Abstract
Background. Human bocavirus 1 (HBoV-1) is frequently detected in young children. The role of HBoV-1 in respiratory illness is unclear, owing to frequent detection in asymptomatic children.
Methods. Weekly oral fluid samples from a longitudinal cohort of infants were tested by quantitative polymerase chain reaction for HBoV-1 DNA. Symptoms during HBoV-1 primary shedding events were compared to those during 14-day control periods occurring 1 month prior to and following the primary event. Eight single-nucleotide polymorphisms were analyzed to assess HBoV-1 variants.
Results. Sixty-six of 87 children (76%), followed for at least 18 months from birth, had a primary HBoV-1 infection. HBoV-1 was consistently detected for >1 month (maximum duration, 402 days) following 42 of 66 primary shedding events. Children were more likely to experience new cough symptoms (odds ratio [OR], 2.7; 95% confidence interval [CI], 1.4–5.5) and to visit a healthcare provider (OR, 2.8; 95% CI, 1.02–7.7) during the 14 days surrounding the time of initial detection of HBoV-1. Recurrent HBoV-1 shedding events were found in 33 children (50%). Twelve of 48 children with HBoV-1 variant data had multiple viral allelic patterns over time.
Conclusions. HBoV-1 primary shedding events are associated with mild respiratory illness with subsequent prolonged detection of HBoV-1 DNA for up to a year. HBoV-1 reinfection contributes to long-term shedding.
Keywords: bocavirus, infant, single nucleotide polymorphism, respiratory tract, oral fluid
Human bocavirus 1 (HBoV-1), a DNA virus in the Parvoviridae family that was first identified in 2005 [1], has been frequently detected in young children experiencing acute respiratory tract illness. HBoV-1 DNA is detected in up to 18% of nasal or nasopharyngeal samples from children with respiratory illness [2] and in a third of samples from children with mild illnesses not requiring hospital admission [3]. Over 85% of children in the United States have antibodies to this virus by 4 years of age [4]. HBoV-1 is infrequently detected in adults [2]. Studies of disease associations have yielded conflicting results. HBoV-1 has been associated with wheezing [5, 6], acute otitis media [7, 8], and severe pneumonia and respiratory failure [9–11]. Studies detecting HBoV-1 DNA in serum [12] or spliced HBoV-1 messenger RNA [13] have shown an association with lower respiratory tract illness in children. However, HBoV-1 is often identified concurrently with other respiratory viruses associated with acute illness [2, 5, 12, 14, 15], and HBoV-1 DNA has frequently been detected in asymptomatic children (up to 44%) [3, 14, 16–19], presenting challenges for cross-sectional analyses.
Long-term viral shedding following symptomatic infection may provide an explanation for HBoV-1 detection in asymptomatic children and children with other acute viral illnesses [3, 19–21]. Limited data are available from studies using longitudinal systematic testing in symptomatic and asymptomatic children. Our objective was to describe HBoV-1 shedding patterns, HBoV-1 variants, and symptoms associated with HBoV-1 detection in a longitudinal cohort of young children with weekly oral fluid samples collected from birth to 2 years of age.
MATERIALS AND METHODS
Study Population
Banked weekly oral fluid samples collected on filter paper, along with demographic and clinical data, were obtained from a previously reported prospective study of human herpesvirus 6 natural history in children [22]. Inclusion criteria for enrollment included pregnancy of the mother, receipt of care at a Seattle area–based obstetrical practice, and provision of informed consent prior to participation. Children were then followed from birth for up to 2 years of age between April 1997 and August 2003. No further exclusion criteria were applied within the birth cohort. This present study was determined to be exempt by the institutional review boards at Seattle Children's Research Institute and Wayne State University. Study methods and findings are reported according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [23].
Study Definitions
An HBoV-1 shedding event was defined as at least 2 sequential HBoV-1–positive samples collected at least 6 days apart with no more than 1 consecutive interim HBoV-1–negative specimen. A primary shedding event was defined as the first such detection of HBoV-1 in each child. Recurrent shedding events were defined as shedding events occurring after the resolution of the primary event, marked by 2 consecutive HBoV-1–negative specimens. Within individual subjects, distinct HBoV-1 variants were defined as those containing any nucleotide polymorphism.
Clinical Data Collection
Demographic data were collected from the mother at enrollment. Symptom data were collected using a daily diary. Symptoms included fever (temperature, >38.0°C), cough, rhinorrhea, vomiting, diarrhea, generalized rash, fussiness above the baseline level for the child, seizure, and physician visit for illness. An additional composite end point, new respiratory illness, was defined as new-onset cough and/or new-onset rhinorrhea. Additional surveys were conducted to collect data on breast-feeding, group child-care attendance, and playgroup attendance.
