Abstract
Objectives. We described disparities in selected communicable disease incidence across area-based poverty levels in New York City, an area with more than 8 million residents and pronounced household income inequality.
Methods. We geocoded and categorized cases of 53 communicable diseases diagnosed during 2006 to 2013 by census tract-based poverty level. Age-standardized incidence rate ratios (IRRs) were calculated for areas with 30% or more versus fewer than 10% of residents below the federal poverty threshold.
Results. Diseases associated with high poverty included rickettsialpox (IRR = 3.69; 95% confidence interval [CI] = 2.29, 5.95), chronic hepatitis C (IRR for new reports = 3.58; 95% CI = 3.50, 3.66), and malaria (IRR = 3.48; 95% CI = 2.97, 4.08). Diseases associated with low poverty included domestic tick-borne diseases acquired through travel to areas where infected vectors are prevalent, such as human granulocytic anaplasmosis (IRR = 0.08; 95% CI = 0.03, 0.19) and Lyme disease (IRR = 0.34; 95% CI = 0.32, 0.36).
Conclusions. Residents of high poverty areas were disproportionately affected by certain communicable diseases that are amenable to public health interventions. Future work should clarify subgroups at highest risk, identify reasons for the observed associations, and use findings to support programs to minimize disparities.
For many conditions of public health importance, differential morbidity and mortality occur across socioeconomic strata,1 but it is not known the extent to which this may be true for a wide variety of reportable infectious diseases. Neighborhood poverty measures reflect a mix of individual-level and area-based socioeconomic effects,1 and may also be a proxy for individual socioeconomic position in the absence of individual-level data.2 Analyzing disease surveillance data according to area-based poverty measures helps define populations at increased risk for disease, an important step toward identifying and tracking disparities and targeting prevention measures.
Several studies used routine surveillance data in New York City (NYC) to identify associations between census tract-based poverty and the incidence of various reportable diseases. For invasive pneumococcal disease,3 invasive Group A streptococcal infection,4 and community-acquired Legionnaires’ disease,5 the highest disease rates occurred in the highest poverty areas. For chronic hepatitis C, the rate of newly reported cases was not associated with poverty among persons aged 21 years or younger, but it was strongly associated with increasing poverty among older age groups.6 Across these studies, investigators chose different study periods, poverty data sources, definitions, approaches to age adjustment, and statistical tests.
Our objective was to build on this previous work to describe disparities across area-based poverty levels by systematically analyzing a larger set of reportable communicable diseases in NYC, following the guidance of the Public Health Disparities Geocoding Project1 as adapted to NYC.2 To identify which reportable diseases had the greatest disparities across area-based poverty levels, we used disease data through 2013, accounted for undomiciled and incarcerated patients, used a consistent source of poverty data, statistically tested for trends in age-adjusted disease rates across poverty levels, and calculated the population attributable fraction (PAF) caused by neighborhood poverty.
METHODS
We identified confirmed, probable, and suspected cases of 53 diseases reportable to the Bureau of Communicable Disease of the NYC Department of Health and Mental Hygiene (DOHMH).7 We considered all events of reportable human diseases currently under surveillance and available in the centralized disease database (Maven, Consilience Software, Austin, TX), except for syndromes that reflect a mix of etiologies (e.g., encephalitis, bacterial meningitis, and viral meningitis) and rarely reported or not routinely investigated diseases (i.e., nonspecific Rickettsia, hepatitis D, hepatitis E, and other or unspecified infectious hepatitis). Data were unavailable for diseases not reportable to the Bureau of Communicable Disease, including tuberculosis, HIV/AIDS, sexually transmitted infections, and some vaccine-preventable infections.
We defined the study period as reported cases with diagnosis dates from January 1, 2006 (the year electronic laboratory reporting was legally mandated in NYC8), through December 31, 2013 (the most recent calendar year of cleaned data available at the time of analysis). We adjusted the study period for certain diseases added to the NYC list of reportable conditions after 2006. For lymphocytic choriomeningitis virus, norovirus, rotavirus, respiratory syncytial virus, and vancomycin-intermediate Staphylococcus aureus, the study period began in 2008. For paratyphoid fever, the study period began in 2009. For invasive pneumococcal disease, the study period began in 2007, because data for 2006 were incomplete in the centralized disease database.
We defined neighborhoods using census tracts. For area-based poverty analyses, we preferred using smaller area units, such as census tracts, over larger areas units such as zip codes to increase socioeconomic homogeneity within units.2 We geocoded cases using the NYC Department of City Planning’s Geosupport System9 to determine the census tract of residence (using 2010 boundaries) at the time of report. The unit of analysis was the disease event; 1 patient could contribute to multiple disease events with potentially different residences at the time of each report. We used the χ2 test to compare demographic characteristics of cases that could versus could not be geocoded10; we excluded the latter cases from analysis.
Following DOHMH recommendations to present neighborhood-level poverty as a standard variable when analyzing routinely collected surveillance data, we defined census tract-based poverty as the percent of residents with incomes below the federal poverty threshold and grouped it into 4 categories: fewer than 10% of residents below the federal poverty threshold, 10% to fewer than 20%, 20% to fewer than 30%, and 30% or more.2 Notably, a 20% or more cutpoint aligned with the federally defined standard definitions of a poverty area and a medically underserved area.11 For cases diagnosed from 2006 to 2008, the poverty data source was the American Community Survey (ACS), 2006 to 2010; for cases diagnosed in 2009, the poverty data source was ACS, 2007 to 2011; and for cases diagnosed in 2010 to 2013, the poverty data source was ACS, 2008 to 2012.12
Two subpopulations were identified to avoid skewing results. We assumed undomiciled individuals were likely to have high individual poverty, and because area-based poverty can be viewed as a proxy for individual socioeconomic position,2 we assigned them to the highest poverty level. We excluded incarcerated individuals from analysis, because such individuals do not contribute to area-based poverty calculations (e.g., the poverty level for the census tract with Rikers Island jail was missing), and patients’ home addresses when not incarcerated were unavailable. Both subpopulations were identified by scanning the address for keywords (e.g., “undom,” “homeless,” “jail,” “prison”), by matching the geocoded address against lists of geocoded facility addresses, and by interviewing patients.
