Abstract
Objectives. We quantified controlled substance donations via permanent drug donation boxes over 2 years in a region with high prescription abuse, assessing medication characteristics, time between dispensing and donation, and weight of medications donated per capita.
Methods. In partnership with Drug Enforcement Administration and local law enforcement, we analyzed permanent drug donation box collections in 8 Northeast Tennessee locations from June 2012 to April 2014. We recorded controlled substance dosage units along with the product dispensing date.
Results. We collected 4841 pounds of pharmaceutical waste, 4.9% (238.5 pounds) of which were controlled substances, totaling 106 464 controlled substance doses. Analysis of dispensing dates for controlled substances indicated a median of 34 months lapsed from dispensing to donation (range = 1–484 months). The mean controlled substance donation rate was 1.39 pounds per 1000 residents. Communities with fewer than 10 000 residents had a statistically higher controlled substance donation rate (P = .002) compared with communities with 10 000 or more residents.
Conclusions. Permanent drug donation boxes can be an effective mechanism to remove controlled substances from community settings. Rural and urban community residents should be provided convenient and timely access to drug disposal options.
Overdose deaths in the United States involving prescription opioid analgesics (POAs) increased more than fourfold from 4030 in 1999 to 16 651 in 2010.1 Deemed an epidemic by the Centers for Disease Control and Prevention, drug-related overdoses are the leading cause of injury death in the United States.2 Nonmedical use of POAs is highly correlated with increases in overdose deaths.3,4 Nonmedical use of prescription drugs, or prescription drug abuse, is defined by the National Institute on Drug Abuse as “the use of a medication without a prescription, in a way other than as prescribed, or for the experiences or feelings elicited.”5(p1) The Office of National Drug Control Policy considers nonmedical prescription drug use to be America’s fastest-growing drug problem, and the 2013 National Survey of Drug Use and Health data report 6700 initiates to nonmedical prescription drug use daily.6,7
Tennessee experienced a 150% increase (422 deaths in 2001, 1059 deaths in 2010) in overdose deaths from 2001 to 2010, accounting for 7% of the nation’s nearly 15 000 prescription drug overdose deaths nationally despite comprising only 2.3% of the nation’s population.8,9 Northeast Tennessee has particularly struggled with prescription drug abuse and drug-related overdose deaths.10–13 If one compares charges for hospital and emergency department treatment of drug dependence, drug abuse, and opioid-related poisonings across all Tennessee counties, Sullivan and Washington County (Northeast Tennessee’s 2 most populous counties), ranked first and third with 10.8 bills and 7.9 bills per 1000 people, respectively (David Reagan, MD, PhD, Tennessee Department of Health, e-mail communication, July 12, 2013). For every 1000 county residents, between $56 000 and $61 000 is charged for drug treatment or opioid-related emergency department visits per year. In addition, in 2011, Sullivan County was highest in the state, and Washington County third highest at 27.6 and 22.3 diagnosed drug poisonings per 1000 people in each county, respectively.
