Abstract
Background
Variant influenza A(H3N2) viruses (H3N2v) have transmitted recently from pigs to humans in the United States. Vaccines strategies are needed.
Methods
Healthy adults received 2 doses of subvirion H3N2v vaccine (15 µg of hemagglutinin/dose) 21 days apart in this open-label trial. Serum hemagglutination inhibition (HAI) and neutralizing (Neut) antibody (Ab) titers were measured before and 8 and 21 days after each dose. Memory B-cell (MBC) responses were assessed.
Results
Vaccine was well tolerated. A total of 40% of subjects had an HAI Ab titer of ≥40 before vaccination. Eight-seven percent (95% confidence interval [CI], 79%–93%) and 73% (95% CI, 63%–81%) of subjects 18–64 years old (98 subjects) and ≥65 years old (90 subjects), respectively, had an HAI titer of ≥40 21 days after dose 1 (P = .01); 51% (95% CI, 41%–61%) and 52% (95% CI, 41%–62%) of younger and older subjects, respectively, developed ≥4-fold rises in titer (P = not significant). Neut Ab response patterns were similar. Geometric mean titers were higher in younger subjects. Dose 2 provided no significant enhancement in responses. Cross-reactive MBCs were detected before vaccination and expanded after vaccination. Preexisting H3N2v-specific MBCs positively correlated with early increases in vaccine-induced Ab.
Conclusions
In most healthy adults, one 15-µg dose of vaccine elicited levels of HAI Abs associated with protection. Studies in children and elderly individuals are indicated to define the immunization needs of these groups.
Clinical Trials Registration
Keywords: influenza, pandemic, H3N2 variant, immune responses, immunization
Since August 2011, 13 US states have reported 342 confirmed human influenza virus infections caused by an influenza A(H3N2) variant that originated in swine (H3N2v) [1–7]. Limited human-to-human transmission has occurred, but sustained transmission has not [3, 8]. Most infections occurred in children with little to no preexisting immunity against the virus [8]. Eighteen people have been hospitalized, and 1 with comorbidities died [8].
The prevalence of putative protective titers of antibody (Ab) against H3N2v in most age groups is reported to be low [9–11]; hence, H3N2v poses a pandemic threat similar to that posed by the 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09). Therefore, an inactivated H3N2v vaccine (H3N2v IIV1) was prepared for human testing. During the A(H1N1)pdm09 vaccine evaluations, 1 dose of H3N2v IIV1 containing 15 μg of hemagglutinin (HA) administered intramuscularly was sufficient to elicit significant Ab responses in adults [12]. Whether 2 doses of H3N2v IIV1 vaccine are needed is unknown. Thus, the goals of this study were to evaluate this approach.
MATERIAL AND METHODS
Study Design
This phase 2 open-label clinical trial to assess the safety and immunogenicity of H3N2v IIV1 was conducted in healthy males and nonpregnant females aged ≥18 years with the goal to enroll up to 120 subjects aged 18–64 years (the younger group) and 120 aged ≥65 years (the older group). The study was conducted at 4 National Institutes of Health–funded Vaccine and Treatment Evaluation Units (VTEUs; 1 each at Baylor College of Medicine, Group Health Research Institute, Emory University School of Medicine, and the University of Iowa) between January 2013 and September 2013. The protocol was approved by the relevant ethics committees, and written informed consent was provided by participants.
Vaccine
Vaccine was provided by the Biomedical Advanced Research and Development Authority, which is headed by Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Vaccine seed stock was produced in eggs, using a strain derived from influenza A/Minnesota/11/2010 produced by classical reassortant technology and provided by the Centers for Disease Control and Prevention (CDC; Atlanta, Georgia) to the vaccine manufacturer, Sanofi Pasteur. The resulting reassortant is designated A/Minnesota/11/2010 NYMC X-203. The manufacturing process for this vaccine was similar to that used to produce their licensed IIV, Fluzone, with slight modifications in the formulation step. Vaccine was formulated to contain 15 μg of HA/0.5-mL dose. HA content was confirmed using a single radial immunodiffusion assay.
Clinical Procedures
Medically stable adults were eligible to participate. Inclusion and exclusion criteria are available in the Supplemental Materials.