Specimen Collection and Processing
Oral fluid samples were collected weekly, using a method developed for the detection of human herpesviruses 6 and 7 in field studies [24] and previously described by our group for detection of HBoV-1 [18]. Oral fluid was collected by parents, using multiple sterile Schirmer-test filter paper strips as previously published [22], which were mailed to the study site and stored at −80°C. DNA was extracted from the filter strips using QIAmp blood DNA miniprep columns (Qiagen, Valencia, California) with a modified protocol [18] and eluted in 100 µL of water. The presence of HBoV-1 DNA was detected by real-time quantitative polymerase chain reaction (PCR) targeting the NP1 gene [25]. Estimates of virus quantity by milliliter of oral fluid were based on the collection of 5 strips that each hold 12 µL of oral fluid, for a total of 60 µL [18, 24]. Initially, samples were selected for HBoV-1 testing at 1-month intervals throughout each child's follow-up to screen for HBoV-1. Once a positive specimen was identified, we tested weekly samples collected before and after that date until the entire shedding event was captured. For the first 10 subjects, all available samples were tested, and HBoV-1 rates were compared to those of the sampled subjects by generalized estimating equation regression with a negative binomial link to assess the sampling strategy. Because no difference was found, we used the aforementioned sampling strategy throughout.
Single-Nucleotide Polymorphism (SNP) Assay
Sixty whole-genomic sequences for HBoV-1 were obtained from GenBank. After alignment of the sequences, 8 single-nucleotide polymorphism (SNP) sites with nucleotide minor allele frequencies of >10% were identified in 2 genes (Table 1). Samples with sufficient remaining volumes of extracted DNA were tested in separate allelic discrimination assays for each of the 8 SNPs. TaqMan Universal PCR Master Mix was used following standard manufacturer instructions for TaqMan SNP Genotyping Assays (Life Technologies, Carlsbad, California), using the following temperature parameters: 95°C followed by 95°C for 15 seconds and 60°C for 1 minute for 40 cycles. The majority of genotyping results (>90%) were obtained from samples containing at least 4 log copies/mL of HBoV-1. Allele determinations were made using plotted data for visual determination, following manufacturer instructions. Samples with fluorescence for >1 reporter dye, indicating mixed alleles, were excluded from the analysis (3 samples).
Table 1.
Allelic Discrimination Assay Primers and Probes, by Single-Nucleotide Polymorphism (SNP) Site
| SNP Site (HBoV-1 Genome Positiona) | HBoV-1 Gene | Minor Allele Frequency, % | Samples Tested, No. | Forward Primer Sequence | Reverse Primer Sequence | Probe 1/Probe2b |
|---|---|---|---|---|---|---|
| 2856 | NP1 | 13 | 202 | GCATTGCTAGAGATGGTACTAATTCAATCT | GCCAGAACATATTTCTGTATTTTTGATCTAGTG | ATAACACTAGAGAACTATTG/ACACTAGAGAGCTATTG |
| 3937 | VP1 | 12 | 218 | GCTGGCGTTCACATCTTTTGT | TGACGTCCTCATCCCATGGA | TGCATTTGGGTAAGCAT/TGCATTTGGATAAGCAT |
| 4195 | VP1 | 27 | 198 | AGAACTGGTGAAAGCACTGAATTTACT | AACTCTTCTTGTTGGGACTTTTGGA | CTCTTTCATTGTTAACCC/CTCTTTCATTATTAACCC |
| 4243 | VP1 | 43 | 211 | GAAAGAGCATACATTCCTCCTGGA | CTGTGCTTCCGTTTTGTCTTATGT | CTTGTTGGGACTTTTG/CTTGTTGGAACTTTTG |
| 4756 | VP1 | 38 | 201 | GGGACAGATTTCCTATCACCAGAGA | AGTGCCTGGAGGATGATCCAT | TGATGGATCAATTGCA/TTTGATGGATCCATTGCA |
| 4813 | VP1 | 37 | 187 | GGATCATCCTCCAGGCACTATTTT | ACAGCTGACTTGTCCAGTACAGTAT | TTCCAGTACCAACTGC/CCAGTTCCAACTGC |
| 4876 | VP1 | 15 | 208 | GGATCATCCTCCAGGCACTATTTT | CCAGTTCTTTGTTGCGTATCTTTCT | CAGCTGTGAAATTGTA/CAGCTGTGAGATTGTA |
| 4888 | VP1 | 38 | 220 | GTACTGGACAAGTCAGCTGTGA | CTGGACGCCAGTTCTTTGTTG | CGTATCTTTCTACTTCCC/CGTATCTTTCTACCTCCC |
Abbreviations: HBoV-1, human bocavirus 1; SNP, single-nucleotide polymorphism.
a Nucleotide position references GenBank accession number DQ000496.
b SNP alleles are underlined.