National Survey of Drug Use and Health data consistently indicate a large percentage of individuals who use POAs nonmedically obtain the medications from friends and family.7 Moreover, research indicates that a majority of patients store unused, unwanted, or expired medications, thereby increasing opportunities for nonmedical use.14–17 Importantly, research indicates that adolescents tend to have unsupervised access to medications in the home,18 an indication that drugs with abuse potential may not be stored in secure locations within homes. McCauley et al. recently reported deficits in patient knowledge of safe POA storage and disposal before a Web-based intervention in a clinic setting.19 Whereas nationally representative knowledge regarding prescription drug storage in US homes is lacking, limited data from the Netherlands support that unsafe medication storage in homes is prevalent.20
From a primary prevention perspective, one method of mitigating the nonmedical use of prescription drugs is by providing means by which individuals can safely dispose of unwanted, unused, or expired medications. In 2009, the Office of National Drug Control Policy published guidelines that sought to educate patients on the proper disposal of medications.21 The following year, the Drug Enforcement Administration (DEA) initiated its national drug Take-Back campaign as a temporary means of collecting medications while legislative and regulatory action was taken to develop permanent disposal methods via enactment of the Secure and Responsible Drug Disposal Act of 2010.22,23 National DEA Take-Back events, which concluded in September 2014, resulted in collection of 2411 tons of pharmaceuticals over 4 years.24 Regional and community-level medication-specific outcomes of Take-Back days have also been reported, including the extent to which controlled substances are collected.16,25
In addition to national Take-Back days, DEA regulations allow registered law enforcement officials (e.g., local law enforcement personnel) to take possession of and destroy controlled substances at any time.26 Over the past 2 to 3 years, permanent drug donation boxes have been installed in multiple law enforcement offices across Tennessee and other states. Whereas national Take-Back days occurred biannually, permanent drug donation boxes serve as a means by which medications can be disposed of in a consistently accessible manner. In a state that significantly exceeds average US consumption of prescription medications (18.4 prescriptions annually per capita in Tennessee vs 12.3 prescriptions annually per capita in United States)27 and in which more than 275 million doses of hydrocodone-containing medication, the most widely dispensed POA medication, were dispensed in 2010,28 permanent drug donation boxes could potentially prevent nonmedical use by decreasing the number of accessible medications. However, to our knowledge, the extent to which medications with abuse potential are being donated in permanent drug donation boxes has not been explored.
Over the past 2 years, we have collaborated with law enforcement officials in 8 localities representing 5 Northeast Tennessee counties to characterize permanent drug donation box donations. Our purpose was to describe (1) donated controlled substance medication characteristics (e.g., active ingredient, therapeutic category, and percentage of total donation weight), (2) time between dispensing and donation across therapeutic categories, and (3) weight of medications donated per capita across study municipalities.
METHODS
The sampling area for this study consisted of the Johnson City–Kingsport–Bristol, Tennessee, combined statistical area. We selected 8 permanent drug donation box locations within this area for research purposes.
Data Collection and Measurement
In partnership with the DEA and local law enforcement, we collected data on permanent drug donation boxes in 8 Northeast Tennessee locations from June 2012 to April 2014. The lead author recruited law enforcement agencies to participate in the study and allow assessment of permanent drug donation box contents. Over the course of 2 years, the research team traveled to 8 collection sites to sort and collect data (37 total visits). Sorting and data collection visits were typically conducted every 60 to 90 days and took a team of 4 approximately 2.5 hours per visit. Law enforcement partners were able to initiate study participation at any time during the 2-year study period. Site 7 had the longest study participation (742 consecutive donation days) and site 3 participated for the fewest number of donation days. We also conducted sorting and collection of data during the week before DEA national Take-Back events.
The research team sorted all donated controlled substances from noncontrolled substances under direct supervision of law enforcement officers. Donation data collected included total donation weight (pounds), controlled substance weight (pounds), active ingredient (controlled substances only), quantity of individual controlled substance unit doses, the date each controlled substance was dispensed and the donation location. Total donation weight included the donated product, labels, and containers when provided. All weight measurements were taken with the same portable medical-grade scale (Detecto DR400, Detecto Scale Company, Webb City, MO).
We visually identified medications outside their original packaging by using drug identification software (Lexicomp Drug ID, Hudson, OH). We defined controlled substance schedule according to Tennessee statutes.29 Schedule I products have no approved medical use, are not available via prescription, and were thus not analyzed. Schedule II medications have an accepted medical use, a high potential for abuse, and severe physical and psychological dependence potential. Examples of Schedule II medications include fentanyl, methadone, morphine, and oxycodone. Schedules III through V have 1 or more accepted medical uses but decrease relatively in their abuse potential (i.e., Schedule V medications have the least relative abuse potential). Briefly, examples for each of these categories include Schedule III: hydrocodone in combination with a nonnarcotic ingredient (Schedule II as of October 6, 2014), buprenorphine; Schedule IV: alprazolam, tramadol; and Schedule V: pregabalin, codeine in limited quantities in combination with a nonnarcotic ingredient.