Age Adjustment
We age-adjusted disease rates, because the baseline disease risk or probability of diagnostic workup might be associated with age, and poverty groups might have different underlying age structures.2 We grouped cases into 7 age categories (< 5, 5–14, 15–24, 25–44, 45–64, 65–74, and ≥ 75 years) to adequately adjust for diseases that disproportionately affect the young and old. We obtained denominator data by age group and poverty level from intercensal population estimates developed by DOHMH; as of 2012, the number of NYC residents in each of the 4 poverty levels, from lowest to highest poverty, were approximately 2.4 million, 2.4 million, 1.7 million, and 1.8 million, respectively.
We calculated disease rates for each of the 4 poverty levels, and adjusted the rates using direct standardization for age at diagnosis and weighting by the US 2000 standard population.13 We calculated 95% confidence intervals (CIs) around the rates based on the γ distribution, which performs better for small counts than the traditional normal approximation method.10,14 Age-adjusted average annual rates were calculated for all diseases, except infant botulism (which only affected < 1-year-old infants) and invasive Group B Streptococcus (for which analyses were restricted to < 7-day-old infants). In addition, subgroup analyses were conducted for campylobacteriosis among children younger than 10 years and salmonellosis among children younger than 5 years to replicate analyses presented in previous literature.15,16
Identifying Disparities in Disease Rates
For each disease, we set up a 2 × 4 table by multiplying the age-adjusted disease rate by the total population in each of the 4 poverty levels to derive age-adjusted counts of disease events and counts of unaffected persons. We then conducted the Cochran–Armitage χ2 test for trend to check for increasing or decreasing trends in disease across poverty levels.2 Results were suppressed for any diseases for which the data were too sparse (i.e., expected disease counts from the χ2 test < 5 for at least 2 of the 4 poverty levels). In addition, for each disease, age-standardized incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were calculated for the highest versus lowest poverty levels.10
We also calculated the PAF, which represented the fraction of all cases that might not have occurred without exposure to higher poverty neighborhoods.10,17 We calculated PAFs for each disease and age category as a function of the case fraction in each poverty level and IRRs, and then aggregated these across age categories. For each disease, the annual number of cases that might be averted if all NYC residents lived in areas with fewer than 10% of residents below the federal poverty threshold was estimated as the PAF multiplied by the average annual number of cases.
Before interpreting results, we identified diseases a priori for which incidence rates by poverty level would be unbiased, because the probability of diagnosis and reporting would not likely depend on poverty level. These diseases were selected because nearly all cases would require inpatient care, and there was no basis for assuming that laboratory testing would depend on poverty level. These included invasive bacterial diseases (i.e., group A Streptococcus, group B Streptococcus, Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae) and selected other severe diseases (legionellosis, listeriosis, malaria, paratyphoid fever, typhoid fever, and West Nile neuroinvasive disease).
RESULTS
Across the 53 reportable diseases, 286 132 cases were identified. On average, each year, more than 9000 newly reported cases each of chronic hepatitis B and C, more than 4000 cases each of laboratory-confirmed influenza and respiratory syncytial virus, and more than 1000 cases each of Lyme disease, campylobacteriosis, and salmonellosis were reported to the Bureau of Communicable Disease (Table 1).
TABLE 1—
Analyses of Communicable Disease Incidence by Census Tract–Level Poverty: New York City, 2006–2013
| Diseasea | Cases,b No. | Years in Study Period,c No. | Cases Geocoded,d % | Highest vs Lowest Poverty Level, IRR (95% CI) | Pe | Population Attributable Fraction | Cases Averted Annually,f No. |
| Fecal–oral | |||||||
| Amebiasis | 3 584 | 8 | 96 | 0.96 (0.87, 1.07) | .25 | 0.03 | 13 |
| Campylobacteriosis | 9 706 | 8 | 97 | 0.94 (0.88, 1.00) | .61 | 0.06 | 71 |
| Cryptosporidiosis | 842 | 8 | 99 | 1.67 (1.38, 2.03) | < .001 | 0.22 | 23 |
| Cyclosporiasis | 140 | 8 | 100 | 0.31 (0.17, 0.55) | < .001 | −0.51 | −9 |
| Giardiasis | 6 958 | 8 | 98 | 0.88 (0.82, 0.94) | < .001 | −0.05 | −44 |
| Hemolytic uremic syndrome | 33 | 8 | 100 | 0.19 (0.06, 0.68) | .004 | −0.34 | −1 |
| Listeriosis | 289 | 8 | 95 | 1.12 (0.80, 1.58) | .55 | 0.06 | 2 |
| Norovirus, laboratory-confirmed | 233 | 6 | 87 | 1.26 (0.85, 1.88) | .24 | 0.07 | 3 |
| Rotavirus, laboratory-confirmed | 673 | 6 | 94 | 1.11 (0.88, 1.40) | .11 | 0.16 | 18 |