Statistical Methods
Analyses were performed using Stata, version 11 (College Station, Texas). The incidence of primary events was calculated with the 95% confidence interval (CI). The risk of a primary event associated with individual characteristics was assessed using univariate Cox proportional hazard regression models. Child-care center attendance and breastfeeding were analyzed as time-varying variables. A step-wise multivariate Cox proportional hazard model was then constructed using an entry P value of .10 and a removal P value of .20. No variables met the criteria for model inclusion. HBoV-1 seasonality was evaluated by comparing time to primary acquisition by season of birth. An individual-level case crossover analysis was performed to assess symptoms associated with HBoV-1 primary events. The 14-day period surrounding the beginning of the primary event in each child (7 days prior to first detection through 7 days thereafter) was compared to 14-day control periods occurring 1 month prior to and following the primary event, using conditional logistic regression. The 7 days prior to the first detection was included in the targeted period to capture symptoms occurring between collection of the last negative specimen and collection of the first positive specimen. Sensitivity analyses were performed to evaluate the impact of viral quantity, respiratory season (defined as October–March), and subsequent recurrence on study estimates. Similar 14-day control periods were also defined to determine symptoms present with the detection of additional HBoV-1 variants. HBoV-1 viral quantity was described as log10 copies per mL of oral fluid. Viral quantity was compared between age strata, using analysis of variance. Viral quantity and duration of viral shedding were compared between primary and recurrent events and after the onset of a new variant, using the Mann–Whitney U test. P values of <.05 were considered statistically significant.
RESULTS
Weekly oral fluid samples were obtained from 87 infants followed from birth to at least 18 months of age. Median follow-up was 705 days (range, 551–778 days). Characteristics of the cohort are shown in Table 2. Almost all children (98%) were breastfed, and the majority of children attended some type of playgroup and/or child-care center. The median age at playgroup start was 146 days, and the median age at child-care center start was 169 days.
Table 2.
Characteristics of Study Participants
| Characteristic | Total (n = 87) | HBoV-1 Primary Event During Study |
Univariate HR (95% CI) for HBoV-1 Primary Event | |
|---|---|---|---|---|
| No (n = 21) | Yes (n = 66) | |||
| Male sex | 54/87 (62) | 13/21 (62) | 41/66 (62) | 0.9 (.5, 1.5) |
| Race/ethnicity | ||||
| White | 63/82 (77) | 16/20 (80) | 47/62 (76) | Reference |
| Black | 2/82 (2) | 0/20 (0) | 2/62 (3) | 1.2 (.3–4.9) |
| Asian | 9/82 (11) | 2/20 (10) | 7/62 (11) | 1.1 (.5–2.3) |
| Hispanic | 6/82 (7) | 1/20 (5) | 5/62 (8) | 2.1 (.8–5.4) |
| Multiple | 2 (2) | 1/20 (5) | 1/62 (2) | 0.4 (.1–3.1) |
| Breastfed | 85/87 (98) | 20/21 (95) | 65/66 (98) | 3.0a (.4–21.8) |
| Age at weaning, mo, mean ± SD | 11 ± 6 | 12 ± 5 | 10 ± 6 | 1.0 (.998–1.001) |
| Child-care center attendance | 33/87 (38) | 8/21 (38) | 25/66 (38) | 1.3a (.8–2.4) |
| Playgroup attendance | 56/86 (65) | 11/21 (52) | 45/65 (65) | 1.4 (.8–2.4) |
| Older siblings in household | 40/87 (46) | 11/21 (52) | 29/66 (44) | 0.9 (.5–1.4) |
| Maternal age, y, mean ± SD | 34 ± 4 | 34 ± 5 | 34 ± 4 | 1.0 (.9–1.1) |
Data are no. of subjects with the characteristic/no. evaluated (%), unless otherwise indicated.
Abbreviations: CI, confidence interval; HBoV-1, human bocavirus 1; HR, hazard ratio; SD, standard deviation.
a Assessed as a time-varying covariate (ie, the HR takes into account actual periods of child-care attendance and breastfeeding).
Risk Factors for HBoV-1 DNA Primary Events
A total of 4425 of 6934 oral fluid samples collected were tested for HBoV-1 DNA. Nearly all children (80 [92%]) had HBoV-1 DNA detected from >1 sample during the study. Sixty-six children (76%) had a primary event identified. The remaining 14 children had only single HBoV-1 detections that did not meet our criteria for a primary event. Thirty-three children (38%) had a recurrent shedding event. The incidence of HBoV-1 primary events was 8.8 events per 100 child-months (95% CI, 6.9–11.2). Median age at HBoV-1 acquisition was 11 months (interquartile range [IQR], 3–20 months); the youngest child with any HBoV-1 detection was 2 days old. No association was found between age at acquisition and patient demographic or family characteristics (Table 2), including birth order or number of older siblings. No differences in age at acquisition were found in children born in the fall, winter, or spring as compared to the summer (hazard ratios [HRs], 1.0 [95% CI, .54–1.86]; 0.7 [95% CI, .31–1.4], and 1.24 [95% CI, .64–2.39], respectively), indicating that seasonal patterns did not influence primary infection.