We recorded doses for each controlled substance as 1 unit per tablet, capsule, milliliter, film, patch, syringe, intravenous drip, nasal spray device, topical application, suppository, or lozenge. We grouped dose units by active ingredient into 4 general pharmacologic categories: opioids, stimulants, sedative/hypnotics, and miscellaneous. We defined time to donation in months as the number of days between the dispensing date printed on the prescription container and the date of the research team’s onsite assessment. Assessment of time to donation was limited to those medications with a discernable dispensing date on the prescription label (1808 of 2399 containers). The research team collected and documented data in a Microsoft Excel versions 2010 and 2013 spreadsheet (Microsoft Corp, Redmond, WA) while onsite.
Analysis
We defined population density for each municipality as the estimated 2012 population per the US Census Bureau because 2012 was the initial year of data collection.30 We summarized total weight per municipality by using a per capita calculation, generating an average monthly rate per 1000 population. We calculated the monthly rate by first generating a daily donation average across the entire sample period for each collection site. This was done based on the total number of site-specific collection days, which ranged from 62 to 742 days. We extrapolated this amount to the monthly per capita rate.
We destroyed all donations (contents, containers, and labels) by incineration after the analysis. We used Microsoft Excel and SAS version 9.2 (SAS Institute, Cary, NC) to analyze data postcollection.
RESULTS
A total of 106 464 prescription controlled substance dosage units were donated across 8 collection sites. Of those, 77 658 were POAs, 22 100 sedative/hypnotics, 1791 stimulants, and 4915 miscellaneous controlled substances. Total pharmaceutical donations weighed 4841 pounds, 238.5 pounds (4.93%) of which were controlled substances.
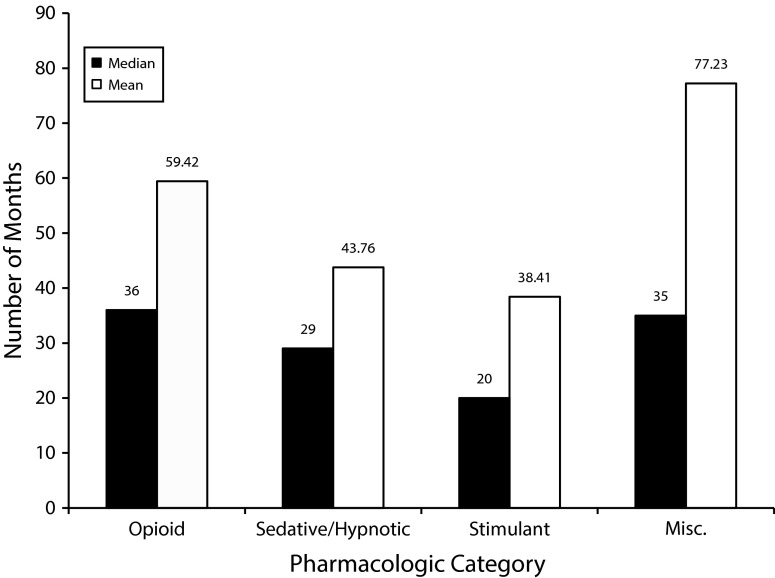
The time from dispensing to donation varied by therapeutic category (Figure 1). Median (mean) time to donation ranged from 36 (59.4) months for POAs to 20 (38.4) months for stimulant medications. We included donations of controlled substances from 6 decades in the mean and median calculations. The most dispensed medication in the United States, hydrocodone, was the most commonly donated controlled substance (20 993 dosage units) followed by tramadol (15 669 dosage units), oxycodone (12 923 dosage units), and alprazolam (6317 dosage units).31 The 10 most common controlled substances donated are listed in Table 1. Of those 10, 5 were POAs and 5 were sedative/hypnotics.
FIGURE 1—
Number of months from dispensing to disposal via permanent drug disposal box by therapeutic category: Northeast Tennessee, 2012–2014.
Note. Misc. = miscellaneous. The sample size was n = 1808.