| Salmonellosis | 9 802 | 8 | 95 | 1.27 (1.20, 1.35) | < .001 | 0.11 | 136 |
| Shiga toxin-producing Escherichia coli | 560 | 8 | 96 | 0.52 (0.41, 0.68) | < .001 | −0.30 | −21 |
| Shigellosis | 3 387 | 8 | 91 | 2.31 (2.08, 2.58) | < .001 | 0.40 | 169 |
| Vibrio species (noncholera) | 132 | 8 | 96 | 0.27 (0.14, 0.52) | < .001 | −0.49 | −8 |
| Yersiniosis | 161 | 8 | 88 | 0.79 (0.48, 1.29) | .23 | −0.01 | 0 |
| Hepatitidies | |||||||
| Hepatitis B, acute | 707 | 8 | 95 | 1.94 (1.56, 2.41) | < .001 | 0.29 | 25 |
| Hepatitis B, chronicg | 74 664 | 8 | 89 | 3.28 (3.20, 3.36) | < .001 | 0.52 | 4 829 |
| Hepatitis C, acute | 69 | 8 | 93 | 1.01 (0.46, 2.21) | .57 | 0.23 | 2 |
| Hepatitis C, chronicg | 75 929 | 8 | 87 | 3.58 (3.50, 3.66) | < .001 | 0.45 | 4 294 |
| International travel-associated | |||||||
| Dengue | 684 | 8 | 94 | 1.54 (1.24, 1.92) | < .001 | 0.17 | 15 |
| Hepatitis Ah | 771 | 8 | 95 | 0.86 (0.68, 1.08) | .07 | 0.07 | 7 |
| Malaria | 1 695 | 8 | 93 | 3.48 (2.97, 4.08) | < .001 | 0.52 | 111 |
| Paratyphoid fever | 66 | 5 | 96 | 1.10 (0.48, 2.53) | .87 | 0.32 | 4 |
| Typhoid fever | 394 | 8 | 97 | 1.31 (0.94, 1.83) | .19 | 0.35 | 17 |
| Zoonotic/vector-borne predominantly acquired in the United States | |||||||
| Anaplasmosis, human granulocytic | 172 | 8 | 95 | 0.08 (0.03, 0.19) | < .001 | −0.81 | −17 |
| Babesiosis | 340 | 8 | 95 | 0.20 (0.13, 0.32) | < .001 | −0.68 | −29 |
| Ehrlichiosis, human monocytic | 82 | 8 | 99 | 0.10 (0.04, 0.29) | < .001 | −0.66 | −7 |
| Lyme disease | 10 763 | 8 | 91 | 0.34 (0.32, 0.36) | < .001 | −0.59 | −789 |
| Rickettsialpox | 134 | 8 | 94 | 3.69 (2.29, 5.95) | < .001 | 0.39 | 7 |
| Rocky Mountain spotted fever | 100 | 8 | 96 | 0.66 (0.38, 1.16) | .09 | −0.26 | −3 |
| West Nile neuroinvasive disease | 109 | 8 | 95 | 0.22 (0.09, 0.51) | < .001 | −0.33 | −4 |
| Invasive bacterial | |||||||
| Group A Streptococcus | 1 640 | 8 | 96 | 2.33 (2.03, 2.68) | < .001 | 0.30 | 61 |
| Group B Streptococcus | 343 | 8 | 97 | 2.29 (1.65, 3.18) | < .001 | 0.41 | 18 |
| Neisseria meningitidis | 218 | 8 | 96 | 2.02 (1.38, 2.97) | < .001 | 0.27 | 7 |
| Haemophilus influenzae | 877 | 8 | 91 | 1.81 (1.50, 2.18) | < .001 | 0.17 | 19 |
| Streptococcus pneumoniae | 5 717 | 7 | 95 | 2.61 (2.42, 2.81) | < .001 | 0.35 | 287 |
| Respiratory | |||||||
| Influenza (laboratory-confirmed) | 38 776 | 8 | 92 | 1.17 (1.14, 1.21) | < .001 | 0.05 | 227 |
| Respiratory syncytial virus (laboratory-confirmed) | 26 479 | 6 | 93 | 1.78 (1.71, 1.84) | < .001 | 0.28 | 1 237 |
| Miscellaneous | |||||||
| Legionellosis | 1 599 | 8 | 98 | 2.04 (1.79, 2.34) | < .001 | 0.24 | 47 |
| Leprosy | 50 | 8 | 88 | 1.84 (0.74, 4.55) | .47 | 0.48 | 3 |
| Transmissible spongiform encephalopathies | 51 | 8 | 80 | 0.24 (0.07, 0.80) | .001 | −0.49 | −3 |
| Vancomycin-intermediate Staphylococcus aureus | 56 | 6 | 88 | 2.51 (1.18, 5.35) | .02 | 0.30 | 3 |
Note. CI = confidence interval; IRR = incidence rate ratio.
In addition to the 41 diseases in the table, an additional 12 diseases were examined, but the data were too sparse for presentation: anthrax; infant botulism; foodborne or other botulism; brucellosis; cholera; ehrlichiosis, not otherwise specified; lymphocytic choriomeningitis virus; leptospirosis; Q fever; trichinosis; toxic shock syndrome; and tularemia.
Number of cases excludes incarcerated individuals and patients not known to reside specifically in 1 of the 5 New York City boroughs (unless undomiciled).
Less than 8 years of data were included for those diseases that were not reportable at the start of the overall study period in 2006.
Undomiciled individuals were considered successfully geocoded. Cases that were geocoded to a census tract with missing poverty level were then excluded from analysis (n = 84).
P values determined by Cochran–Armitage test for trend.
Average number of annual cases that might be averted if all NYC residents lived in areas with < 10% of residents below the federal poverty threshold.
Chronic hepatitis B and C cases represent cases newly reported during the study period and should not be interpreted as incident cases.
Hepatitis A among New York City residents is predominantly acquired during international travel.18
Across all diseases, 5640 (2.0%) patients were identified as undomiciled and assigned to the highest poverty level, whereas 7039 (2.5%) patients were identified as incarcerated and excluded from analysis. Chronic hepatitis C accounted for the majority of reportable disease cases among undomiciled individuals (3757; 67%), representing 4.6% of all newly reported chronic hepatitis C patients in NYC. Similarly, the majority of reportable disease cases among incarcerated individuals (6433; 91%) were chronic hepatitis C, which represented 7.8% of all patients with this disease. The remaining undomiciled cases were reported with 29 other diseases, each representing fewer than 6% of patients with that disease. The remaining incarcerated cases were reported with 15 diseases, each representing fewer than 2% of patients with that disease.
