivity rates. Our results are more close to those of black Americans, Pakistanis and Chinese. A Jordanian study revealed 50.8% ER-positive tumors and 57.5% of PR-positive tumors in their study sample (14). A prevalence of 32.6% for ER-positive and 46.1% for PR-positive breast cancers has been documented in a study carried out in India (15). This could be partially explained by the age at diagnosis of breast cancer. In our study, 70.6% of the ER-negative cases were below the age of 50 years. Biological and lifestyle factors are also likely to contribute to these findings.
Although several studies have reported that the expression of hormone receptors increases with the increase in the age of the patient which in part was explained by the higher proportion of low grade and more differentiated carcinomas seen in older women (16-21). However, in our study, we did not find any significant association between the age of the patient and ER and PR expression which was due to increased prevalence of higher grade tumor s and late diagnosis in our population leading to more cases without ER/PR.
In contrast to previous studies, our study showed no correlation between ER/PR expression and menopausal status of the patient (17, 22, 23). The likelihood of fault in taking menopausal history from the patient and other technical causes, though possible for this difference in findings, cannot completely erase the need to consider other biological reasons for increased receptor negative tumors in our population.
Our results and previous reports show no association of tumor size with ER and PR (18, 19, 21, 24-27). Although axillary lymph node status is the single most important prognostic factor for patients with breast cancer, the coefficient of correlation for both ER and PR showed statistically insignificant relationship with lymph node stage, which is in agreement with most of the other series (24-27). Marked heterogeneity of ER and PR expression in primary breast tumor has been linked with involved nodes that do not contain ER and PR, due to the hypothesis that it’s the hormone receptor negative cell from the primary tumor that metastasizes to the nodes.
The presence of definite vascular invasion is not only of prognostic significance in long term survival of patients, but is also useful in predicting local recurrences after conservative surgery. Though no association was noted when we compared lymphovascular invasion with ER/PR status in our study, the independent prognostic significance of lymphovascular invasion cannot be undermined as it identifies a subset of patients at increased risk for axillary lymph node involvement.
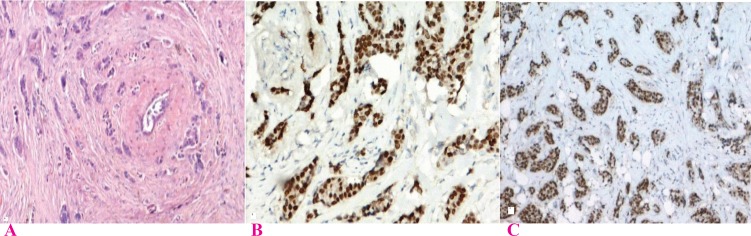
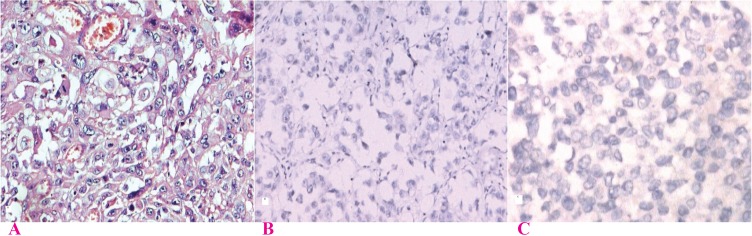
Several authors have reported a strong inverse correlation between hormone receptors expression and MBR grade (14, 16, 18, 19, 21, 22, 24-27) (Fig. 2, 3). No reports have been found with contradicting findings. This study and few other authors have also correlated intensity of staining and proportion of stained cells with grade (27, 28). A significant correlation was also found independently with the three criteria used in grading, tubule formation, nuclear pleomorphism and mitotic count (Table 3).
Fig. 2.
Expression of ER & PR in breast carcinoma MRB Grade I; A) Grade I Ca Breast H & E (×100); B) ER expression in Grade I Ca breast (×200); showing nuclear staining with intensity score 3 and proportion score 5(Quick Score 8); C) PR expression in Grade I Ca Breast (×100); showing nuclear staining with intensity score 3 and proportion score 5(Quick Score 8)
Fig. 3.