Subjects were stratified into 2 groups on the basis of age (younger and older) and then given 2 intramuscular doses of H3N2v IIV1 21 days apart. Oral temperature and injection site and systemic symptoms and signs were recorded daily for 7 days after each vaccination. Adverse events (AEs) were assessed through 42 days after dose 1; serious adverse events (SAEs) and new-onset chronic medical conditions were assessed through 7 months after dose 1. Sera for Ab assays were collected before and 8 and 21 days after each dose. Blood samples for memory B-cell (MBC) studies were collected on days 0 and 42 from a subset of younger adults enrolled at Emory University School of Medicine.
Laboratory Methods
Serum hemagglutination inhibition (HAI) and neutralizing (Neut) Ab assays were performed at Cincinnati Children's Hospital Medical Center, using previously described methods [13–15]. Antigen used for the HAI assay (A/Minnesota/11/2010 X-203, BPL-Inactivated) and the virus used for the Neut assay (A/Minnesota/11/2010[H3N2v]) were obtained from the Influenza Reagent Resource (IRR; available at: http://www.influenzareagentresource.org) of the CDC.
Assay for Enumeration of Influenza Virus–Specific MBCs
Influenza virus–specific MBC responses were ascertained at days 0 and/or 42, using 2 complementary methods.
MBC Analysis Using Enzyme-Linked Immunospot (ELISpot) Assay
H3N2 A/Minnesota/11/2010 vaccine antigen was provided by Sanofi Pasteur. Recombinant HA (rHA) from H3N2 A/Perth/16/2009 (NR-19442) was provided by Biodefense and Emerging Infections Research Repository (available at: http://www.beiresources.org); rHA from H3N2 A/Victoria/361/2011 (IRR number, FR-1059) was provided by the IRR. At Emory University School of Medicine, cryopreserved peripheral blood mononuclear cells (PBMCs) collected on day 0 from a subset of participants were thawed in a 37°C water bath and washed. Cells were counted and checked for viability by Trypan blue dye exclusion. MBC assays were performed as previously described [16, 17]. In brief, PBMCs were plated in 24-well dishes at 5 × 105 cells/well in R-10 medium supplemented with an optimized mix of polyclonal mitogens: pokeweed mitogen extract (made in-house), phosphorothiolated CpG ODN-2006 (Integrated DNA Technologies), and Staphylococcus aureus Cowan (Sigma). Eight wells were cultured per individual for 6 days. Stimulated cells were harvested, washed, and assayed using the ELISpot assay. Developed plates were scanned and analyzed using an automated ELISpot counter (Cellular Technologies). Data are presented as the percentage of influenza virus–specific immunoglobulin G (IgG)–secreting cells among total IgG-secreting cells.
Enzyme-Linked Immunosorbent Assay (ELISA) of MBCs After Epstein–Barr Virus (EBV) Transformation of PBMCs
PBMCs isolated from blood collected on days 0 and 42 from a subset of participants at Emory University School of Medicine were aliquoted into cryovials, cryopreserved, stored in liquid nitrogen, and shipped to Vanderbilt University for testing. Cells were thawed in a 37°C water bath and washed prior to transformation with Epstein–Barr virus (EBV) in the presence of Chk2 inhibitor (Sigma catalog no. C3742), cyclosporin A (Sigma), and CpG10103. The CpG10103 was synthesized as an oligonucleotide, TCGTCGTTTTTCGGTCGTTTT, containing phosphorothioate bonds (Invitrogen). EBV-transformed cells were plated in 384-well microtiter plates, grown for 10 days, and screened by ELISA for binding to each of the H3 rHAs (described below). The minimal frequency of HA-reactive B cells was estimated on the basis of the number of wells with HA-reactive supernatants, compared with the total number of lymphoblastoid cell line (LCL) colonies in the transformation plates (calculation: HA-reactive B-cell frequency = [number of wells with HA-reactive supernatants] ÷ [number of LCL colonies in the plate] × 100).
rHA Production
DNA copies of the genes encoding the extracellular portion of HAs from A/Minnesota/11/2010 H3N2v and 2 seasonal H3N2 strains (A/Victoria/361/2011 and A/Wisconsin/57/2005) were synthesized with a trimerization domain and a 6× histidine epitope tag and subcloned into the pCDNA3.1+ mammalian cell expression vector (Invitrogen). Protein was expressed by transient transfection of 293F cells (Invitrogen) according to the manufacturer's instructions, with the exception that polyethyleneimine was used as the transfection reagent instead of 293Fectin. Cells were grown for 7 days and then harvested by centrifugation at 2500g. Supernatant was passed through a 0.45-µm membrane. Clarified supernatant was applied to a HiTrap Talon crude HP column (GE Healthcare). Purified rHAs were concentrated and buffer exchanged twice with Dulbecco's phosphate-buffered saline, using an Amicon Ultra centrifugal concentrator with a 30-kD cutoff membrane (Millipore).
