Abstract
Objectives. We examined the impact of undetected infections, adult immunity, and waning vaccine-acquired immunity on recent age-related trends in pertussis incidence.
Methods. We developed an agent-based model of pertussis transmission in Dakota County, Minnesota using case data from the Minnesota Department of Health. For outbreaks in 2004, 2008, and 2012, we fit our model to incidence in 3 children’s age groups relative to adult incidence. We estimated parameters through model calibration.
Results. The duration of vaccine-acquired immunity after completion of the 5-dose vaccination series decreased from 6.6 years in the 2004 model to approximately 3.0 years in the 2008 and 2012 models. Tdap waned after 2.1 years in the 2012 model. A greater percentage of adults were immune in the 2008 model than in the 2004 and 2012 models. On average, only 1 in 10 adult infections was detected, whereas 8 in 10 child infections were detected.
Conclusions. The observed trends in relative pertussis incidence in Dakota County can be attributed in part to fluctuations in adult immunity and waning vaccine-acquired immunity. No single factor accounts for current pertussis trends.
The United States is experiencing a resurgence of pertussis despite concerted public health efforts to prevent infection. Outbreaks have gradually increased in size since the late 1970s, with substantial increases occurring during the past decade.1,2 National incidence rates have remained highest among infants younger than 1 year, but the remaining burden of disease has shifted between age groups.2 The average age of someone with pertussis before the vaccine era was 5 years. Recently, outbreaks have been recorded with an increased percentage of cases in those older than 5 years. In particular, children aged 7 to 10 years have recently begun to experience a greater burden of disease than have other age groups.2,3
Similar to national trends, in Minnesota changes in pertussis burden by age have been observed despite relatively consistent overall vaccination rates.4 In 2004, children (5–12 years) accounted for 28% of reported pertussis cases, adolescents (13–17 years) accounted for 35% of cases, and adults (≥ 18 years) accounted for 25% of cases. During Minnesota’s subsequent peak year of 2008, 42% of cases were in children, 23% of cases were in adolescents, and 23% of cases were in adults. Incidence rates in all age groups increased from 2004 to 2008. The age distribution of cases in 2012 was nearly identical to that in 2008.5
Although protecting the vulnerable infant population remains a public health priority, understanding the driving factors behind incidence trends in older age groups is necessary to develop and implement effective control measures. The transition from a whole cell pertussis vaccine, DTP, to an acellular vaccine, DTaP, in the 1990s appears to have played a role in increasing incidence among those aged 7–10 years.6 The recommended ages for children to receive DTaP are the same as for DTP (2, 4, 6, and 15–18 months and 4–6 years), but immune protection acquired from DTaP has been reported to wane sooner than that acquired from DTP.7
In addition to vaccine changes, improved laboratory testing, increased awareness, and the introduction of a booster for adolescents and adults (Tdap) have played a role in altering incidence trends.8–10 Research is needed to explain how these factors have collectively contributed to the resurgence of pertussis11; however, this research is complicated by uncertainty in other contributing factors that are difficult to assess directly, such as undetected infections, adult immunity, and waning vaccine-acquired immunity.
Adults with pertussis often present with mild symptoms, and a nondistinctive cough may be the only manifestation of infection.12 Consequently, a large number of adult infections remain undetected.13 Although adults do not constitute a large percentage of reported cases, their mild and asymptomatic infections are believed to play a significant role in transmission.14 Among children, mild or asymptomatic infections in previously immunized individuals may similarly remain undetected.15
Childhood vaccination records for adults are often incomplete and likely do not represent current immune status because of waning immunity. Records of Tdap vaccination are often the only documentation relevant to current immunity in adults, and these records are more challenging to locate than are records for children.16 Seroprevalence data have been relied on to generate estimates of infection and immunity within adult populations, but serologic testing is not standardized and antibodies may reach undetectable levels before immunity wanes.7,17,18
DTP was used for all pertussis vaccinations before the 1990s and is estimated to provide protection for 4 to 12 years beyond the last dose.7 DTaP was initially approved in 1991 for administration to children aged 15 months to 6 years who had received a DTP primary series (shots 1, 2, and 3), and in 1997 DTaP was approved for all 5 doses in children.19 Estimates for the duration of DTaP-induced immunity suggest that protection lasts 5 to 6 years after completion of the DTaP series, with efficacy decreasing each year.20,21 In 2005, Tdap was licensed as a booster shot for adolescents and adults; the longevity of its protection is still being assessed.