HBoV-1 Detection and Viral Quantity
Among all 842 HBoV-1–positive oral fluid samples, viral quantities ranged from 2.3 to 10.7 log10 copies/mL (median, 4.1 log10 copies/mL; mean, 4.4 log10 copies/mL). Peak copies within shedding events ranged from 3.1 to 10.7 log10 copies per mL (median, 5.2 log10 copies/mL). Peak viral concentrations were higher in primary events (median, 6.1 log10 copies/mL; IQR, 4.8–7.8 log10 copies/mL), compared with recurrent events (median, 4.4 log10 copies/mL; IQR, 3.8–5.1 log10 copies/mL; P < .001). Peak viral concentration during primary events was associated with older age; children who experienced a primary event later in infancy had higher peak viral quantities (median, 4.2, 5.8, 6.8, and 7.4 log10 copies/mL in children aged 0–5, 6–11, 12–18, ≥18 months, respectively; P = .003). The median viral quantity among 111 single HBoV-1 DNA detections that did not constitute a primary event was 4.0 log10 copies/mL (IQR, 3.5–4.1 log10 copies/mL).
Association Between HBoV-1 Detection and Respiratory Illness
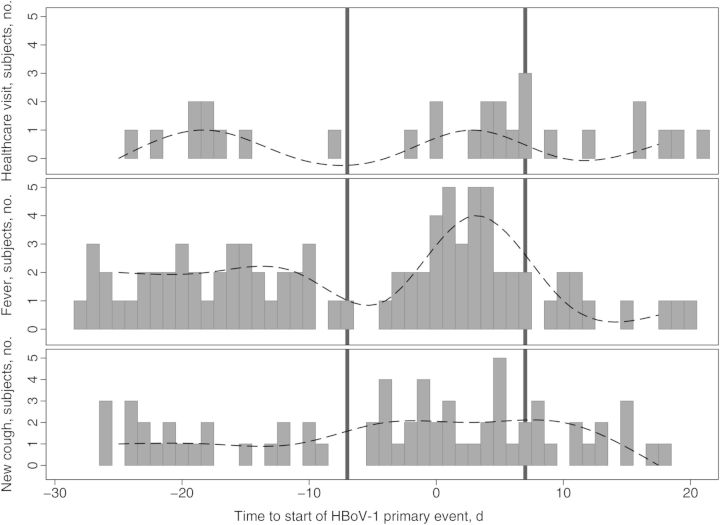
Fifty-two percent (425) of 824 HBoV-1 detections with diaries completed for that day or adjacent days were in asymptomatic children. During HBoV-1 primary events at any quantity of HBoV-1 DNA detection, children were significantly more likely to experience new cough symptoms (odds ratio [OR], 2.7; 95% CI, 1.4–5.5) and to visit a healthcare provider (OR, 2.8; 95% CI, 1.02–7.7; Figure 1 and Table 3). Specific reasons for healthcare visits reported by parents of children with an HBoV-1 primary event included upper respiratory tract illness (4 cases), conjunctivitis (2 cases), otitis media (2 cases), initiation of antibiotics (2 cases), erythema infectiosum (1 case), and unspecified viral illness (1 case). When restricting the analysis to primary events with higher peak viral loads (≥106 copies/mL; 36 events) and paired control periods, HBoV-1 acquisition was significantly associated with new onset of respiratory symptoms (OR, 5.6; 95% CI, 2.1–15.1), which was defined as new cough (OR, 4.1; 95% CI, 1.6–10.7) and/or new rhinorrhea (OR, 2.9, 95% CI, 1.3–6.7), as well as healthcare visits (OR, 7.5; 95% CI, 1.6–34.8). No significant differences were found in symptom prevalence between recurrent events and primary events or between recurrent events and paired control periods. A sensitivity analysis was performed, restricting the data to include only those primary events occurring during the respiratory virus season (October–March). These estimates were similar to those for primary events occurring outside of the respiratory virus season.
Figure 1.
Symptom timing around the human bocavirus 1 (HBoV-1) primary event. Height of gray bars indicates number of children with a healthcare visit, fever, or new-onset cough (y-axis) on a given day (x-axis) relative to the beginning of the HBoV-1 primary event. The continuous line was generated using a median cubic spline (Stata 11) and represents a smoothed curve of the daily symptom prevalence. The area between the black reference lines represents the predefined primary event period.
Table 3.