Of 279 093 cases remaining after excluding incarcerated persons, we successfully geocoded 252 395 cases (90.4%). The geocoding success rate was higher for cases of diseases that are routinely investigated in NYC (95.6%) than for diseases that are not investigated (e.g., influenza or chronic hepatitis; 89.4%; χ2 P < .001). Non-geocodable cases were more likely to be male, to be 25 to 64 years old, and to reside in the Bronx (P < .001 for each). The average geocoding success rate across all diseases was 92.8% (range = 80.4% for transmissible spongiform encephalopathies to 100% for cyclosporiasis and hemolytic uremic syndrome).
Diseases Associated With Increasing Poverty
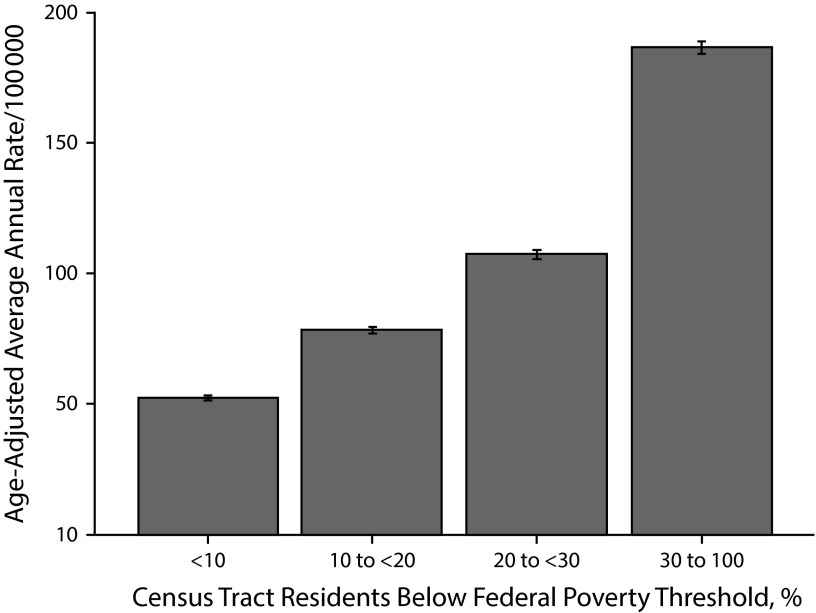
Age-adjusted rates of acute and chronic hepatitis B and chronic hepatitis C increased as area-based poverty increased (Table 1). Of all the diseases examined, chronic hepatitis C had the second highest IRR for the highest versus lowest poverty level (IRR for new reports = 3.58; 95% CI = 3.50, 3.66; Figure 1). The PAF for chronic hepatitis C was 0.45, which suggested that 45% of chronic hepatitis C cases might not have occurred if all NYC census tracts had fewer than 10% of residents below the federal poverty threshold.
FIGURE 1—
Newly reported chronic hepatitis C cases per 100 000 population by census tract–level poverty: New York City, 2006–2013.
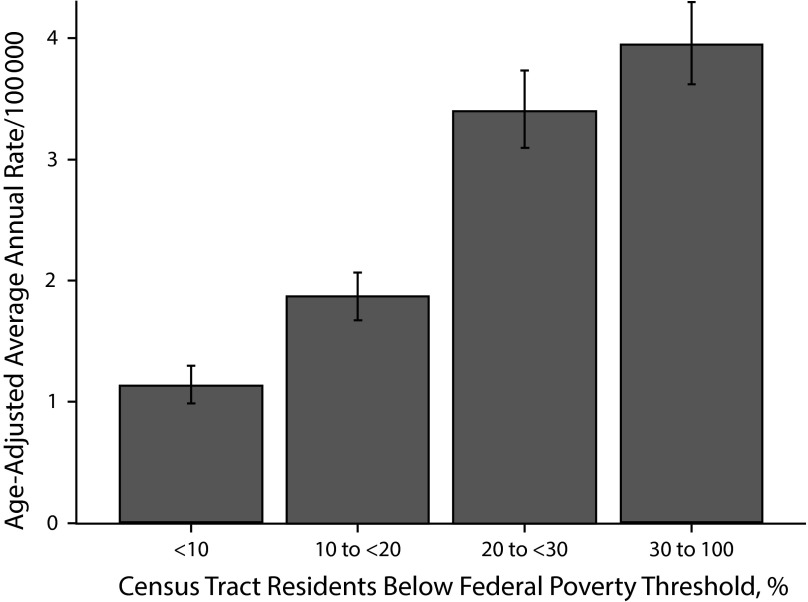
Among diseases predominantly acquired during international travel, malaria and dengue were associated with increasing poverty; the association between malaria and increasing poverty was especially strong (Figure 2), with a PAF of 0.52. Among zoonotic or vector-borne diseases predominantly acquired in the United States, only rickettsialpox was associated with increasing poverty; of all diseases examined, rickettsialpox had the greatest disparity between the highest and lowest poverty levels (IRR = 3.69; 95% CI = 2.29, 5.95).
FIGURE 2—
Malaria incidence by census tract–level poverty: New York City, 2006–2013.
All 5 invasive bacterial diseases were strongly associated with increasing poverty, as were laboratory-confirmed influenza, laboratory-confirmed respiratory syncytial virus, and legionellosis (Table 1). Among diseases acquired through fecal–oral transmission, cryptosporidiosis, salmonellosis (for all ages and restricted to those < 5 years old), and shigellosis were associated with increasing poverty.