Expression of ER & PR in breast carcinoma MRB Grade III; A) Grade III Ca Breast H & E (×200); B) ER expression in Grade III Ca breast (×100); showing negligible nuclear staining with intensity score 1 and proportion score 1 (Quick Score 2) ; C) PR expression in Grade III Ca Breast (×400); showing negligible nuclear staining with intensity score 1 and proportion score 1 (Quick Score 2)
The NPI is a powerful and reproducible method of assessing prognosis. The higher the value of NPI, the worse is the prognosis (7, 10). Although tumor size and lymph node stage, both components of NPI, do not correlate with the hormone receptor expression, our study revealed strong association between ER/PR expression and NPI, indicating that it is MBR grade’s strong correlation with ER and PR which leads to the observed results. Similar correlation has been observed by previous authors (25, 27).
The presence of tumor necrosis is significantly related with larger tumor size and higher tumor grade, both features of poor prognosis, thus explaining the presence of tumor necrosis in tumors without hormone receptor expression.
In our study, significant correlation was seen between prominent lymphomononuclear cell infiltrate and ER/PR. Out of 50 cases, 34 had an associated lymphomononuclear infiltrate and 27 of these were negative for both the receptors whereas none was positive for both. However, out of 16 cases which did not show lymphomononuclear infiltrate, 15 were positive for both ER and PR (Table 2). Our findings are in accordance with other authors indicating that lymphomononuclear infiltrate is directly related to tumor differentiation (29-32).
A highly significant positive association was observed between elastosis and ER & PR expression. Elastosis positive lesions thus seem to constitute good prognosis and relate to presence of estrogen and progesterone receptors suggesting that elastosis results from the presence of hormone dependent cells in breast carcinomas (33, 34).
Despite the great variation in levels of HER2/neu positivity, nearly all investigators have reported a negative relationship between HER2/neu status and steroid receptors levels (26, 27). This inverse association has been linked to the fact that estrogens are required to suppress HER2/neu. This leads to lower or absent hormone receptors in women with HER2/neu positive breast cancers. Out of 34 HER2/neu positive cases, 68.75% were ER-ve/PR-ve and out of 16 HER2/neu negative cases, 66.6% were positive for both the receptors (Table 2).
Thus, we observed significant correlation of ER/PR expression with various prognostic parameters which were directly or indirectly related to tumor differentiation suggesting that ER and PR represent another index of tumor differentiation. Table 4 shows a comparison between previous studies done so far and the present study to reflect the correlation between various prognostic factors and ER/PR. In our study, we encountered a few significant observations in the expression pattern of ER and PR which are discussed below:
Table 4.
Correlation of Er and Pr Expression With Various Prognostic Parameters
| Study | Year |
Prognostic parameters
|
|||||
|---|---|---|---|---|---|---|---|
| Tumor Necrosis | Elastosis | Her2neu | MBR | LN Stage | NPI | ||
| Fisher et al30 | 1981 | Present | Present | Present | -- | -- | -- |
| Howat et al31 | 1983 | -- | Present | -- | Present | -- | -- |
| Schmitt et al32 | 1992 | Present | -- | -- | Present | -- | -- |
| Pichon et al22 | 1996 | -- | -- | -- | Present | Present | -- |
| Thike et al25 | 2001 | -- | -- | -- | Present | Absent | Present |
| Barnes et al28 | 2005 | Present | -- | -- | Present | -- | -- |
| Azizun-Nisa et al21 | 2008 | -- | -- | Present | Present | -- | -- |
| Lobna et al 26 | 2008 | -- | -- | Present | Present | Absent | -- |
| Mudduwa et al27 | 2009 | -- | -- | Present | Present | Absent | Present |
| Present study | 2011 | Present | Present | Present | Present | Absent | Present |
Present : correlation was found, Absent : no correlation was found, : parameter not studied
ER-ve/PR+ve TUMORS
Expression of PR is rarely reported in ER negative tumors and its perplexing occurrence is often attributed to laboratory error. We found 6 ER-ve/PR+ve tumors. These cases though PR positive, had a relatively lower expression of PR (Quick score of either 6 or less), were infiltrating ductal carcinoma (NOS), grade II and had intermediate prognostic index. 66.6 % were in patients below 40 years of age, had a tumor size > 4 cm, and were associated with lymphatic vessel invasion. All cases had an associated lymphomononuclear infiltrate and strong HER2/neu expression.
Therefore, these tumors although are similar to ER-ve/PR-ve tumors, may have a slightly better prognosis as compared to them and may show benefit from hormonal therapy.