TABLE 1—
Ten Most Common Controlled Substances Collected From Permanent Drug Donation Boxes, Northeast Tennessee, 2012–2014
| Active Ingredient | Therapeutic Category | Controlled Substance Schedule | Units, No. |
| Hydrocodone | Opioid | 3 | 20 993 |
| Tramadol | Opioid | 4 | 15 669 |
| Oxycodone | Opioid | 2 | 12 923 |
| Alprazolam | Sedative/hypnotic | 4 | 6 317 |
| Propoxyphene | Opioid | 4 | 4 724 |
| Lorazepam | Sedative/hypnotic | 4 | 3 987 |
| Diazepam | Sedative/hypnotic | 4 | 3 842 |
| Morphine | Opioid | 2 | 3 728 |
| Clonazepam | Sedative/hypnotic | 4 | 3 592 |
| Zolpidem | Sedative/hypnotic | 4 | 2 081 |
Note. The sample size was n = 77 856 units.
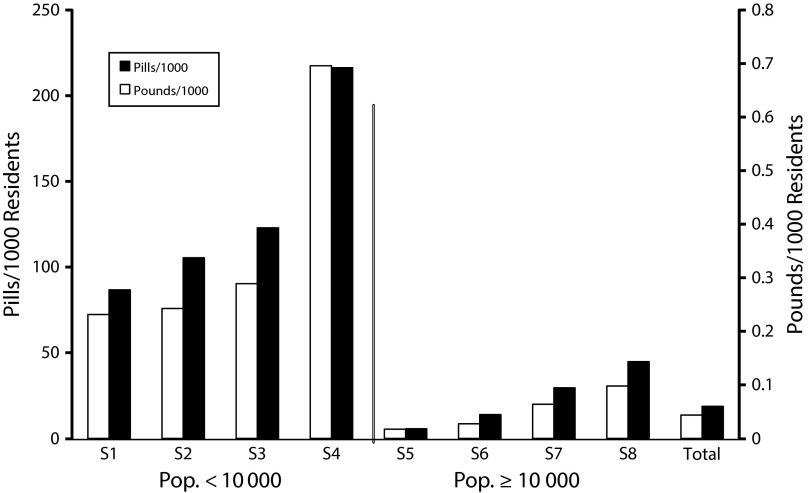
The 8 permanent donation locations encompassed an area in which 172 148 Tennesseans formally reside within city limits. Of the 8 municipal locations, 4 contained resident populations greater than or equal to 10 000 and 4 of them had fewer than 10 000. The municipalities with 10 000 or more population donated in aggregate 153 pounds of controlled substance prescriptions compared with 85.5 pounds for those cities with fewer than 10 000 residents (Table 2). Collection sites in geographic areas with fewer than 10 000 residents donated significantly more controlled substances in pounds (P = .002) and individual doses (P < .001) per capita than collection sites with 10 000 residents or more (Figure 2). In aggregate, residents from the 8 donation sites donated 1.39 pounds or 618.5 units of controlled substances per 1000 residents.
TABLE 2—
Total Pounds and Dosage Units of Prescription and Controlled Substances Collected at Each of 8 Permanent Drug Disposal Box Collection Sites: Northeast Tennessee, 2012–2014
| Collection Site | Population (2012), No. | Local DEA Drug Take-Back Event | Collection Period, d | Total Collected, lbs | Controlled Substances Collected, lbs | Controlled Substances Collected, Units |
| S3 | 2 512 | Yes | 62 | 42 | 2.5 | 2 628 |
| S4 | 3 193 | Yes | 567 | 850 | 51 | 16 940 |
| S2 | 4 397 | Yes | 225 | 95.5 | 9 | 3 826 |
| S1 | 5 138 | Yes | 164 | 301.5 | 23 | 13 096 |
| S8 | 14 204 | Yes | 216 | 401 | 16 | 7 796 |
| S5 | 26 675 | Yes | 229 | 158.5 | 5.5 | 2 469 |
| S7 | 51 501 | No | 742 | 2 048 | 89 | 39 899 |
| S6 | 64 528 | Yes | 553 | 944.5 | 42.5 | 19 810 |
| Total | 172 148 | 4 841 | 238.5 | 106 464 |
Note. DEA = Drug Enforcement Administration.