Statistical Analyses
The study sample size was selected to obtain preliminary estimates in a time-critical manner; the study was not powered to test a specific hypothesis. Analyses of primary safety end points of vaccine-related SAEs and solicited reactogenicity were primarily descriptive. Immunogenicity end points included the proportions of subjects achieving seroconversion (ie, subjects with either a prevaccination titer of <10 and a postvaccination titer of ≥40 or a prevaccination titer of ≥10 and a minimum 4-fold increase in the postvaccination titer [18]), the proportion of subjects with a titer of ≥40, and the geometric mean titer (GMT) 8 and 21 days after each vaccination. Immune responses were compared between age groups, using the Fisher exact test for proportional end points or the t test for log-transformed titers. Statistical significance was considered at an α level of 0.05, without adjustment for multiple comparisons; all tests were 2 sided. SAS, version 9.3, was used for analysis.
Multivariate models were fit to explore the relationship between immune responses and baseline titer (log transformed, continuous), sex, prior receipt of seasonal influenza vaccine (none in 2011/2012 or 2012/2013; 1 in 2011/2012 only; 1 in 2012/2013 only; or 1 in both 2011/2012 and 2012/2013), and VTEU site. Separate linear regression models were fit within each age stratum for log-transformed HAI or Neut Ab titers 21 days after dose 1.
To estimate the increase in MBC frequency over time, a generalized least squares model was fit, accounting for correlation due to repeated observations on the same subject for multiple visits and multiple antigens.
Analyses are presented for the per-protocol subset, which includes data for subjects who received ≥1 dose of study vaccine and had valid HAI results before vaccination and at ≥1 postvaccination visit, with the following exclusions: data for subjects found to be ineligible at baseline; data obtained after dose 2 if dose 2 was not received at the appropriate time; and data for visits following the receipt of nonstudy vaccine or corticosteroids. Safety data were included for all subjects who received study vaccination.
RESULTS
Study Population
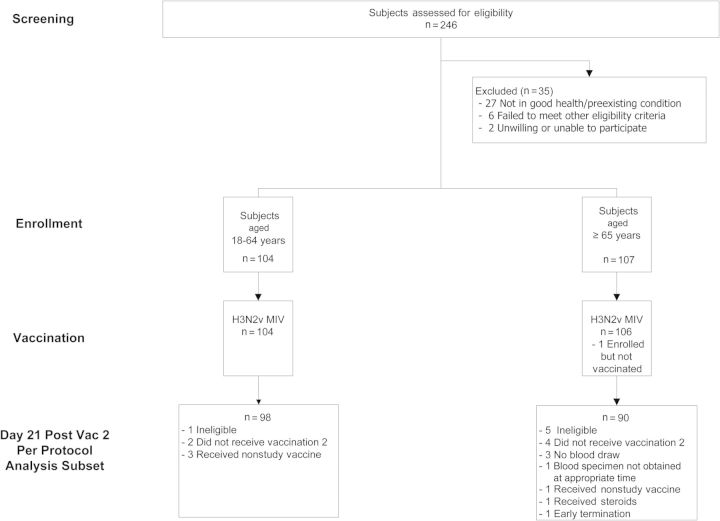
Two hundred eleven subjects were enrolled in January 2013: 104 were in the younger group, and 107 were in the older group. Two hundred and ten subjects received dose 1, and 193 received dose 2 (Figure 1). Demographic characteristics of enrolled subjects are shown in Table 1. A total of 209 subjects completed the study; 2 subjects terminated early.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of subject disposition.
Table 1.