Prevalence of Healthcare Visits and Symptoms During Human Bocavirus 1 (HBoV-1) Primary and Recurrent Events, Compared With Matched Control Periods
| Variable | HBoV-1 Primary Event |
HBoV-1 Primary Events, Peak Viral Load ≥6 Logs Only |
HBoV-1 Recurrent Event |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HBoV-1 Event, No. (%) (n= 66)a | Control Period, No. (%) (n = 132)a | OR (95% CI)b | HBoV-1 High Copy Event, No. (%) (n = 36) | Control Period, No. (%) (n = 72)a | OR (95% CI)b | First HBoV-1 Recurrence, n (%) (n = 33) | Control Period,a No. (%) (n = 66) | OR (95% CI)b | |
| Healthcare visit | 12 (18) | 11 (8) | 2.8 (1.02–7.7) | 10 (28) | 5 (7) | 7.5 (1.6–34.8) | 5 (15) | 9 (14) | 1 (.3–3.5) |
| Fever | 17 (26) | 20 (15) | 2.1 (.9–4.6) | 14 (39) | 14 (20) | 2.5 (1.0–6.1) | 9 (27) | 9 (14) | 3.0 (.9–10.4) |
| New rhinorrhea | 31 (47) | 44 (33) | 1.7 (.9–3.2) | 23 (64) | 24 (34) | 2.9 (1.3–6.7) | 13 (39) | 22 (34) | 0.9 (.4–2.4) |
| New cough | 29 (44) | 32 (24) | 2.7 (1.4–5.5) | 20 (56) | 17 (24) | 4.1 (1.6–10.7) | 15 (45) | 26 (41) | 1.6 (.6–4.2) |
| Vomiting | 3 (5) | 12 (9) | 0.5 (.1–1.7) | 2 (6) | 9 (13) | 0.4 (.08–1.9) | 1 (3) | 5 (8) | 0.3 (.04–3.4) |
| Diarrhea | 4 (6) | 9 (7) | 0.8 (.2–3.2) | 3 (8) | 7 (10) | 0.7 (.1–3.7) | 3 (9) | 6 (9) | 1.0 (.2–6.4) |
| General rash | 3 (5) | 5 (4) | 1.2 (.3–5.0) | 1 (3) | 3 (4) | 0.7 (.07–6.4) | 2 (6) | 4 (6) | 1.0 (.2–6.4) |
| Local rash | 1 (2) | 2 (2) | 1.0 (.09–11.0) | 1 (3) | 1 (1) | 2.0 (.1–32.0) | 1 (3) | 2 (3) | 1.0 (.1–11.0) |
| Fussiness | 25 (38) | 44 (33) | 1.2 (.6–2.5) | 17 (47) | 22 (31) | 2.0 (.9–4.8) | 12 (36) | 23 (36) | 1.0 (.4–2.7) |
| New respiratory symptomc | 41 (62) | 54 (41) | 2.3 (1.2–4.2) | 30 (83) | 29 (41) | 5.6 (2.1–15.1) | 20 (61) | 38 (59) | 1.0 (.4–2.5) |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Symptom data missing for 2 of 132 control periods, 2 of 72 high-copy control periods, 2 of 66 recurrence control periods, and 1 of 66 primary case events. The valid percentage is reported.
b The OR was calculated using conditional logistic regression, to account for correlation in case and control periods matched by individual child.
c Defined as new-onset cough and/or new-onset rhinorrhea.
HBoV-1 Shedding
The median shedding duration was 50 days, and HBoV-1 was consistently detected for >30 days following 42 of 66 primary events (64%). The longest duration of consistent shedding following a primary event was 402 days. Thirty-three children had recurrent HBoV-1 events, and 12 children had multiple recurrent events, including 3 children who had 4 recurrences each. The median duration between the end of the primary event and the beginning of the recurrent event was 48 days (range, 21–420 days). The median duration of shedding for recurrent events was significantly shorter than for primary events (26 vs 50 days; P < .01).
HBoV-1 Variants
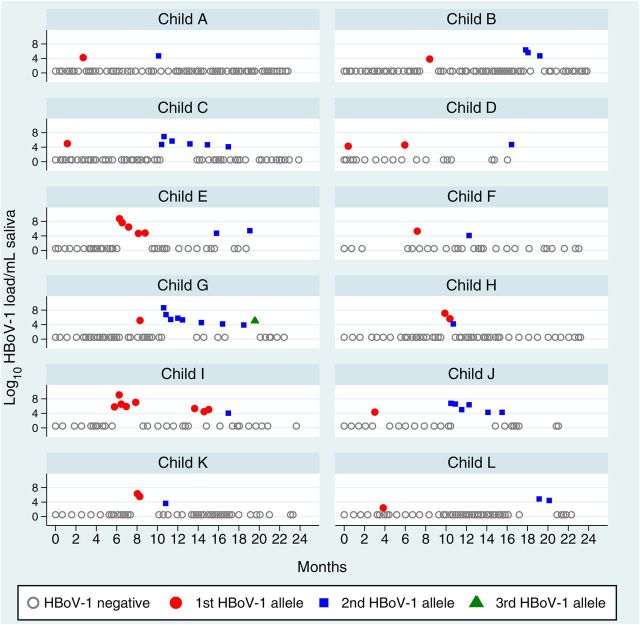
Allele data for at least 4 of the 8 identified HBoV-1 SNPs were available from 224 samples from 71 children. Of these, 48 children had SNP data from ≥2 HBoV-1 samples (range, 2–10 samples; mean, 3 samples) collected over a median of 146 days (range, 7–497 days). Twelve of 48 (25%) had HBoV-1 DNA demonstrating multiple allelic patterns, or variants, over time (Figure 2), suggesting reinfection with different HBoV-1 variants. In 11 of 12 cases, 1 variant persisted without change to the end of a shedding event with a different variant identified at the beginning of a recurrent event, including 1 child (child G) with 3 different sequential variants (Figure 2). One of the 12 children (child H; Figure 2) had 2 variants detected sequentially during the same primary event. Differences between variants included variations at >1 SNP site in samples from 10 of 12 individuals, and the second variant was sustained for multiple weeks in 6 (Table 4). The median time between shedding events due to different variants was 212 days. Symptoms reported among the 11 children with data available during the 2-week period surrounding the second HBoV-1 variant detection included fever (7 cases [64%]), new-onset cough (6 [55%]), new-onset runny nose (8 [73%]), vomiting (1), diarrhea (3), general rash (1), local rash (1), and fussiness (9 [82%]). Four of 11 children (36%) reported an illness-related primary care provider visit.