With the relatively new field of agent-based modeling, factors that are difficult to measure directly (undetected infections, adult immunity, and waning immunity) can be investigated with computer simulations that can capture high levels of detail. A variety of software platforms have been developed to support this modeling technique in which individuals, or “agents,” in an agent-based model (ABM) move and act independently in a simulated environment on the basis of a set of rules that are determined by their assigned characteristics and model parameters.22 Each individual is programmed to have characteristics (e.g., age and vaccination status) that mimic an individual in a particular population that reflects a geographic region (e.g., a county or a state). ABMs can thus provide insight into local drivers of pertussis outbreaks.
Using data from the Minnesota Department of Health (MDH), we developed an explanatory ABM of pertussis transmission. We simulated outbreaks that occurred in Dakota County, Minnesota during 2004, 2008, and 2012 (Table 1). Dakota County has a population of approximately 400 000 residents and is relatively ethnically diverse (18% non-White) with a mixture of urban and rural settings (approximately one third each of land use is rural, urban, and suburban).23 This population was representative of the state with regard to age-related incidence trends. We calibrated the ABM to attain parameter values for undetected infections, adult immunity, and waning vaccine-acquired immunity so that model output was consistent with age-related relative incidence trends observed during the Dakota County outbreaks.
TABLE 1—
Age Distribution of Reported Pertussis Cases During Outbreaks: Minnesota Department of Health, Dakota County, Minnesota, 2004, 2008, 2012
| Age Group | 2004, % | 2008, % | 2012, % |
| 6 mo–6 y | 9.1 | 11.9 | 14.4 |
| 7–10 y | 11.2 | 30.9 | 29.2 |
| 11–18 y | 58.1 | 40.3 | 33.6 |
| ≥ 19 y | 21.2 | 17.0 | 23.2 |
| Missing | 0.4 | 0.0 | 0.0 |
| Total | 100.0 | 100.0 | 100.0 |
Note. Data are percentages of total cases. Total population was n = 241 for 2004, n = 395 for 2008, and n = 272 for 2012. Total percentages may exceed 100.0 because of rounding.
We identified these factors’ influence on pertussis’s changing incidence trends with an ABM.
METHODS
We implemented an ABM of pertussis transmission in NetLogo, version 5.0.4.24 Three versions of the model were created to independently simulate the 2004, 2008, and 2012 Dakota County outbreaks. The model structure was identical except for the inclusion of Tdap vaccinations in the 2008 and 2012 simulations. To parameterize the model, we used data that MDH collected on confirmed and probable cases as defined by the Council of State and Territorial Epidemiologists. Probable cases met the clinical case definition for pertussis.25 Confirmed cases met the clinical case definition for pertussis and were laboratory confirmed or had contact with a laboratory confirmed case. Approximately 16% of cases had a case status of probable in 2004, whereas 18% had a case status of probable in 2008 and 7% in 2012. Additional details of the Dakota County outbreaks can be found in a previously published article.26
Because pertussis is a reportable disease in Minnesota, laboratories, clinics, and schools are required to report pertussis cases to MDH. All individuals with pertussis are interviewed for clinical and epidemiological information, and in situations in which a family cannot be reached, follow-up through the provider is attempted. Between 2004 and 2012, no major changes occurred in the data collection methods for the variables in our analysis.
We programmed our model population with ages that reflected the age distribution of Dakota County.27 We categorized the population into 1 of 5 age groups (0–6 months, 6 months to 6 years, 7–10 years, 11–18 years, and 19 years and older) on the basis of the recommended ages for pertussis vaccination. Vaccination coverage in the children’s age groups (< 19 years) was on the basis of the percentage of confirmed and probable Dakota County pertussis cases that were known to have received age-appropriate vaccinations. We assumed proportionate coverage among children’s cases with unknown vaccination status. Vaccination and other model parameters are detailed in Table 2.