FIGURE 2—
Average monthly controlled substance donations per 1000 residents by pounds and number of pills: Northeast Tennessee, 2012–2014.
Note. Pop. = population.
DISCUSSION
A convenient medication Take-Back process via law enforcement–based permanent drug donation boxes has been established in many communities nationwide. Our analysis of permanent drug donation boxes in our region of South Central Appalachia indicated that a substantial amount of pharmaceutical products was donated, including controlled substances. Our collection analyses over the course of approximately 24 months at 8 different sites yielded on average 1.39 pounds or 618.5 units of controlled substances per 1000 residents. To our knowledge, this is the first report of outcomes associated with permanent drug donation boxes.
Residents who possess prescription medications in the United States are encouraged to dispose of their medications in an environmentally safe manner.26 Unfortunately, those recommended methods for destruction via incineration (live DEA Take-Back or permanent drug disposal boxes) often require significantly more effort on the part of the owner than much simpler methods of disposal at home, including discarding medications in the trash or by flushing. Moreover, patients may be uninformed regarding appropriate disposal methods. Thus, active communication between patients and their providers and pharmacists will be needed to improve patient awareness of appropriate medication disposal practices in addition to altering the patients’ established disposal habits and motivations.
Evaluating outcomes of primary prevention efforts is particularly important in states such as Tennessee, where there is a high rate of controlled substance prescribing and dispensing, prescription drug abuse–associated mortality, and neonatal abstinence syndrome cases per capita.1,32,33 Prescribing rates for POAs in Tennessee (143 opioid prescriptions per 100 residents per year) are among the highest of all 50 states and at a rate of nearly 3 times the lowest prescribing state (Hawaii, 52 opioid prescriptions per 100 residents per year).32 Thus, residents in our Tennessee-based collection area are likely to have unused, unwanted, or expired POAs available for disposal.
Results from our analysis suggest that many patients store their controlled substance prescriptions at home for 3 or more years. Storage of medications beyond the expiration date or beyond the period of indicated use may contribute to unintended diversion or accidental poisonings. Furthermore, inconsistent storage practices have been documented in patient subgroups associated with high-risk controlled substance use. Practices and differences across controlled substance pharmacotherapy categories suggest a wide and difficult-to-predict range in the number of months from dispensing to donation.34 Multiple community and individual factors are likely to influence drug disposal behaviors, including but not limited to regional prescribing patterns, relative morbidity, community pharmacy access, convenience of drug donation locations, placement of twice-annual DEA-sponsored Take-Back events, perceived anonymity at the donation site, community-level messaging (e.g., coalition activity, public service announcements), and a patient’s perceived value of the medications. Whereas increased exploration of these factors is warranted, increased emphasis on appropriate disposal messaging when medications are prescribed and dispensed could serve as an effective primary prevention mechanism against nonmedical use.
Uniquely, rural communities with populations of fewer than 10 000 residents donated medications at a higher rate per capita than communities of 10 000 residents or more. Factors associated with this dichotomy may include prescribing patterns, relative access to drug donation boxes, substance abuse coalition activity, concern of environmental disturbance by alternate disposal methods, and the value a patient places on the medication itself. Perceived value can be influenced by differences in nonmedical demand or street value for the controlled substance.
Medical practice screening patterns or dispensing restrictions may also give the impression that the medications may be difficult to replace. Donation metrics linked to live DEA Take-Back events versus permanent drug donation boxes are similar in that hydrocodone is the most donated controlled substance, nonprescription and noncontrolled prescription substances represent more than 90% of all donations, and controlled substances are consistently donated for destruction via incineration.16,25,35,36
Limitations
We based analysis and conclusions on the collections from 8 sites over a defined period in a single combined statistical area. Those 8 sites represented all but 1 recently installed (2013) permanent drug donation collection site in a 5-county geographical region. Because of geographical limitations, results of our analysis cannot be generalized to other areas in Tennessee or the United States.