Demographic Characteristics of the Study Participants
| Characteristic | All Subjects (n = 211) | Younger Subjects (n = 104) | Older Subjects (n = 107) |
|---|---|---|---|
| Sex | |||
| Male | 91 (43) | 45 (43) | 46 (43) |
| Female | 120 (57) | 59 (57) | 61 (57) |
| Ethnicity | |||
| Non-Hispanic | 202 (96) | 96 (92) | 106 (99) |
| Hispanic | 9 (4) | 8 (8) | 1 (1) |
| Race | |||
| Asian | 13 (6) | 13 (13) | 0 |
| Black/African American | 18 (9) | 13 (13) | 5 (5) |
| White | 176 (83) | 74 (71) | 102 (95) |
| Multiracial | 2 (1) | 2 (2) | 0 |
| Other/unknown | 2 (1) | 2 (2) | 0 |
| Age, y | 57.4 ± 19.6 | 40.9 ± 14.6 | 73.3 ± 5.8 |
Data are no. (%) of subjects, or mean ± SD. Younger subjects were aged 18–64 years, and older subjects were aged ≥65 years.
Safety and Reactogenicity
Vaccine was safe and well tolerated. A description of safety and reactogenicity is provided for 210 vaccinated subjects in the Supplementary Materials.
Serum Ab Responses
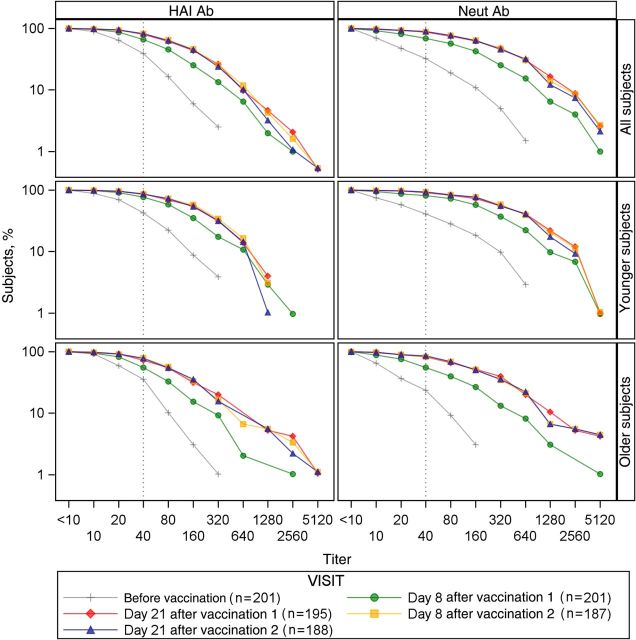
HAI GMTs and proportions of subjects with a serum HAI titer of ≥40 against H3N2v before dose 1 were similar in both age strata (Table 2, Figure 2, and Supplementary Figure 2). Ab responses after vaccination were generally higher among younger subjects, compared with older subjects, with the largest differences seen on day 8 after dose 1. GMTs were higher at every time point after receipt of dose 1 among younger subjects, compared with older subjects (P < .01 for each time point). A higher proportion of younger subjects achieved a serum HAI titer of ≥40 on day 8 (P = .001) and day 21 (P = .01) after dose 1, but the differences were not significant following dose 2. Reverse cumulative distribution curves demonstrated that most adults (93%) had Ab to H3N2v before immunization and that a second dose of vaccine conferred little additional benefit (Figure 2). The geometric mean fold increase from day 8 to day 21 for older subjects (HAI, 1.93 [95% confidence interval {CI}, 1.7–2.3]; Neut, 3.1 [95% CI, 2.6–3.8]) was higher than that observed for younger subjects (HAI, 1.6 [95% CI, 1.4–1.8; P = .04]; Neut, 2.1 [95% CI, 1.7,–2.5; P = .001]).
Table 2.