TABLE 2—
Input Parameter Values for Agent-Based Model of Pertussis Transmission: Minnesota Department of Health, Dakota County, Minnesota, 2004, 2008, 2012
| Parameter | 2004 | 2008 | 2012 |
| Infectious if antibiotics taken, d | 11 | 13 | 13 |
| Infectious if no antibiotics taken, d | 21 | 21 | 21 |
| Detected cases on antibiotics, % | 58 | 62 | 73 |
| Vaccine efficacy, % | 85 | 85 | 85 |
| Case vaccination status known, No. (%) | |||
| 6 mo–6 y | 21 (95) | 36 (80) | 33 (85) |
| 7–10 y | 26 (96) | 92 (75) | 72 (91) |
| 11–18 y | 119 (85) | 115 (72) | 76 (84) |
| ≥ 19 y | 1 (2) | 3 (5) | 5 (8) |
| Cases with age-appropriate vaccination if status known, no. (%) | |||
| 6 mo–6 y | 18 (82) | 29 (81) | 26 (78) |
| 7–10 y | 22 (85) | 81 (88) | 68 (94) |
| 11–18 y | 113 (95) | 104 (90) | 76 (91) |
| ≥ 19 y | 0 (0) | 1 (33) | 3 (60) |
The vaccination schedule was determined by the Advisory Committee on Immunization Practices recommendations and modeled as a child receiving shots at aged 2, 4, 6, and 15 months and 4 years. We did not assume vaccine-acquired immunity until infants received their third shot at aged 6 months. Among children being vaccinated, we assumed full completion of the 5-dose vaccination series with no delays and 85% vaccine efficacy.4 Because our model does not incorporate increasing vaccine efficacy with each shot in the primary series, we restricted analysis to individuals older than aged 6 months, because they have had the opportunity to complete the primary series. We modeled loss of immunity as instantaneous and complete. We determined Tdap coverage from National Immunization Survey estimates for Minnesota adolescents.4
We assumed that individuals interacted randomly each day without restrictions. Susceptible individuals had an age- and location-dependent probability of becoming infected. We derived transmission probabilities from a previously published pertussis model.28 We assumed the latent period of pertussis was negligible, and we did not represent variable degrees of infectiousness.
Once infected, both symptomatic and asymptomatic individuals remained infectious for 21 days unless antibiotics were taken.29 Case data determined the percentage of infectious individuals who received antibiotics. The duration of infectiousness among individuals on antibiotics was the median number of days between cough onset and 5 days past the date of first antibiotic, as calculated from Dakota County case data.30 Individuals did not interact randomly for the 5 days while on antibiotics. This restriction in movement reflects the MDH recommendation to remain home until 5 days of antibiotics are completed. MDH’s recommendation presumably changes normal activities, thereby limiting interactions of infectious individuals. We made the simplifying assumption that loss of infectiousness coincided with the development of complete immunity.
Parameter Values Estimated by the Model
Because of the lack of empirical data, we estimated the percentage of immune adults and the percentages of undetected infections in both adults and children during the Dakota County outbreaks through model calibration.
We achieved this by varying these unknown parameters to find combinations that resulted in model output that matched age-related outbreak patterns in Dakota County. We also estimated the duration of vaccine-acquired immunity through model calibration and compared it with published estimates, which served as validation for the model.
Fitting the Model
We varied parameters that the model derived until the model’s relative incidence by age group (with the adult age group as reference) fit Dakota County case data. We initially explored parameter values within wide plausible ranges identified by the expert opinion of public health epidemiologists and then iteratively narrowed the ranges on the basis of 95% confidence intervals (CIs) of best fitting target parameters. We tested 12 000 parameter combinations for each outbreak. We ran each parameter combination for 100 simulations and averaged the output, because repeated simulations with identical initial conditions produced different results owing to the probabilistic nature of the model.