Figure 2.
Human bocavirus 1 (HBoV-1) alleles over time in 12 children. The first, second, and third allele patterns in each individual child are represented by changes in shape and color. Gray circles indicate tested samples with no HBoV-1 detected.
Table 4.
Allele Patterns of Human Bocavirus 1 Variants Over Time in 12 Children
| Child | Age at Earliest Detection, mo | Variant | Times Detected, No. | SNP Position |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2842 | 3923 | 4181 | 4229 | 4742 | 4799 | 4862 | 4874 | ||||
| A | 3 | 1 | 1 | A | C | T | T | A | T | … | G |
| 10 | 2 | 1 | A | C | [C] | [C] | A | … | A | G | |
| B | 8 | 1 | 1 | A | C | C | C | A | … | A | G |
| 18 | 2 | 3 | A | C | [T] | [T] | [C] | A | A | [A] | |
| C | 1 | 1 | 1 | A | C | C | T | C | … | G | A |
| 10 | 2 | 6 | A1 | C | C1 | [C] | [A] | T | [A]1 | [G] | |
| D | 0 | 1 | 2 | A1 | C1 | C | C | A1 | T | A | G |
| 16 | 2 | 1 | A | C | C | [T] | [C] | T | [G] | [A] | |
| E | 6 | 1 | 5 | A | C | C1 | C | A | T | A2 | G |
| 16 | 2 | 2 | A | C | [T] | [T] | [C] | [A] | A | [A] | |
| F | 7 | 1 | 1 | A | C | T | T | C | A | A | A |
| 12 | 2 | 1 | … | [T] | [C] | [C] | [A] | … | A | [G] | |
| G | 8 | 1 | 1 | A | C | T | T | C | A | A | A |
| 11 | 2 | 8 | [G]1 | [T]1 | [C]3 | [C] | [A]1 | [T] | A1 | [G] | |
| 20 | 3 | 1 | [A] | [C] | C | C | A | … | A | G | |
| H | 10 | 1 | 2 | A | C | T1 | … | C1 | A1 | A | A |
| 11 | 2 | 1 | … | C | … | T | C | … | [G] | A | |
| I | 6 | 1 | 8 | A | C | C | C1 | A1 | T2 | A | G |
| 17 | 2 | 1 | A | C | [T] | [T] | [C] | … | A | [A] | |
| J | 3 | 1 | 1 | … | C | T | T | C | A | … | A |
| 10 | 2 | 6 | A1 | C | [C] | [C] | [A]1 | [T]2 | A1 | [G] | |
| K | 8 | 1 | 2 | A | C | T | T | C1 | A1 | A | A |
| 11 | 2 | 1 | … | C | [C] | T | C | A | … | A | |
| L | 4 | 1 | 1 | A | C | … | T | C | … | … | A |
| 19 | 2 | 2 | A1 | C | C | [C]1 | [A] | T1 | A | [G] | |
Single-nucleotide polymorphisms (SNPs) are enclosed in brackets. Superscript numerals indicate the number of samples with missing data for that allele in that child. Dashes indicate missing data for all samples for that child and allele.
DISCUSSION
This prospective cohort study documents the natural history and clinical symptoms associated with HBoV-1 shedding in young children followed longitudinally from birth, measured using HBoV-1 DNA in oral fluid. We were able to evaluate the symptoms associated with the onset of HBoV-1 detection, as samples were collected frequently and systematically during both symptomatic and asymptomatic periods. Through the use of molecular methods, we demonstrated that reinfection with different HBoV-1 variants may occur following primary infection. Sequential infection may be partially responsible for instances of prolonged HBoV-1 shedding.
Our epidemiologic analyses show that HBoV-1 primary events occur in very young children and are associated with mild respiratory illness. The median age of initial HBoV-1 acquisition in our study population was 11 months, reflecting findings by Meriluoto et al showing that most primary HBoV-1 immune responses occur after 6 months of age [8]. The virus was detected year-round, with no evidence of seasonality, as demonstrated by the lack of differences in viral acquisition by season of birth. We found a significant association with new-onset cough during primary infection. HBoV-1 primary events with higher viral quantities were also associated with new-onset rhinorrhea and healthcare visits. Previous studies including children of a range of ages have linked HBoV-1 primary infection to wheezing [5, 6] and lower respiratory tract illness [12]. It is unexpected that our analyses of HBoV-1 load found higher peak viral loads in children who were older at the time of their primary event, in contrast to studies of other respiratory viruses in children, including respiratory syncytial virus [26–28]. Given the high seroprevalence of HBoV-1 antibody in the US population [4], it is possible that maternal antibody may pay a role in modifying primary HBoV-1 infection in the very youngest children.