Diseases Associated With Decreasing Poverty
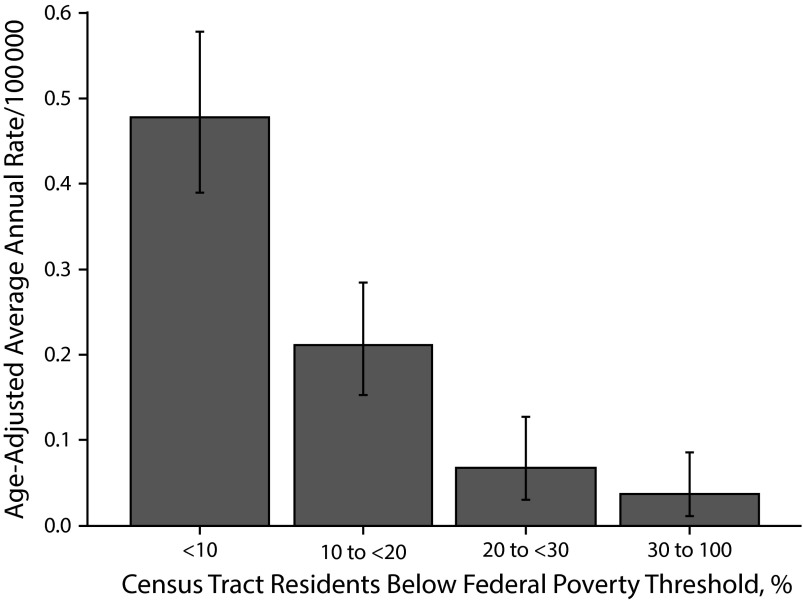
Rates of most domestic zoonotic or vectorborne diseases were higher in neighborhoods with low poverty (Table 1). In particular, human granulocytic anaplasmosis (Figure 3) had the most extreme disparity with this directionality between the highest and lowest poverty levels (IRR 0.08; 95% CI = 0.03, 0.19). Similar patterns were observed for babesiosis, human monocytic ehrlichiosis, Lyme disease, and West Nile neuroinvasive disease. Because residing in low poverty areas was associated with infection, the PAFs for these diseases were strongly negative (Table 1).
FIGURE 3—
Human granulocytic anaplasmosis incidence by census tract–level poverty: New York City, 2006–2013.
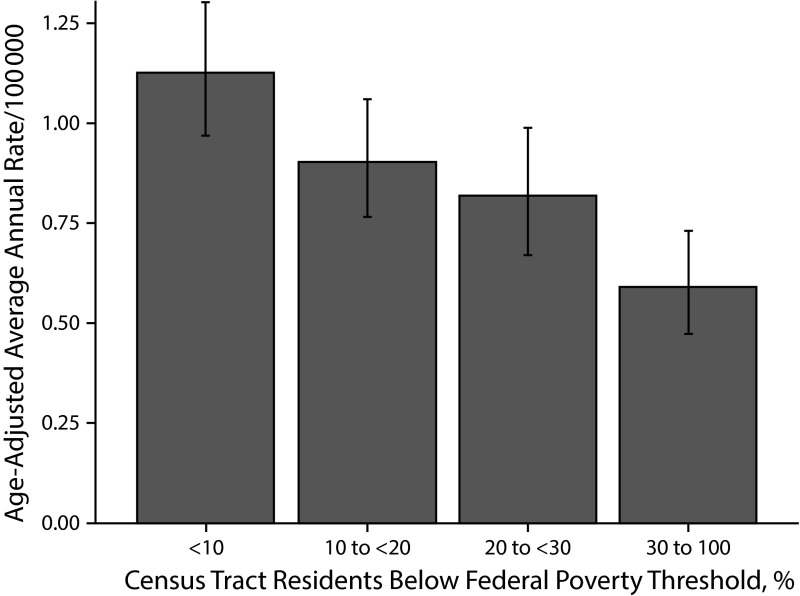
Among diseases acquired through fecal–oral transmission, cyclosporiasis, giardiasis, hemolytic uremic syndrome, Shiga toxin-producing Escherichia coli (STEC; Figure 4) and Vibrio species (noncholera) were all associated with decreasing poverty. A similar trend across poverty levels was observed for transmissible spongiform encephalopathies.
FIGURE 4—
Shiga toxin-producing Escherichia coli incidence by census tract–level poverty: New York City, 2006–2013.
Diseases Not Associated With Poverty
For listeriosis and laboratory-confirmed norovirus, the trend across poverty levels was not statistically significant, although the highest poverty level appeared to have the highest rates (Table 1). By contrast, for amebiasis and yersiniosis, the highest poverty level appeared to have the lowest rates, but again, the trend was not statistically significant.
For some diseases, apparent trends across poverty levels were neither monotonic nor statistically significant. For campylobacteriosis and laboratory-confirmed rotavirus, there appeared to be an increasing trend across the lower 3 poverty levels, but the trend did not continue into the highest poverty level, where the rate was lower. (After restricting campylobacteriosis to those < 10 years old, the overall pattern was similar, although the association with increasing poverty was statistically significant at P < .001.) By contrast, for typhoid fever and paratyphoid fever, disease rates increased with decreasing poverty across the highest 3 poverty levels, but the trend did not continue into the lowest poverty level, where the rate was lower. For Rocky Mountain spotted fever, disease rates decreased with decreasing poverty, but the trend did not continue into the lowest poverty level, where the rate was higher.
For 2 diseases, rates were similar across poverty levels, except rates appeared to be higher in a middle poverty level. For acute hepatitis C, the highest rate was in the second highest poverty level, although the case count was low. For hepatitis A, the highest rate was in the second lowest poverty level.
For leprosy, no clear association with poverty was observed. Data for an additional 12 diseases were too sparse to conclude whether there was any association with poverty (Table 1).
DISCUSSION
We systematically examined associations between area-based poverty and a large number of reportable communicable diseases in NYC, an area with a population of more than 8 million people and one of the largest measures of household income inequality in the United States.19 Of 41 diseases with an adequate sample size to assess an association with poverty, 18 diseases (44%) were statistically significantly associated with high poverty areas, and 11 diseases (27%) were associated with low poverty areas. Identifying which diseases were most concentrated in high or low poverty areas might be informative for targeting outreach or educational efforts.