We defined best model fit as the average of the 50 parameter combinations that produced the lowest percentage error of relative incidence summed across age groups, and we calculated 95% CIs using means and SDs of the 50 best fitting parameter combinations. We weighted the percentage error in each age group by the distribution of cases across the age groups. We assessed model internal validity by conducting 1-way sensitivity analyses.
RESULTS
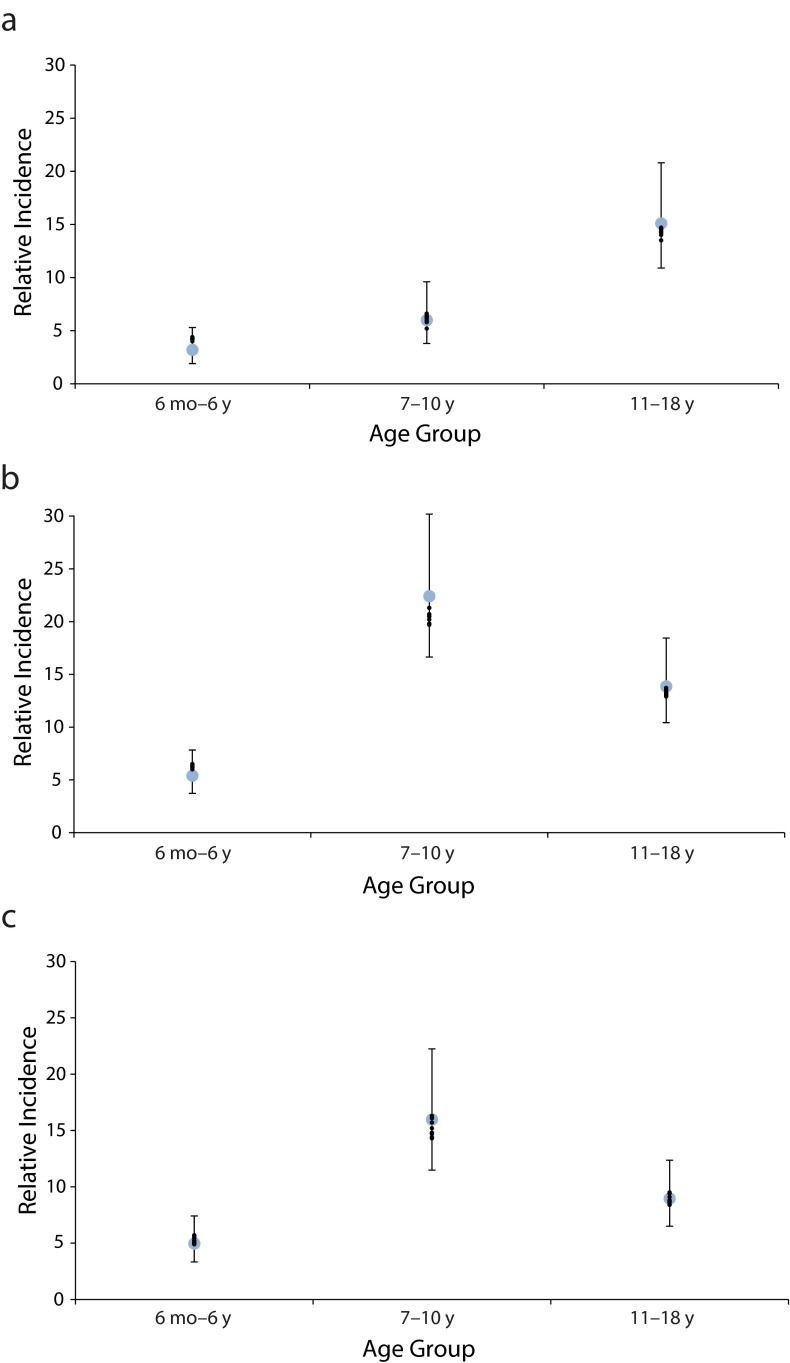
Our model produced trends in age group–relative incidence that were similar to trends observed during the Dakota County outbreaks (Figure 1). Specifically, the increased incidence in the aged 7 to 10 years group relative to the adult age group was captured in the 2008 and 2012 models.