Serum Hemagglutination Inhibition Antibody Responses Following Immunization With H3N2v IIV1
| Subjects, Study Visit | Subjects, No. | GMT (95% CI) | Titer ≥40 |
Seroconversion |
||
|---|---|---|---|---|---|---|
| Subjects, No. | Proportion (95% CI) | Subjects, No. | Proportion (95% CI) | |||
| All subjects | ||||||
| Before vaccination | 201 | 24.2 (21.1–27.8) | 79 | 0.39 (0.33–0.46) | … | … |
| Day 8 after vaccination 1 | 201 | 58.0 (48.6–69.1) | 133 | 0.66 (0.59–0.73) | 59 | 0.29 (0.23–0.36) |
| Day 21 after vaccination 1 | 195 | 98.7 (81.9–118.9) | 156 | 0.80 (0.74–0.85) | 100 | 0.51 (0.44–0.58) |
| Day 8 after vaccination 2 | 187 | 103.3 (85.4–125.0) | 154 | 0.82 (0.76–0.88) | 100 | 0.53 (0.46–0.61) |
| Day 21 after vaccination 2 | 188 | 101.1 (84.5–121.0) | 154 | 0.82 (0.76–0.87) | 98 | 0.52 (0.45–0.59) |
| Younger subjects | ||||||
| Before vaccination | 103 | 27.5 (22.3–34.1) | 44 | 0.43 (0.33–0.53) | … | … |
| Day 8 after vaccination 1 | 103 | 82.2 (64.2–105.2) | 79 | 0.77 (0.67–0.84) | 38 | 0.37 (0.28–0.47) |
| Day 21 after vaccination 1 | 100 | 126.4 (99.4–160.8) | 87 | 0.87 (0.79–0.93) | 51 | 0.51 (0.41–0.61) |
| Day 8 after vaccination 2 | 97 | 131.5 (101.8–169.7) | 83 | 0.86 (0.77–0.92) | 54 | 0.56 (0.45–0.66) |
| Day 21 after vaccination 2 | 98 | 126.2 (99.5–160.2) | 84 | 0.86 (0.77–0.92) | 50 | 0.51 (0.41–0.61) |
| Older subjects | ||||||
| Before vaccination | 98 | 21.2 (17.9–25.1) | 35 | 0.36 (0.26–0.46) | … | … |
| Day 8 after vaccination 1 | 98 | 40.1 (31.8–50.7) | 54 | 0.55 (0.45–0.65) | 21 | 0.21 (0.14–0.31) |
| Day 21 after vaccination 1 | 95 | 76.0 (57.4–100.7) | 69 | 0.73 (0.63–0.81) | 49 | 0.52 (0.41–0.62) |
| Day 8 after vaccination 2 | 90 | 79.7 (60.2–105.5) | 71 | 0.79 (0.69–0.87) | 46 | 0.51 (0.40–0.62) |
| Day 21 after vaccination 2 | 90 | 79.4 (60.7–103.8) | 70 | 0.78 (0.68–0.86) | 48 | 0.53 (0.43–0.64) |
Younger subjects were aged 18–64 years, and older subjects were aged ≥65 years.
Abbreviations: CI, confidence interval; GMT, geometric mean titer.
Figure 2.
Reverse cumulative distribution curves of serum hemagglutination inhibition (HAI) and neutralizing (Neut) antibodies (Abs) following immunization with H3N2v IIV1.
Before vaccination, younger subjects had a Neut Ab GMT twice as high as older subjects and maintained higher levels for all postvaccination measurements (P < .01 for all time points; Table 3). A significantly higher proportion of younger subjects had Neut Ab titers of ≥40 for all time points except day 21 following dose 2. The proportion of subjects with a ≥4-fold rise in Neut Ab titer was higher in younger subjects on day 8 after the first dose (P = .03), but there were no significant differences between age groups for later time points. There was a significant correlation between HAI and Neut Ab levels (r = 0.85 and P < .001), although postvaccination Neut Ab titers were significantly higher than HAI titers (P < .001, by the paired t test, for all visits).
Table 3.