The 5 diseases with the greatest disparities between the highest versus lowest poverty levels were rickettsialpox, chronic hepatitis C, malaria, chronic hepatitis B, and invasive pneumococcal disease. Rickettsialpox is transmitted to humans from mice via mites, and residents of high poverty areas likely have greater exposure to house mice.20 For chronic hepatitis C, the strong association with poverty in NYC was previously demonstrated to be driven by older age groups, particularly among those born between 1945 and 1965, and by injection drug use.6,21 Chronic hepatitis C prevalence is very high,22 prevention programs need strengthening,23 and curative treatment is available. Patterns of chronic hepatitis B in NYC are largely driven by immigration patterns from China.24,25 All malaria cases in NYC during the study period were travel-related, with the majority of patients infected while traveling to visit friends and relatives or before recent immigration to NYC18; the association with high poverty neighborhoods again likely reflected immigration patterns. Antimalarial prophylaxis should be promoted among at-risk travelers residing in these neighborhoods, who might be less likely to access pretravel care because of costs or perceived lack of need.26 For invasive pneumococcal disease, a similar association with poverty was also been reported elsewhere, including in Connecticut27 and areas across 9 states.28 Higher invasive pneumococcal disease rates might be related to lower pneumococcal vaccination rates, higher rates of underlying medical conditions and smoking, or crowding in higher poverty areas.3,27
Four of the 5 diseases with the greatest disparities in the other direction (i.e., residents of the highest poverty areas were much less likely to be infected than residents of the lowest poverty areas) were domestic vector-borne diseases. The association with low poverty for human granulocytic anaplasmosis, human monocytic ehrlichiosis, and babesiosis likely reflected a population wealthy enough to travel to areas outside of NYC where infected vectors are prevalent.29 By contrast, most West Nile neuroinvasive disease cases among NYC residents were locally acquired; the association with low poverty was similar to findings in Chicago, Illinois, and Suffolk County, New York,30,31 and might reflect environmental factors contributing to increased West Nile virus-infected Culex mosquitoes in affected areas. Residents of low poverty areas were also more likely to be reported with having STEC, hemolytic uremic syndrome, and vibriosis, which were findings that might be related to differential exposure to contaminated foods, food preferences, or restaurant dining patterns.
Comparisons With Previous Studies
Our results were broadly consistent with previous published reports. Our finding that laboratory-confirmed influenza in NYC was associated with increasing poverty was consistent with previous observations for influenza-associated hospitalizations during the 2009 H1N1 pandemic in NYC,32 and among children33 and adults34 in Connecticut. For example, this finding could be related to lower vaccination rates or higher case ascertainment among residents of higher poverty areas. Increased prevalence of comorbidities or delayed care seeking could result in complications, prompting presentation to emergency departments or hospitals, where influenza testing is more likely.
Among foodborne bacterial diseases, our finding that STEC was associated with decreasing poverty in NYC was consistent with findings in Connecticut.35 For campylobacteriosis, the directionality of the association with poverty in Connecticut was age-dependent and monotonic, such that incidence was associated with increasing poverty among those younger than 10 years, but with decreasing poverty among those aged 10 years or older.15 By contrast, in NYC, the trend of campylobacteriosis incidence across poverty levels was not monotonic; the overall findings were similar between NYC and Connecticut for those younger than 10 years, but the trend among all age groups in NYC was not statistically significant. For salmonellosis, the directionality of the association was opposite, and was associated with decreasing poverty in Connecticut,16,36 yet with increasing poverty in NYC. Possible explanations for this different finding between Connecticut and NYC might relate to differences in dining patterns or in predominant serotypes; in Connecticut, Salmonella Heidelberg was associated with increasing poverty, whereas S. Enteriditis and S. Newport were associated with decreasing poverty.36
Limitations
Our findings might be influenced by detection bias, because the probability of receiving a diagnosis might be related to area-based poverty. Residents of high poverty areas could be less likely to seek health care (e.g., because of inadequate health insurance or cultural factors) or receive expensive or specialized diagnostic testing (e.g., for transmissible spongiform encephalopathies). Alternatively, residents of high poverty areas could be more likely to receive certain diagnoses because patients with acute diarrheal illness and low household income are reportedly more likely to seek care and submit a stool specimen for culture,37 and low-income New Yorkers disproportionately use emergency departments and hospitals,38,39 where testing for certain diseases might be more common than in primary care settings. Also, some residents of high poverty areas in NYC might have greater access to disease screening (e.g., routine hepatitis C screening in correctional facilities, drug treatment programs, and needle exchanges). Of the 11 diseases for which we believed a priori that the probability of diagnosis and reporting would not depend on poverty level, 7 (legionellosis, malaria, and the 5 invasive bacterial diseases) were associated with increasing poverty, 1 (West Nile neuroinvasive disease) was associated with decreasing poverty, and 3 (listeriosis, paratyphoid fever, and typhoid fever) were not statistically significantly associated with poverty.
Our analyses were subject to at least 4 additional limitations. First, cases that were successfully geocoded might be nonrepresentative (e.g., patient addresses using a PO Box cannot be geocoded), and geocodable and non-geocodable cases might be distributed differentially across poverty areas. However, geocoding rates were high for all diseases, so this was unlikely to strongly bias the results. Second, data were sparse for some diseases, poverty levels, and age groups, which could limit the robustness of our findings. Third, the temporal relationship between disease occurrence and neighborhood poverty could be unclear for chronic infections; we analyzed cases according to residence at the time of report, because residence at the time of infection was unknown. Fourth, because we conducted independent analyses for 53 diseases, 2 to 3 diseases could be expected to be significantly associated with poverty by chance alone. Because we did not wish to miss possible associations with this exploratory analysis, we did not formally adjust for multiple testing.