FIGURE 1—
Relative incidence of pertussis in child age groups compared with corresponding estimates generated by top 10 model fits during simulations of outbreaks in (a) 2004, (b) 2008, and (c) 2012: Minnesota Department of Health, Dakota County, Minnesota.
Note. Estimates from top 10 fits were similar to estimates generated by top 50 fits. Top 10 fits are used for ease of presentation. Adult group is the reference.
Varying only a single model parameter (whether percentages of undetected infections in adults and children, duration of vaccine-acquired immunity, or percentage of immune adults) was not sufficient to replicate the age distribution of Dakota County cases in the 3 outbreaks. The 2008 model had a higher percentage of immune adults than did the 2004 and 2012 models. The percentage of undetected infections among children was similar between all 3 models. Waning of Tdap was negligible in the 2008 model, and Tdap provided protection for 2.1 years in the 2012 model. Vaccinated children in the 2008 and 2012 models were susceptible sooner after completion of the 5-dose vaccination series than were vaccinated children in the 2004 model. Parameter values derived from best model fits are shown in Table 3.
TABLE 3—
Pertussis Parameters Estimated by Model: Minnesota Department of Health, Dakota County, Minnesota, 2004, 2008, 2012
| Best Fitting Parameter Values, Parameter Means (95% CI) |
||||
| Model Parameter | Plausible Ranges Explored by Model | 2004 | 2008 | 2012 |
| Immunity provided by first 5 shots, y | 1.0–10.0 | 6.6 (6.6, 6.7) | 3.2 (3.2, 3.2) | 3.3 (3.3, 3.3) |
| Immunity provided by Tdap, y | 1.0–6.0 | NA | NA | 2.1 (2.0, 2.2) |
| Undetected infections in children, % | 10.0–99.0 | 23.0 (22.4, 23.5) | 22.2 (21.9, 22.5) | 22.1 (21.7, 22.5) |
| Undetected infections in adults, % | 50.0–99.0 | 89.4 (89.0, 89.7) | 90.9 (90.6, 91.2) | 90.0 (89.6, 90.3) |
| Immune adults, % | 10.0–90.0 | 49.6 (47.4, 51.7) | 57.7 (55.7, 59.7) | 48.2 (46.0, 50.5) |
Note. CI = confidence interval; NA = not applicable. Data are for top 50 model fits.
Model output was highly sensitive to parameters pertaining to adults. Changing the duration of immunity after completion of the 5-dose vaccination series influenced the relative incidence in predominantly the aged 7 to 10 years group. Testing the assumption that all cases with unknown vaccination status were not vaccinated did not heavily influence the results. Model outputs were stable for any number of averaged simulations greater than 100.
DISCUSSION
Our ABM successfully replicated age-related trends in pertussis incidence that were observed during the 2004, 2008, and 2012 Dakota County outbreaks. The model identified factors pertaining to adult immunity levels and waning vaccine-acquired immunity that may have contributed to differences between these outbreak years. To our knowledge, this is the first ABM of pertussis transmission developed to characterize the influence of these parameters within a population. Previous models have generated similar estimates of the duration of vaccine immunity, and serologic studies have provided population-specific estimates of immunity and subclinical infections.31–33 Using an ABM and surveillance data routinely collected by MDH, we were able to generate estimates for all these parameters simultaneously for specific outbreaks within a single population.
The loss of immunity in children approximately 6.5 years after final vaccination in the 2004 model is consistent with published estimates of the duration of DTP acquired immunity.7 The loss of immunity in children approximately 3.0 years after completion of the DTaP series in the 2008 and 2012 models supports assessments that the acellular vaccine wanes sooner than does the whole cell vaccine.7 The estimate for the duration of vaccine-acquired immunity significantly changed between the 2004 outbreak and subsequent outbreaks. Our model supports the assertion that the change in vaccine formula contributed to shifting the burden of pertussis from adolescents to those aged 7 to 10 years, a finding that is consistent with previous case–control and cohort studies.6,21
Although vaccine changes appear to be a driving force in shifting the age group–relative incidence trends, they do not explain the increased incidence of pertussis in children relative to adults that was observed in the 2008 outbreak compared with the 2012 outbreak. Our model suggests that fluctuations in adult immunity were responsible for these observed changes in relative incidence. Specifically, increased adult immunity in 2008 reduced the pool of potential infections among adults, leading to increased incidence in children relative to adults.