ER+ve/PR-ve TUMORS
Our study had just one ER+ve/PR-ve case. The Quick score was 4 for ER. This case had 4.5 cm sized tumor, was infiltrating ductal type carcinoma (NOS), grade II, lymph node stage III, belonged to poor prognostic group, with no tumor necrosis and elastosis, associated with lymphatic vessel invasion and lympho-mononuclear invasion and strong HER2/neu expression.
The findings suggest that ER+ve/PR-ve tumors had a profile different from ER+ve/PR+ve tumors and reflect a correlation with relatively poor prognostic factors. Dunnwald et al. (20) reported that the risks of breast cancer-specific mortality are elevated among women with ER+ve/PR-ve, ER-ve/PR+ve, and ER-ve/PR-ve tumors relative to women with ER+ve/PR+ve tumors. Therefore, PR should be analysed in conjunction with ER and although these tumors respond to endocrine therapy but the response may be lower as compared to ER+ve/PR+ve tumors.
Triple Negative Tumors
Triple-negative (TN) breast cancer is defined as a group of breast carcinomas that are negative for ER, PR and HER2/neu.
In our study, of 50 cases, we found 6 TN tumors. Five out of these 6 cases were IDC-NOS (one was medullary carcinoma), had grade of either II or III and were in intermediate or poor prognostic group. Half had lymph node metastasis and lymphatic vessel invasion, 66.7% had tumor necrosis, 83% had no elastosis and all had lymphomononuclear infiltrate.
Our results showed that TNBC had poor prognostic characteristics as compared with other subtypes of breast cancers. Other authors have also found similar results (35-38).
Although patients with TNBC tend to have a poor prognosis, only chemotherapy is expected to be effective because no therapeutic targets have yet been established. DNA microarray analyses have proved that TNBCs are composed of the basal-like subtype and normal breast (or unclassified) subtype, the former being correlated with an aggressive clinical course. Histological types of TNBCs are reported to be common with those of basal-like subtype, comprising high-grade invasive ductal carcinoma, no special type (solid-tubular carcinoma (or atypical medullary carcinoma), invasive ductal carcinoma with a large central acellular zone), typical medullary carcinoma, and metaplastic carcinomas. The basal-like subtype is characterized by the expression of myoepithelial/basal markers and molecular changes including TP53 gene mutations, BRCA1 inactivation, and many chromosomal alterations. New target molecules for the treatment of TNBCs are under extensive investigation, and their clinical application is awaited (35).
Triple Positive Tumors
HER2-positivity does not always translate to worse survival, as noted when one evaluates the triple positive (TP) subtype (ER+ve/PR+ve/HER2+ve).
We found 3 TP tumors. These cases belonged to either grade I or II, had IDC-NOS. Two of them were in good prognostic group and one in poor, none of them was associated with tumor necrosis and lympho-mononuclear infiltrate. It shows that TP tumors have better prognostic features as compared to the tumors which were negative for ER. Similarly, Bauer et al found excellent survival of patients with ER-positive cancers irrespective of HER2/neu status (39). Therefore, ER-negativity appears to be a stronger predictor of poor survival than HER2/neu-positivity.
Conclusion
ER and PR expression is seen in benign breast lesions and in breast carcinoma associated with good prognostic parameters. Steroid receptor assays in breast tumors represent the very first step of a general strategy to decipher the biological behaviour of human breast cancer for clinical purposes. To this date, none of the other biological prognostic factors have gained general acceptance for clinical practice. Steroid receptor status, although an imperfect predictor of patients' outcome, still remains the only single biological parameter in use to suggest therapeutic directives for subgroups of breast cancer patients.
Acknowledgment
We hereby acknowledge the contributions of our fellow colleagues, seniors, faculty and the technical staff. The authors declare that there is no conflict of interests.