Conclusions
Systematically describing positive, negative, and null associations between area-based poverty and a large number of reportable diseases is feasible. In NYC, residents of high poverty areas were disproportionately affected by 18 communicable diseases. Future work should clarify populations at highest risk through targeted subgroup analyses by pathogen subtype or by patient case status, age, sex, travel history, underlying medical conditions, and household crowding,40 and by factors that might be unavailable from routine surveillance, such as race/ethnicity or household income. In addition, analyzing possible environmental exposures in relation to area-based poverty might help further elucidate the reasons behind some of the observed disparities in communicable disease incidence and suggest risk factors amenable to intervention. Additional public health jurisdictions could consider joining national efforts to devote more attention to health disparities,1 including the Healthy People 2020 Public Health Infrastructure objective to increase data available by socioeconomic status41 and the work of the health disparities subcommittee of the Council of State and Territorial Epidemiologists.11,16 These findings could serve as a baseline for monitoring disparities over time and across jurisdictions and for targeting prevention measures.
Acknowledgments
S. K. Greene and A. Levin-Rector were supported by the Public Health Emergency Preparedness (PHEP) Cooperative Agreement (grant 5U90TP221298-08) from the Centers for Disease Control and Prevention (CDC).
All Bureau of Communicable Disease staff employed during the study period contributed to the data included in this analysis. In addition, Katherine Bornschlegel, MPH, Jennifer Hsieh, MSPH, Lucretia Jones, DrPH, MPH, and Sally Slavinski, DVM, MPH, contributed to interpreting findings for specific diseases.
This work was presented at the 2015 Annual Conference of the Council of State and Territorial Epidemiologists; June 15, 2015; Boston, MA.
Note. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of Centers for Disease Control and Prevention.
Human Participant Protection
Because this work was conducted in the course of routine public health practice, institutional review board approval was not required.
References
- 1.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toprani A, Hadler JL. Selecting and applying a standard area-based socioeconomic status measure for public health data: analysis for New York City. New York City Department of Health and Mental Hygiene: Epi Research Report, May 2013. Available at: http://www.nyc.gov/html/doh/downloads/pdf/epi/epiresearch-SES-measure.pdf. Accessed December 31, 2013.
- 3.Dentinger C, Lane K, Cordoba E, Lee E, Wang S. Invasive pneumococcal disease surveillance in New York City. Epi data brief. 2011. Available at: http://www.nyc.gov/html/doh/downloads/pdf/epi/databrief7.pdf. Accessed June 4, 2014.
- 4.Dentinger C, Al-Dulaimi R, Dorsinville M, Yea J. Invasive Group A streptococcal infection in New York City. Epi data brief. 2013. Available at: http://www.nyc.gov/html/doh/downloads/pdf/epi/databrief23.pdf. Accessed March 14, 2014.
- 5.Farnham A, Alleyne L, Cimini D, Balter S. Legionnaires’ disease incidence and risk factors, New York, New York, USA, 2002–2011. Emerg Infect Dis. 2014;20(11):1795–1802. doi: 10.3201/eid2011.131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prussing C, Bornschlegel K, Balter S. Hepatitis C surveillance among youth and young adults in New York City, 2009–2013. J Urban Health. 2015;92(2):387–399. doi: 10.1007/s11524-014-9920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.New York City Department of Health and Mental Hygiene. Communicable disease surveillance data: diseases reportable to the Bureau of Communicable Disease. Available at: http://www.nyc.gov/html/doh/html/data/cd-epiquery.shtml. Accessed October 27, 2014.
- 8.Nguyen TQ, Thorpe L, Makki HA, Mostashari F. Benefits and barriers to electronic laboratory results reporting for notifiable diseases: the New York City Department of Health and Mental Hygiene experience. Am J Public Health. 2007;97(suppl 1):S142–S145. doi: 10.2105/AJPH.2006.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geographic Systems Section, Information Technology Division, New York City Department of City Planning. Geosupport Desktop Edition™ copyrighted by the New York City Department of City Planning. Version 14.1, release 14A. 2014. Available at: http://www.nyc.gov/html/dcp/html/bytes/gdeguide.shtml. Accessed September 16, 2014.
- 10.Krieger N, Waterman PD, Chen JT, Rehkopf DH, Subramanian SV. Geocoding and monitoring US socioeconomic inequalities in health: an introduction to using area-based socioeconomic measures–the Public Health Disparities Geocoding Project monograph. 2004. Available at: http://www.hsph.harvard.edu/thegeocodingproject. Accessed January 9, 2014.
- 11.Council of State and Territorial Epidemiologists. Consultant’s guidance document for CSTE utilization of the Public Health Disparities Geocoding Project health disparities monitoring methodology. 2012. Available at: http://www.cste.org/?GuidanceforStates. Accessed December 31, 2013.
- 12.Bureau of Epidemiology Services, New York City Department of Health and Mental Hygiene. Recommendations for use of the agency’s neighborhood poverty measure. 2013. Available at: http://www.nyc.gov/html/doh/downloads/pdf/epi/neighpov_recs.pdf. Accessed January 13, 2014.
- 13.Klein RJ, Schoenborn CA. Age Adjustment Using the 2000 Projected US Population. Vol. 20. Hyattsville, MD: National Center for Health Statistics; 2001. [PubMed] [Google Scholar]
- 14.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Bemis K, Marcus R, Hadler JL. Socioeconomic status and campylobacteriosis, Connecticut, USA, 1999–2009. Emerg Infect Dis. 2014;20(7):1240–1242. doi: 10.3201/eid2007.131333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadler JL. Council of State and Territorial Epidemiologists webinar: analysis of public health data using census tract-level poverty. 2014. Available at: http://www.cste.org/default.asp?page=WebinarLibrary. Accessed March 14, 2014.
- 17.Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55(7):508–514. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamson R, Reddy V, Jones L et al. Epidemiology and burden of hepatitis A, malaria, and typhoid in New York City associated with travel: implications for public health policy. Am J Public Health. 2010;100(7):1249–1252. doi: 10.2105/AJPH.2009.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg DH. American Community Survey Reports: US neighborhood income inequality in the 2005–2009 period. US Census Bureau; 2011. Available at: http://www.census.gov/prod/2011pubs/acs-16.pdf. Accessed November 19, 2014.