Although our model does not distinguish between vaccine-acquired immunity and natural immunity, the increase in adult immunity in 2008 compared with 2004 and 2012 can be explained in part by the introduction and uptake of Tdap in 2005. A 2008 National Immunization Survey of self-reported Tdap coverage in adults reported that approximately 6% of adults had been vaccinated with Tdap.34 This percentage is similar to the percentage increase in adult immunity observed between the 2004 and 2008 models. Adults who were vaccinated with Tdap soon after its licensure would no longer be immune by 2012 considering the model’s estimate of approximately 2 years of protection from Tdap.
The short duration of immunity provided by Tdap is further supported by the model’s similar estimates of adult immunity in the 2004 and 2012 outbreaks and is consistent with estimates from recent research.35 Despite this short duration of immunity, the Advisory Committee on Immunization Practices does not currently recommend repeat boosters because the cost of vaccination is considered along with duration of immunity when assessing benefit.36 Providers should consider pertussis among adults with a prolonged cough illness, especially among those who were vaccinated with Tdap more than 2 years before illness. This increased awareness will help to reduce the frequency of undetected adult infections.
Although the estimated degree of underreporting varies by population, serologic studies consistently show substantial underreporting of adult infections.37,38 Our model suggests that in our study population, approximately 8 in 10 pertussis infections in children were detected, whereas only 1 in 10 adult infections was detected. This age-related discrepancy in reporting was consistent across the 3 outbreaks, and variable detection of pertussis infections in children did not appear to have been a factor in driving relative incidence trends. The model’s high percentage of undetected adult infections supports the notion that adults serve as a reservoir for pertussis despite the infrequency with which their infections are identified.14 Because of this discrepancy in detection, additional detection efforts targeted at adults are warranted in combination with promoting Tdap vaccine.
Limitations
We determined vaccination coverage in our model by pertussis case data that included detailed information on age. Although projecting the vaccination status of cases to the entire study population is not ideal, it allowed us to calculate vaccination coverage by customized age groups, enabling us to examine the effect of waning immunity. Our current assumption of random mixing may be improved by the addition of geographic components and school-based behaviors that would provide a more comprehensive representation of population mixing, as age-dependent contact rates affect the timing and speed of an outbreak.39,40
Our model can further be developed by representing variable degrees of immunity and infectiousness; however, because our model produced estimates of the duration of vaccine-acquired immunity that are similar to published estimates, we do not believe that the simplifying assumption of instantaneous loss of immunity greatly influenced our results. Delays in the vaccination schedule and failure to complete the full vaccination series may contribute to outbreaks, so these aspects of vaccination should be included in future research. Further expansion of the model to compare years with and without outbreaks should also be explored.
Previous studies have proposed that the resurgence of pertussis can be attributed to a history of partial vaccination coverage with an imperfect vaccine and age-specific contact patterns.39,40 Our model instead focused on assessing the impact of undetected infections, adult immunity, and waning vaccine-acquired immunity in recent outbreaks. Although we found that these factors should also be considered as a potential explanation for the resurgence of pertussis, our model does not address resurgence pertaining to historical vaccination coverage or contact patterns.
A recent model that assessed the impact of contact network structure in pertussis outbreaks produced results that had the greatest deviation from observed population incidence in its aged 6 to 10 years group.40 Our model suggests that the vaccine change largely affected a similar age group (those aged 7–10 years). The combination of these 2 findings further suggests that the resurgence of pertussis is the result of a combination of factors that will prove difficult to capture in any single model.