We have previously reported a lack of association between HBoV-1 detection and respiratory symptoms in older children attending a child-care center [3]. It is possible that the association found here is specific to primary infection in very young children, and the contribution of HBoV-1 to respiratory illness becomes less pronounced as the child ages. Primary shedding events lasted >1 month in a majority of cases and included shedding events that lasted for >1 year. The shedding events described here are notably longer than those previously reported [3, 19, 20], likely because sampling continued even after symptoms resolved. This extended shedding makes it difficult to attribute HBoV-1 detection to acute illness when longitudinal testing and collection of blood specimens [12] has not been performed prior to the onset of illness [3]. Given the size of our study and the generally healthy nature of the infants who participated, it is likely that we have not captured the full range of diseases related to HBoV-1. We have not excluded the possibility that HBoV-1 may play a role in more-severe disease in some children and cause milder disease in others, akin to other DNA viruses with prolonged shedding patterns, namely human herpesvirus 6 [22, 29].
We used a novel molecular assay to describe HBoV-1 sequence variation across time in 48 children, including children with prolonged shedding and/or recurrent HBoV-1 events. This analysis indicated possible reinfection with a second HBoV-1 variant in 12 children (Table 4). The changes detected by our assay may be due to random single-event mutations. However, we found that changes occurred simultaneously at multiple SNP sites and were sustained over time, providing evidence that an infection with a second variant occurred. The majority of children had fever, new-onset cough and runny nose, and fussiness in the 2 weeks surrounding the appearance of the second variant. Unfortunately, our sample size was too limited to do comparative testing of symptoms at the time of a potential second infection, similar to that done by Meriluoto et al, who found no association between illness and HBoV-1 secondary immunoactivation or reconversion, based on immunoassay findings [8]. It is possible this difference is due to the younger age of our study participants (up to 2 years vs up to 8 years) and the increased specificity enabled by the weekly sample collections in our study. Further work using deep-sequencing methods is needed to determine the degree to which HBoV-1 primary infections represent mixed populations of variants versus a single homologous strain.
Although the oral fluid samples used in this study were collected as long as 13 years before testing, DNA dried onto filter paper is very stable [30]. A sensitivity analysis showed that the peak viral quantity and rate of primary events was not associated with year of enrollment, indicating that DNA detection has been consistent across the study period (data not shown). However, the use of dried samples of oral fluid restricted our ability to detect other respiratory viruses that may have contributed to the symptoms observed during the primary HBoV-1 event. For this reason, we selected control periods in each child that were 1 month prior to and following the primary event. We anticipate that this comparison controls for the impact of seasonal variation in other respiratory pathogens on our final results. We also performed subanalyses of primary events occurring during respiratory season as compared to those occurring outside the season and did not see a significant difference. We are unable to comment on how generalizable our findings are to children with different epidemiologic risk factors, particularly children who are not breastfed. Sampling of oral fluid provides advantages, including the ability to regularly collect and easily store weekly samples without requiring frequent contact with healthcare providers or researchers. We have found that our oral fluid assay was more sensitive than nasopharyngeal swabs for the detection of HBoV-1 DNA [18]. By performing complete testing on the first 10 subjects, we confirmed that the rate of HBoV-1 detection was not reduced as a result of our sampling strategy. We did not have serum samples available to confirm acute HBoV infection through serological analysis or detection of HBoV-1 DNA, as reported elsewhere [6, 8, 12, 31, 32]. Despite this, our use of longitudinal weekly samples beginning shortly after birth allowed us to clearly define the timing of the onset of first and ongoing HBoV-1 detection. Our study is also limited to young children in the first 2 years of life, when HBoV-1 infection is most frequent, and therefore we were unable to examine the impact of HBoV-1 on syndromes typically found in older children, such as asthma. Furthermore, as parents were not asked to collect daily symptom data on wheezing, we were limited in our ability to investigate an association with wheezing as a potential precursor to asthma.
The onset of HBoV-1 DNA detection in this cohort of young children was associated with new cough symptoms and primary care visits. Our results add to the growing evidence that HBoV-1 is an etiologic cause of respiratory illness. However, we have also demonstrated the pitfalls of using a single positive respiratory specimen to establish HBoV-1 as an etiologic cause of disease, in the absence of more-robust clinical diagnostic analyses. Because of the long duration of HBoV-1 shedding and the potential for reinfection with additional variants, detection of HBoV-1 at a single time point is not sufficient to diagnose an incident HBoV-1 infection and should be interpreted with care.