- 20.Paddock CD, Zaki SR, Koss T et al. Rickettsialpox in New York City: a persistent urban zoonosis. Ann N Y Acad Sci. 2003;990:36–44. doi: 10.1111/j.1749-6632.2003.tb07334.x. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner J, Bornschlegel K, Devinney K Hepatitis B and C surveillance report: New York City, 2013. 2015. Available at: http://www.nyc.gov/html/doh/html/data/cd-hepabc-reports.shtml. Accessed April 13, 2015.
- 22.Balter S, Stark JH, Kennedy J, Bornschlegel K, Konty K. Estimating the prevalence of hepatitis C infection in New York City using surveillance data. Epidemiol Infect. 2014;142(2):262–269. doi: 10.1017/S0950268813000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagan H. Agent, host, and environment: hepatitis C virus in people who inject drugs. J Infect Dis. 2011;204(12):1819–1821. doi: 10.1093/infdis/jir654. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Surveillance for chronic hepatitis B virus infection – New York City, June 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2012;61(1):6–9. [PubMed] [Google Scholar]
- 25.France AM, Bornschlegel K, Lazaroff J, Kennedy J, Balter S. Estimating the prevalence of chronic hepatitis B virus infection–New York City, 2008. J Urban Health. 2012;89(2):373–383. doi: 10.1007/s11524-011-9653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones LE. Malaria in NYC residents: examining the determinants of chemoprophylaxis use and adherence among immigrants who travel abroad to visit friends and relatives. 2012. Dissertation for City University of New York. Available at: http://gradworks.umi.com/35/41/3541839.html. Accessed April 13, 2015.
- 27.Soto K, Petit S, Hadler JL. Changing disparities in invasive pneumococcal disease by socioeconomic status and race/ethnicity in Connecticut, 1998–2008. Public Health Rep. 2011;126(suppl 3):81–88. doi: 10.1177/00333549111260S313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton DC, Flannery B, Bennett NM et al. Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am J Public Health. 2010;100(10):1904–1911. doi: 10.2105/AJPH.2009.181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdool AJ, Fernandez R, Addei-Maanu C, Slavinski S, Fine A. Lyme disease in New York City – is it locally acquired? (poster). Presented at 6th International Conference on Emerging Infectious Diseases; March 16–18, 2008; Atlanta, GA.
- 30.Ruiz MO, Tedesco C, McTighe TJ, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3(1):8. doi: 10.1186/1476-072X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochlin I, Turbow D, Gomez F, Ninivaggi DV, Campbell SR. Predictive mapping of human risk for West Nile virus (WNV) based on environmental and socioeconomic factors. PLoS ONE. 2011;6(8):e23280. doi: 10.1371/journal.pone.0023280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy NS, Nguyen TQ, Westheimer E, Layton M. Disparities in the severity of influenza illness: a descriptive study of hospitalized and nonhospitalized novel H1N1 influenza-positive patients in New York City: 2009–2010 influenza season. J Public Health Manag Pract. 2013;19(1):16–24. doi: 10.1097/PHH.0b013e31824155a2. [DOI] [PubMed] [Google Scholar]
- 33.Yousey-Hindes KM, Hadler JL. Neighborhood socioeconomic status and influenza hospitalizations among children: New Haven County, Connecticut, 2003–2010. Am J Public Health. 2011;101(9):1785–1789. doi: 10.2105/AJPH.2011.300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam K, Yousey-Hindes K, Hadler JL. Influenza-related hospitalization of adults associated with low census tract socioeconomic status and female sex in New Haven County, Connecticut, 2007–2011. Influenza Other Respir Viruses. 2014;8(3):274–281. doi: 10.1111/irv.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitney B, Hadler JL, Hurd S, Niccolai L. Census tract-level poverty and rurality and Shiga toxin-producing Escherichia coli incidence: Connecticut, 2000–2011 (poster). Presented at: Council of State and Territorial Epidemiologists Annual Conference; June 23, 2014; Nashville, TN.
- 36. Hadler JL, Mainero C, Humes E, Hurd S. Disparities in the incidence of salmonellosis overall and by leading serogroups with census tract-level poverty, Connecticut 2000–2011 (poster). Presented at: Council of State and Territorial Epidemiologists Annual Conference; June 23, 2014; Nashville, TN.
- 37.Scallan E, Jones TF, Cronquist A et al. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog Dis. 2006;3(4):432–438. doi: 10.1089/fpd.2006.3.432. [DOI] [PubMed] [Google Scholar]
- 38.Billings J, Zeitel L, Lukomnik J, Carey TS, Blank AE, Newman L. Impact of socioeconomic status on hospital use in New York City. Health Aff (Millwood) 1993;12(1):162–173. doi: 10.1377/hlthaff.12.1.162. [DOI] [PubMed] [Google Scholar]
- 39.Billings J, Parikh N, Mijanovich T. Emergency department use in New York City: a substitute for primary care? Issue Brief (Commonw Fund) 2000;(433):1–5. [PubMed] [Google Scholar]
- 40.Baker MG, McDonald A, Zhang J, Howden-Chapman P. Infectious diseases attributable to household crowding in New Zealand: a systematic review and burden of disease estimate. Wellington: He Kainga Oranga/Housing and Health Research Programme, University of Otago; 2013. Available at http://www.healthyhousing.org.nz/wp-content/uploads/2010/01/HH-Crowding-ID-Burden-25-May-2013.pdf. Accessed October 24, 2014.
- 41.US Department of Health and Human Services. Healthy People 2020 topics and objectives: public health infrastructure subobjective PHI-7.3. 2014. Available at: http://www.healthypeople.gov/node/5150/data_details. Accessed October 30, 2014.