Conclusions
We developed an ABM of pertussis transmission to identify factors that may have contributed to differences between 2004, 2008, and 2012 Dakota County outbreaks. Our results suggest that current trends in pertussis incidence in children relative to adults can be attributed in part to waning vaccine-acquired immunity, consistently undetected adult infections, and fluctuations in adult immunity. Additionally, our model suggests that no single factor accounts for these observed trends. Public health intervention strategies will need to consider the interaction of these factors to successfully address the resurgence of pertussis. Agent-based modeling is a useful tool for this future research.
Acknowledgments
This work was supported by the Minnesota Department of Health and a University of Minnesota Academic Health Center seed grant.
Human Participant Protection
Institutional review board approval was not required because we used de-identified county-level data.
References
- 1.Centers for Disease Control and Prevention. Pertussis—United States, January 1992–June 1995. MMWR Morb Mortal Wkly Rep. 1995;44(28):525–529. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Pertussis (whooping cough) 2014. Available at: http://www.cdc.gov/pertussis/surv-reporting.html. Accessed January 28, 2014.
- 3.Gordon JE, Hood RI. Whooping cough and its epidemiological anomalies. Am J Med Sci. 1951;222(3):333–361. doi: 10.1097/00000441-195109000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. US vaccination coverage reported via NIS. 2013. Available at: http://www.cdc.gov/vaccines/stats-surv/nis/default.htm#nisteen. Accessed February 18, 2014.
- 5.Department of Health. Minnesota health statistics annual summary. 2010. Available at: http://www.health.state.mn.us/divs/chs/annsum. Accessed January 30, 2013.
- 6.Tartof SY, Lewis M, Kenyon C et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics. 2013;131(4):e1047–e1052. doi: 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 7.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24(5 suppl):S58–S61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- 8.Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics. 2005;115(5):1422–1427. doi: 10.1542/peds.2004-2648. [DOI] [PubMed] [Google Scholar]
- 9.Cherry JD. The present and future control of pertussis. Clin Infect Dis. 2010;51(6):663–667. doi: 10.1086/655826. [DOI] [PubMed] [Google Scholar]
- 10.Bamberger ES, Srugo I. What is new in pertussis? Eur J Pediatr. 2008;167(2):133–139. doi: 10.1007/s00431-007-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiologic trends and their potential causes. Epidemiol Infect. 2014;142(4):672–684. doi: 10.1017/S0950268812003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senzilet LD, Halperin SA, Spika JS et al. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin Infect Dis. 2001;32(12):1691–1697. doi: 10.1086/320754. [DOI] [PubMed] [Google Scholar]
- 13.Deville JG, Cherry JD, Christenson PD et al. Frequency of unrecognized Bordetella pertussis infections in adults. Clin Infect Dis. 1995;21(3):639–642. doi: 10.1093/clinids/21.3.639. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JD. The changing epidemiology of pertussis in infants: the role of adults as reservoirs of infection. Am J Dis Child. 1978;132(4):371–373. doi: 10.1001/archpedi.1978.02120290043006. [DOI] [PubMed] [Google Scholar]
- 15.Yaari E, Yafe-Zimerman Y, Schwartz SB et al. Clinical manifestations of Bordetella pertussis infection in immunized children and young adults. Chest. 1999;115(5):1254–1258. doi: 10.1378/chest.115.5.1254. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Vaccine information for adults. Available at: http://www.cdc.gov/vaccines/adults/vaccination-records.html. Accessed April 11, 2015.
- 17.Olin P, Hans HO, Gustafsson L, Reizenstein E, Storsaeter J. How to make sense of pertussis immunogenicity data. Clin Infect Dis. 2001;33(suppl 4):S288–S291. doi: 10.1086/322564. [DOI] [PubMed] [Google Scholar]
- 18.de Melker HE, Versteegh FG, Schellekens JF, Teunis PF, Kretzschmar M. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect. 2006;53(2):106–113. doi: 10.1016/j.jinf.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children recommendations of the Advisory Committee on Immunizations Practices (ACIP) MMWR Recomm Rep. 1997;46(RR-7):1–25. [PubMed] [Google Scholar]
- 20.Pickering LK, Clark T. DTaP vaccine effective for about 5 years, then booster needed. AAP News. 2011;32(12):7. [Google Scholar]
- 21.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 22.Railsback SF, Lytinen SL, Jackson SK. Agent-based simulation platforms: review and development recommendations. Simulation. 2006;82(9):609–623. [Google Scholar]
- 23.Dakota County. About us. 2012. Available at: https://www.co.dakota.mn.us/Pages/default.aspx. Accessed April 15, 2013.