Serum Neutralizing Antibody Responses Following Immunization With H3N2v IIV1
| Study Visit | Subjects, No. | GMT (95% CI) | Titer ≥40 |
Seroconversion |
||
|---|---|---|---|---|---|---|
| Subjects, No. | Proportion (95% CI) | Subjects, No. | Proportion (95% CI) | |||
| All subjects | ||||||
| Before vaccination | 201 | 22.9 (19.0–27.7) | 65 | 0.32 (0.26–0.39) | … | … |
| Day 8 after vaccination 1 | 201 | 102.3 (80.7–129.7) | 138 | 0.69 (0.62–0.75) | 70 | 0.35 (0.28–0.42) |
| Day 21 after vaccination 1 | 195 | 255.0 (202.9–320.6) | 169 | 0.87 (0.81–0.91) | 123 | 0.63 (0.56–0.70) |
| Day 8 after vaccination 2 | 187 | 260.1 (206.9–326.8) | 164 | 0.88 (0.82–0.92) | 124 | 0.66 (0.59–0.73) |
| Day 21 after vaccination 2 | 188 | 256.7 (204.9–321.6) | 168 | 0.89 (0.84–0.93) | 122 | 0.65 (0.58–0.72) |
| Younger subjects | ||||||
| Before vaccination | 103 | 31.9 (23.7–42.9) | 42 | 0.41 (0.31–0.51) | … | … |
| Day 8 after vaccination 1 | 103 | 182.4 (132.8–250.5) | 84 | 0.82 (0.73–0.89) | 43 | 0.42 (0.32–0.52) |
| Day 21 after vaccination 1 | 100 | 362.8 (267.7–491.7) | 91 | 0.91 (0.84–0.96) | 59 | 0.59 (0.49–0.69) |
| Day 8 after vaccination 2 | 97 | 375.6 (277.9–507.6) | 90 | 0.93 (0.86–0.97) | 64 | 0.66 (0.56–0.75) |
| Day 21 after vaccination 2 | 98 | 364.6 (270.6–491.4) | 91 | 0.93 (0.86–0.97) | 62 | 0.63 (0.53–0.73) |
| Older subjects | ||||||
| Before vaccination | 98 | 16.2 (13.0–20.2) | 23 | 0.23 (0.15–0.33) | … | … |
| Day 8 after vaccination 1 | 98 | 55.7 (40.6–76.5) | 54 | 0.55 (0.45–0.65) | 27 | 0.28 (0.19–0.37) |
| Day 21 after vaccination 1 | 95 | 176.0 (126.2–245.4) | 78 | 0.82 (0.73–0.89) | 64 | 0.67 (0.57–0.77) |
| Day 8 after vaccination 2 | 90 | 175.0 (125.4–244.2) | 74 | 0.82 (0.73–0.89) | 60 | 0.67 (0.56–0.76) |
| Day 21 after vaccination 2 | 90 | 175.2 (126.1–243.3) | 77 | 0.86 (0.77–0.92) | 60 | 0.67 (0.56–0.76) |
Younger subjects were aged 18–64 years, and older subjects were aged ≥65 years.
Abbreviations: CI, confidence interval; GMT, geometric mean titer.
Separate linear regression models for log-transformed titers were fit within each age stratum. For models of both HAI and Neut Abs in both age strata, baseline titer had a significant positive association with the day 21 titer after dose 1 (P < .001 for all models), but there were no associations with sex, prior receipt of seasonal vaccine, or VTEU site. Thus, data for both age strata were combined, and additional linear regression models were fit for log HAI or log Neut Abs on day 21 after dose 1 with the covariates age (18–64 years and ≥65 years) and baseline titer. The interaction of age and baseline titer was not significant. Models suggested that, on average, older subjects had HAI titers 1.4 times lower than those of younger subjects and that each log increase in baseline HAI titer resulted in an approximately one-half log increase in HAI titer on day 21. Results for the model of Neut Ab titers were similar.
A similar modeling strategy was used to examine the associations of the covariates with seroconversion in HAI or Neut Ab. In separate logistic regression models for HAI or Neut Ab responses in younger subjects, baseline titer had a significant negative association with a ≥4-fold rise on day 21 after dose 1 (HAI, P = .008; Neut, P = .0003), but this association was not significant for older subjects. No associations with sex, prior receipt of seasonal vaccine, or VTEU site were observed for either assay. In logistic regression models combining data from both age strata, the interaction of age and baseline titer was not significant. Results of the logistic regression models suggest that there was no difference in proportions with ≥4-fold rises between age groups and that, as the baseline HAI titer increased, the odds of a response decreased. Results for the model of Neut Ab response were similar.
Overall, for both HAI and Neut Ab assays, higher baseline titers were associated with higher titers on day 21 after dose 1 but a lower probability of seroconversion. After adjustment for baseline titers, the younger age group had significantly higher titers on day 21, but there was no difference in the probability of a ≥4-fold response between age groups. Sex, prior receipt of seasonal vaccine, and VTEU site were not associated with immune responses in this study.
B-Cell Studies
The 2 MBC assays reported here provided estimates of the H3N2v-specific B-cell populations that were complementary and consistent. The ELISpot method was performed on 20 participants at baseline only. The B cell transformation method was performed at baseline (12 participants) and day 42 (25 participants).