The reliance on sorting of prescription medication by hand may have slightly underrepresented the actual number of controlled substance dosage units donated. However, the research team was very familiar with the identity of controlled substance medications. Time from dispensing to donation in months may be slightly inflated (1–3 months) as we were not able to determine the exact date of disposal for each medication. Population comparisons used formal population statistics for each municipality, missing potential donors from nearby county residences. Finally, data are presented in aggregate form and should thus be interpreted as such and not to be applied to individuals.
Conclusions
Permanent drug donation boxes can be an effective mechanism to collect controlled substances from communities, even if the same community hosts biannual DEA Take-Back activities. The prevalence of controlled substances donated in small population communities (fewer than 10 000 residents) was higher compared with more populous communities. Future studies should compare the efficiency of disposal mechanisms and investigate the factors that influence the donation of controlled substances across diverse regions and municipalities.
Acknowledgments
Research reported in this article was supported by the National Institute on Drug Abuse of the National Institutes of Health under award R24DA036409.
The authors would like to thank Rob Pack, MPH, PhD, our many regional law enforcement partners, and the student pharmacists who assisted with medication sorting and analysis during the study period.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Human Participant Protection
The East Tennessee State University institutional review board approved the study. Human participant information was not collected.
References
- 1.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Injury Prevention and Control. Prescription drug overdose in the United States: fact sheet. 2014. Available at: http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html. Accessed September 12, 2014.
- 3.Jones CM. Frequency of prescription pain reliever nonmedical use: 2002–2003 and 2009–2010. Arch Intern Med. 2012;172(16):1265–1267. doi: 10.1001/archinternmed.2012.2533. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 5.National Institute on Drug Abuse. Prescription drug abuse. 2014. NIH Research Report Series 15–4881. Available at: http://d14rmgtrwzf5a.cloudfront.net/sites/default/files/prescriptiondrugrrs_11_14.pdf. Accessed April 13, 2014.
- 6.Epidemic: Responding to America’s Prescription Drug Abuse Crisis. Washington, DC: Office of National Drug Control Policy; 2011. [Google Scholar]
- 7.Center for Behavioral Health Statistics and Quality. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Results from the 2013 National Survey on Drug Use and Health: summary of national findings. HHS publication no. 14–4863, NSDUH Series H–48. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morbid Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 9.Nashville, TN: Tennessee Department of Health Safety Subcabinet Working Group; 2012. Prescription drug abuse in Tennessee. [Google Scholar]
- 10.Wu LT, Pilowsky DJ, Patkar AA. Non-prescribed use of pain relievers among adolescents in the United States. Drug Alcohol Depend. 2008;94(1-3):1–11. doi: 10.1016/j.drugalcdep.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havens JR, Young AM, Havens CE. Nonmedical prescription drug use in a nationally representative sample of adolescents: evidence for greater use among rural adolescents. Arch Pediatr Adolesc Med. 2011;165(3):250–255. doi: 10.1001/archpediatrics.2010.217. [DOI] [PubMed] [Google Scholar]
- 12.Young AM, Havens JR, Leukefled CG. A comparison of rural and urban nonmedical prescription opioid users’ lifetime and recent drug use. Am J Drug Alcohol Abuse. 2012;38(3):220–227. doi: 10.3109/00952990.2011.643971. [DOI] [PubMed] [Google Scholar]
- 13.Wunsch MJ, Nakamoto K, Behonick G, Massello W. Opioid deaths in rural Virginia: a description of the high prevalence of accidental fatalities involving prescribed medications. Am J Addict. 2009;18(1):5–14. doi: 10.1080/10550490802544938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seehusen DA, Edwards J. Patient practices and beliefs concerning disposal of medications. J Am Board Fam Med. 2006;19(6):542–547. doi: 10.3122/jabfm.19.6.542. [DOI] [PubMed] [Google Scholar]
- 15.Kuspis DA, Krenzelok EP. What happens to expired medications? A survey of community medicine disposal. Vet Hum Toxicol. 1996;38(1):48–49. [PubMed] [Google Scholar]
- 16.Gray JA, Hagemeier NE. Prescription drug abuse and DEA-sanctioned drug take-back events: characteristics and outcomes in rural Appalachia. Arch Intern Med. 2012;172(15):1186–1187. doi: 10.1001/archinternmed.2012.2374. [DOI] [PubMed] [Google Scholar]
- 17.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185(2):551–555. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 18.Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medications in the home. J Adolesc Health. 2013;53(2):260–264. doi: 10.1016/j.jadohealth.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addict Behav. 2013;38(6):2230–2235. doi: 10.1016/j.addbeh.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beirens TM, van Beecka EF, Dekker R, Brug J, Raat H. Unsafe storage of poisons in homes with toddlers. Accid Anal Prev. 2006;38(4):772–776. doi: 10.1016/j.aap.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Washington, DC: Office of National Drug Control Policy; 2009. Proper disposal of prescription drugs. [Google Scholar]
- 22.US Department of Justice, Drug Enforcement Administration, Office of Diversion Control. National Take-Back initiative. 2011. Available at: http://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. Accessed November 3, 2011.