- 24.Wilensky U. NetLogo. 1999. Available at: http://ccl.northwestern.edu/netlogo. Accessed January 15, 2013.
- 25.Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS) Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=795&DatePub=1/1/2010%2012:00:00%20AM. Accessed June 27, 2014.
- 26.Kenyon C, Banerjee E, Sweet K, Miller C, Ehresmann K. Assessing the impact of a pertussis active surveillance program on provider testing behavior, Minnesota 2005–2009. Am J Public Health. 2014;104(4):e34–e39. doi: 10.2105/AJPH.2013.301815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. United States July 1st resident population by state, county, age, sex, bridged-race, and Hispanic origin. Available at: http://wonder.cdc.gov/bridged-race-v2011.html. Accessed June 20, 2013.
- 28.Hethcote HW. An age-structured model for pertussis transmission. Math Biosci. 1997;145(2):89–136. doi: 10.1016/s0025-5564(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Washington, DC: Public Health Foundation; 2012. [Google Scholar]
- 30.Centers for Disease Control and Prevention. VPD Surveillance Manual. 5th ed. Atlanta, GA: 2011. [Google Scholar]
- 31.Lavine JS, Bjørnstad ON, de Blasio BF, Storsaeter J. Short-lived immunity against pertussis, age-specific routes of transmission, and the utility of a teenage booster vaccine. Vaccine. 2012;30(3):544–551. doi: 10.1016/j.vaccine.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saffer MJ, Khalilian AR, Rafee AR et al. Bordetella pertussis IgG and IgA antibodies seroprevalence among 1–35 y-old populations: the role of subclinical pertussis infection. Indian J Pediatr. 2012;79(3):353–357. doi: 10.1007/s12098-011-0593-8. [DOI] [PubMed] [Google Scholar]
- 33.Zaĭtsev EM, Mazurova IK, Krasnoproshina LI, Astakhova TI, Zakharova NS. [Humoral immunity against pertussis and its prevalence in population] Zh Mikrobiol Epidemiol Immunobiol. 2009;(1):56–58. [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Tetanus and pertussis vaccination coverage among adults aged ≥18 years—United States, 1999 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59(40):1302–1306. [PubMed] [Google Scholar]
- 35.Koepke R, Eickhoff JC, Ayele RA et al. Estimating the effectiveness of Tdap vaccine for preventing pertussis: evidence of rapidly waning immunity and differences in effectiveness by Tdap brand. J Infect Dis. 2014;210(6):942–953. doi: 10.1093/infdis/jiu322. [DOI] [PubMed] [Google Scholar]
- 36.Advisory Committee on Immunization Practices. Summary report: June 19–20, 2013. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-jun13.pdf. Accessed April 19, 2015.
- 37.Stefanoff P, Paradowska-Stankiewicz IA, Lipke M et al. Incidence of pertussis in patients of general practitioners in Poland. Epidemiol Infect. 2014;142(4):714–723. doi: 10.1017/S0950268813001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGirr AA, Tuite A, Fisman D. Estimation of the underlying burden of pertussis in adolescents and adults in Southern Ontario, Canada. PLoS ONE. 2013;8(12):e83850. doi: 10.1371/journal.pone.0083850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riolo MA, King AA, Rohani P. Can vaccine legacy explain the British resurgence of pertussis? Vaccine. 2013;31(49):5903–5908. doi: 10.1016/j.vaccine.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohani P, Zhong X, King A. Contact network structure explains the changing epidemiology of pertussis. Science. 2010;330(6006):982–985. doi: 10.1126/science.1194134. [DOI] [PubMed] [Google Scholar]