Prevaccination MBCs were quantified using the influenza virus antigen–specific ELISpot method in 20 participants from the younger group. Data for 5 additional participants were missing owing to limitations in the availability of cells. MBCs that produced IgG that recognized the vaccine antigen (A/Minnesota/11/2010) or recent seasonal H3 rHA proteins (A/Perth/16/2009 and A/Victoria/361/2011) were identified (Figure 3A). Nineteen of 20 subjects had MBCs that produced IgG that recognized the H3N2v vaccine antigen at baseline. The frequencies of IgG-secreting MBCs that recognized H3N2v ranged from approximately 0.05% to 1% of total IgG-secreting MBC and did not correlate with baseline Ab levels against the vaccine strain (HAI, r = 0.06 and P = .80; Neut Ab, r = −0.03 and P = .90). However, the percentage of IgG-secreting MBCs that produced Ab against H3N2v did correlate with the fold change in day 8 HAI Ab levels relative to baseline (r = 0.62 and P = .003; Figure 3A). By day 21, this correlation was no longer present (r = 0.31 and P = .18). The corresponding percentages of IgG-secreting MBCs producing IgG against seasonal H3N2 strains did not significantly correlate with either day 8 or day 21 HAI responses.
Figure 3.
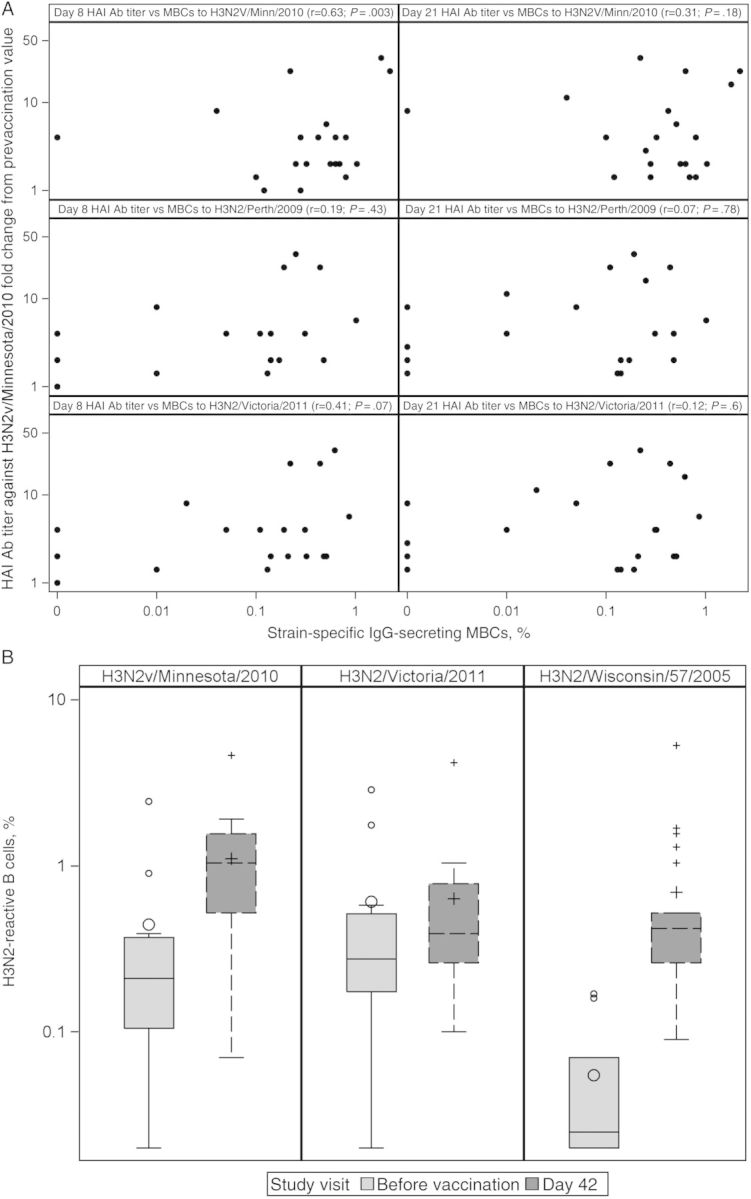
Memory B-cell (MBC) responses before and after vaccination. A, Relationship of fold change in hemagglutination inhibition (HAI) antibody (Ab) titer against H3N2v 8 and 21 days after vaccination and day 0 MBCs against H3N2v or 2 seasonal H3 strains measured before vaccination (enzyme-linked immunospot MBC assay; n = 20). B, Percentage of H3N2-reactive B cells before vaccination and at day 42 (B-cell transformation method; n = 13 and n = 25 at day 0 and day 42, respectively). Abbreviation: IgG, immunoglobulin G.