- 23.US Congress. Secure and Responsible Drug Disposal Act. 21 USC §822 (2010)
- 24.US Drug Enforcement Administration. DEA and partners collect 309 tons of pills on ninth prescription drug take-back day. 2014. Available at: http://www.dea.gov/divisions/hq/2014/hq110514.shtml. Accessed November 21, 2014.
- 25.Perry LA, Shinn BW, Stanovich J. Quantification of ongoing community-based medication take-back program. J Am Pharm Assoc (2003) 2014;54(3):275–279. doi: 10.1331/JAPhA.2014.13143. [DOI] [PubMed] [Google Scholar]
- 26. US Department of Justice. Disposal of controlled substances, 79 Federal Register (2014). [PubMed]
- 27.Kaiser Family Foundation. State health facts—retail prescription drugs filled at pharmacies (annual per capita) 2013. Available at: http://kff.org/other/state-indicator/retail-rx-drugs-per-capita/#table. Accessed April 13, 2015.
- 28.Tennessee Department of Health. Nashville, TN: Controlled Substance Database Advisory Committee, Tennessee Board of Pharmacy; 2011. Report to the General Assembly: Controlled Substance Monitoring Database Report. [Google Scholar]
- 29. Tennessee Drug Control Act, TCA 39-17-part 4 (2014)
- 30. US Census Bureau. Annual estimates of the population of Combined Statistical Areas: April 1, 2010 to July 1, 2012. 2012. Available at: http://www.census.gov/popest/data/metro/totals/2012. Accessed November 4, 2013.
- 31.Medicine Use and Shifting Costs of Healthcare: A Review of the Use of Medicines in the United States in 2013. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2014. [Google Scholar]
- 32.Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines—United States, 2012. MMWR Morbid Mortal Wkly Rep. 2014;63(26):563–568. [PMC free article] [PubMed] [Google Scholar]
- 33.State of Tennessee. Neonatal abstinence syndrome. 2014. Available at: http://health.state.tn.us/MCH/NAS/index.shtml. Accessed November 24, 2014.
- 34.Reddy A, de la Cruz M, Rodriguez EM et al. Patterns of storage, use, and disposal of opioids among cancer outpatients. Oncologist. 2014;19(7):780–785. doi: 10.1634/theoncologist.2014-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma CS, Batz F, Juarez DT, Ladao LC. Drug take back in Hawai’i: partnership between the University of Hawai’i Hilo College of Pharmacy and the Narcotics Enforcement Division. Hawaii J Med Public Health. 2014;73(1):26–31. [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart H, Malinowski A, Ochs L, Jaramillo J, McCall K, III, Sullivan M. Inside Maine’s medicine cabinet: findings from the Drug Enforcement Administration’s Medication Take-Back Events. Am J Public Health. 2015;105(1):e65–e71. doi: 10.2105/AJPH.2014.302207. [DOI] [PMC free article] [PubMed] [Google Scholar]