Notes
Acknowledgments. Single-nucleotide polymorphism assays were performed by Susan Land and Matthew Hess at the Applied Genomics Technology Core, Wayne State University.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Financial support. This work was supported by the Thrasher Research Fund (early career award to E. T. M.), the Eugene Applebaum College of Pharmacy and Health Sciences Faculty Research Award Program (to E. T. M.), the Seattle Children's Center for Clinical and Translational Research (Pediatric Pilot Fund Award to E. T. M. and D. M. Z.), and the National Institute of Allergy and Infectious Diseases, NIH (award K23 AI01679 to D. M. Z.).
Potential conflicts of interest. E. T. M. receives research support from Pfizer and Sage Products. D. M. Z. has received research support from Sage Products. J. A. E. has served as a consultant for GlaxoSmithKline and has received research support from Chimerix, Gilead, and Roche. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 2005; 102:12891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Soderlund-Venermo M. Human bocavirus-the first 5 years. Rev Med Virol 2012; 22:46–64. [DOI] [PubMed] [Google Scholar]
- 3.Martin ET, Fairchok MP, Kuypers J, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 2010; 201:1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn JS, Kesebir D, Cotmore SF, et al. Seroepidemiology of human bocavirus defined using recombinant virus-like particles. J Infect Dis 2008; 198:41–50. [DOI] [PubMed] [Google Scholar]
- 5.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007; 44:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soderlund-Venermo M, Lahtinen A, Jartti T, et al. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis 2009; 15:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehtoranta L, Soderlund-Venermo M, Nokso-Koivisto J, et al. Human bocavirus in the nasopharynx of otitis-prone children. Int J Pediatr Otorhinolaryngol 2012; 76:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meriluoto M, Hedman L, Tanner L, et al. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 2012; 18:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ursic T, Steyer A, Kopriva S, Kalan G, Krivec U, Petrovec M. Human bocavirus as the cause of a life-threatening infection. J Clin Microbiol 2011; 49:1179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edner N, Castillo-Rodas P, Falk L, Hedman K, Soderlund-Venermo M, Allander T. Life-threatening respiratory tract disease with human bocavirus-1 infection in a 4-year-old child. J Clin Microbiol 2012; 50:531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jula A, Waris M, Kantola K, et al. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis 2013; 19:1328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen A, Nordbo SA, Krokstad S, Rognlien AG, Dollner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 2010; 49:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen A, Dollner H, Skanke LH, Krokstad S, Moe N, Nordbo SA. Detection of spliced mRNA from human bocavirus 1 in clinical samples from children with respiratory tract infections. Emerg Infect Dis 2013; 19:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry AM, Lu X, Chittaganpitch M, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 2007; 195:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses 2012; 6:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Garcia ML, Calvo C, Pozo F, et al. Human bocavirus detection in nasopharyngeal aspirates of children without clinical symptoms of respiratory infection. Pediatr Infect Dis J 2008; 27:358–60. [DOI] [PubMed] [Google Scholar]
- 17.Longtin J, Bastien M, Gilca R, et al. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis 2008; 14:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin ET, Taylor J, Kuypers J, et al. Detection of bocavirus in saliva of children with and without respiratory illness. J Clin Microbiol 2009; 47:4131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Linstow ML, Hogh M, Hogh B. Clinical and epidemiologic characteristics of human bocavirus in Danish infants: results from a prospective birth cohort study. Pediatr Infect Dis J 2008; 27:897–902. [DOI] [PubMed] [Google Scholar]
- 20.Brieu N, Guyon G, Rodiere M, Segondy M, Foulongne V. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 2008; 27:969–73. [DOI] [PubMed] [Google Scholar]
- 21.Koskenvuo M, Mottonen M, Waris M, Allander T, Salmi TT, Ruuskanen O. Human bocavirus in children with acute lymphoblastic leukemia. Eur J Pediatr 2008; 167:1011–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005; 352:768–76. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 24.Zerr DM, Huang ML, Corey L, Erickson M, Parker HL, Frenkel LM. Sensitive method for detection of human herpesviruses 6 and 7 in saliva collected in field studies. J Clin Microbiol 2000; 38:1981–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. Human bocavirus in French children. Emerg Infect Dis 2006; 12:1251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall CB, Douglas RG, Jr, Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr 1976; 89:11–5. [DOI] [PubMed] [Google Scholar]
- 27.Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 2004; 31:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis 2008; 62:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall CB, Long CE, Schnabel KC, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med 1994; 331:432–8. [DOI] [PubMed] [Google Scholar]
- 30.Chaisomchit S, Wichajarn R, Janejai N, Chareonsiriwatana W. Stability of genomic DNA in dried blood spots stored on filter paper. Southeast Asian J Trop Med Public Health 2005; 36:270–3. [PubMed] [Google Scholar]
- 31.Don M, Soderlund-Venermo M, Valent F, et al. Serologically verified human bocavirus pneumonia in children. Pediatr Pulmonol 2010; 45:120–6. [DOI] [PubMed] [Google Scholar]
- 32.Kantola K, Hedman L, Allander T, et al. Serodiagnosis of human bocavirus infection. Clin Infect Dis 2008; 46:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]