References
- 1.Agarwal G, Ramakant P. Breast cancer care in India: The current scenario and the challenges for the future. Breast Care. 2008;3:21–7. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosai J. Rosai and Ackerman’s Surgical Pathology. 9th ed. Missouri: Mosby; 2004. [Google Scholar]
- 3.Jensen EV. Hormone dependency of breast cancer. Cancer. 1981;47(10):2319–26. doi: 10.1002/1097-0142(19810515)47:10<2319::aid-cncr2820471002>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Jacquemier JD, Rolland PH, Vague D, Lieutaud R, Spitalier JM, Martin PM. Relationships between steroid receptor and epithelial cell proliferation in benign fibrocystic disease of the breast. Cancer . 1982;49(12):2534–6. doi: 10.1002/1097-0142(19820615)49:12<2534::aid-cncr2820491221>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Giani C, D'Amore E, Delarue JC, Mouriesse H, May-Levin F, Sancho-Garnier H, et al. Estrogen and progesterone receptors in benign breast tumor s and lesions: relationship with histological and cytological features. Int J Cancer. 1986;37(1):7–10. doi: 10.1002/ijc.2910370103. [DOI] [PubMed] [Google Scholar]
- 6.Umekita Y, Yoshida H. Immunohistochemical study of hormone receptor and hormone-regulated protein expression in phyllodes tumor: comparison with fibroadenoma. Virchows Arch. 1998;433(4):311–4. doi: 10.1007/s004280050254. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CDM. Diagnostic Histopathology of Tumors. 3rd ed. Philadelphia: Churchill Livingstone; 2007. [Google Scholar]
- 8.Henson DE, Fielding LP, Grignon DJ, Page DL, Hammond ME, Nash G, et al. College of American Pathologists Conference XXVI on clinical relevance of prognostic markers in solid tumor s Summary Members of the Cancer Committee. Arch Pathol Lab Med. 1995;119(12):1109–12. [PubMed] [Google Scholar]
- 9.Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, et al. Immunohistochemical detection of steroid receptors in breast cancer: A working protocol. J Clin Pathol. 2000;53:634–5. doi: 10.1136/jcp.53.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg SG, DeLellis RA, Frable WJ, Livolsi VA, Wick MR. Silverberg’s Principles and Practice of Surgical Cytopathology. 4th ed. Philadelphia: Churchill Livingstone; 2006. [Google Scholar]
- 11.Bancroft JD, Gamble M. Theory and Practice of Histologic techniques. 6th ed. Philadelphia: Churchill Livingstone; 2008. [Google Scholar]
- 12.Mudduwa LK. Quick score of hormone receptor status of breast carcinoma: correlation with other clinicopathological prognostic parameters. Indian J Pathol Microbiol. 2009;52:159–63. doi: 10.4103/0377-4929.48906. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed RH, Lakatua DJ, Haus E, Yasmineh WJ. Estrogen and progesterone receptors in human breast cancer Correlation with histologic subtype and degree of differentiation. Cancer. 1986;58(5):1076–81. doi: 10.1002/1097-0142(19860901)58:5<1076::aid-cncr2820580516>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Sughayer MH, Al-Khawaja M, Massarweh S, Al-Masri M. Prevalence of hormone receptors and HER2/neu in breast cancer cases in Jordan. Pathol Oncol Res. 2006;12:83–6. doi: 10.1007/BF02893449. [DOI] [PubMed] [Google Scholar]
- 15.Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF. Hormone receptor status of breast cancer in India: A study of 798 tumor s. Breast. 2000;9:267–70. doi: 10.1054/brst.2000.0134. [DOI] [PubMed] [Google Scholar]
- 16.Fisher ER, Redmond CK, Liu H, Rockette H, Fisher B. Correlation of estrogen receptor and pathologic characteristics of invasive breast cancer. Cancer. 1980;45:349–53. doi: 10.1002/1097-0142(19800115)45:2<349::aid-cncr2820450226>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone – related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151(7):703–14. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- 18.Fatima S, Faridi N, Gill S. Breast cancer: steroid receptors and other prognostic indicators. J Coll Physicians Surg Pak. 2005;15(4):230–3. [PubMed] [Google Scholar]
- 19.Rosenberg LU, Einarsdo´ ttir K, Friman EI, Wedren S, Dickman PW, Hall P, et al. Risk factors for hormone receptor-defined breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2482–8. doi: 10.1158/1055-9965.EPI-06-0489. [DOI] [PubMed] [Google Scholar]
- 20.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):1–10. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azizun-Nisa , Bhurgri Y, Raza F, Kayani N. Comparison of ER, PR and HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancer. Asian Pac J Cancer Prev. 2008;9(4):553–6. [PubMed] [Google Scholar]
- 22.Pichon MF, Broet P, Magdelenat H, Delarue JC, Spyratos F, Basuyau JP, et al. Prognostic value of steroid receptors after long-term follow-up of 2257 operable breast cancers. Br J Cancer. 1996;73:1545–51. doi: 10.1038/bjc.1996.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enger SM, Ross RK, Paganini-Hill A, Longnecker MP, Bernstein L. Alcohol consumption and breast cancer oestrogen and progesterone receptor status. Br J Cancer. 1999;79(7/8):1308–14. doi: 10.1038/sj.bjc.6690210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contesso G, Delarue JC, Mouriesse H, May-Levin F, Garnier H. Anatomopathology of breast cancer and hormone receptors. Pathol Biol. 1983;31(9):747–54. [PubMed] [Google Scholar]
- 25.Thike AA, Chng MJ, Fook-Chong S, Tan PH. Immunohistochemical expression of hormone receptors in invasive breast carcinoma: correlation of results of H-score with pathological parameters. Pathology. 2001;33(1):21–5. [PubMed] [Google Scholar]
- 26.Ayadi A, Khabir A, Amouri H, Karray S, Dammak A, Guermazi M, et al. Correlation of HER-2 over-expression with clinico-pathological parameters in Tunisian breast carcinoma. World J Surg Oncol . 2008;6 doi: 10.1186/1477-7819-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudduwa LK. Quick score of hormone receptor status of breast carcinoma: correlation with other clinicopathological prognostic parameters. Indian J Pathol Microbiol. 2009;52:159–63. doi: 10.4103/0377-4929.48906. [DOI] [PubMed] [Google Scholar]
- 28.Barnes NLP, Boland GP, Davenport A, Knox WF, Bundred NJ. Relationship between hormone receptor status and tumor size, grade and comedo necrosis in ductal carcinoma in situ. Br J Surg. 2005;92:429–34. doi: 10.1002/bjs.4878. [DOI] [PubMed] [Google Scholar]
- 29.Millis RR. Correlation of hormone receptors with pathological features in human breast cancer. Cancer. 1980;46:2869–71. doi: 10.1002/1097-0142(19801215)46:12+<2869::aid-cncr2820461426>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Fisher ER, Osborne CK, McGuire WL, Redmond C, Knight WA 3rd, Fisher B, et al. Correlation of primary breast cancer histopathology and estrogen receptor content. Breast Cancer Res Treat. 1981;1:37–41. doi: 10.1007/BF01807890. [DOI] [PubMed] [Google Scholar]
- 31.Howat JM, Barnes DM, Harris M, Swindell R. The association of cytosol estrogen and progesterone receptors with histologic features of breast cancer and early recurrence of disease. Br J Cancer. 1983;47:629–40. doi: 10.1038/bjc.1983.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt FC, Andrade LM, de Lucca LA. Detection of estrogen receptor in formalin fixed and paraffin embedded breast carcinoma: correlation with histologic patterns. Rev Paul Med. 1992;110:158–62. [PubMed] [Google Scholar]
- 33.Rolland PH, Jacquemier J, Martin PM. Histological differentiation in human breast cancer is related to steroid receptors and stromal elastosis. Cancer Chemother Pharmacol. 1980;5(2):73–7. doi: 10.1007/BF00435407. [DOI] [PubMed] [Google Scholar]
- 34.Lima-de-Almeida FM, Brentani MM, Velludo MA, Goes JC, Baruffi I. Elastosis and steroid receptors in primary breast cancer. Braz J Med Biol Res. 1985;18(3):279–83. [PubMed] [Google Scholar]
- 35.Sasaki Y, Tsuda H. Clinicopathological characteristics of triple-negative breast cancers. Breast Cancer . 2009;16(4):254–9. doi: 10.1007/s12282-009-0153-5. [DOI] [PubMed] [Google Scholar]
- 36.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and HER2/neu expression: Comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1-2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwase H, Kurebayashi J, Tsuda H, Ohta T, Kurosumi M, Miyamoto K, et al. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer . 2010;17(2):118–24. doi: 10.1007/s12282-009-0113-0. [DOI] [PubMed] [Google Scholar]
- 38.Chacón R, Costanzo M. Triple-negative breast cancer. Breast Cancer Res . 2010;12 (Suppl 2) doi: 10.1186/bcr2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer K, Parise C, Caggiano V. Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. BMC Cancer . 2010;10 doi: 10.1186/1471-2407-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]