PBMCs collected on days 0 and 42 from the same 25 subjects were transformed with EBV, and LCL supernatants were tested for the presence of Ab against 3 H3 rHAs by ELISA (Figure 3B). Day 0 data are missing for 13 subjects owing to a freezer malfunction. MBCs were detected at day 0 with comparable frequencies among circulating transformable MBCs against H3N2v rHA (0.4%; 95% CI, .04%–.8%) and the A/Victoria/361/2011 rHA (0.6%; 95% CI, .3%–1.1%). There was little prevaccination reactivity detected for the seasonal strain A/Wisconsin/57/2005 rHA. Following vaccination (day 42), there was a rise in the frequency of reactive B cells (likely representing MBCs that were present before vaccination and a new pool of MBCs induced by vaccination). Following vaccination, the mean frequency of H3N2v-reactive B cells increased to 1.1% (95% CI, .7%–1.5%; P = .03). The rise in reactivity was lower for the seasonal strain A/Wisconsin/57/2005 rHA, to a mean frequency of 0.7% (95% CI, .3–1.1; P = .02). As a group, subjects did not show a significant change in the frequency of MBCs to A/Victoria/361/2011 rHA (P = .98).
MBC data obtained using both methods were available for 10 subjects at day 0 for the H3N2v Minnesota/2010 and H3N2 Victoria/2011 strains. The Spearman correlations between the 2 methods were as follows: H3N2v Minnesota/2010: r = 0.83 (P = .003); H3N2 Victoria/2011: r = 0.36 (P = .30).
DISCUSSION
Serum IgG Ab to the influenza virus HA elicited following infection or vaccination has a major role in protective immunity against influenza virus infection [19]. Protection against infection and disease caused by seasonal influenza virus strains correlates directly with both serum HAI and Neut Ab levels, and measurements of these Abs are used to assess the immunogenicity of IIVs. These data are used to predict the likelihood that a vaccine will provide protection against influenza. The putative protective HAI Ab titer is generally regarded to be ≥40. Our results suggest that most adults will be protected against infection with H3N2v following 1 dose of vaccine containing 15 μg of H3N2v HA.
Vaccine factors affecting the immunogenicity of influenza vaccines include dose, number of doses given, and type of product used. Host factors include age, sex, prior priming, preexisting Ab levels, comorbidities, and disease treatments that could influence immune responses. The results of our study are consistent with the concept that prior priming by infection or vaccination with antigenically related vaccine strains is associated with brisk and robust immune responses following immunization of adults with a new variant of that subtype, using the standard dosage for currently licensed IIVs.
Most subjects in the subgroup had preexisting MBCs against the H3 HAs that have circulated in recent years and have been in seasonal IIVs. Furthermore, preexisting cross-reactive MBCs that recognized H3N2v HA were demonstrated for most subjects, and the level of these preexisting MBCs had a significant correlation with early anamnestic Ab responses against H3N2v in this small substudy. By day 21, this correlation was lost, perhaps because of a subsequent (slower) rise of primary Ab responses against new epitopes in H3N2v rHA. We also demonstrated that the frequencies of MBCs recognizing H3N2v rHA and rHA from 1 of 2 seasonal viruses tested increased after vaccination. Thus, vaccinated individuals appeared as a group quite capable of developing new MBCs to H3N2v, irrespective of prior exposure to seasonal vaccines or infection. Following vaccination, most subjects responded to H3N2v vaccine antigen, and the MBC pool expanded to include not only B cells recognizing seasonal H3N2 HA, but also newly generated H3N2v-specific cells that encoded Abs that do not recognize the HAs of previous seasonal H3N2 viruses. The frequency of preexisting MBCs observed here for seasonal antigens and the frequency of MBCs to H3N2v after vaccination are typical of the human response to IIVs. It was interesting that the frequency of MBCs to H3N2v vaccine antigen after vaccination did not correlate with the magnitude of serum Ab responses. Abs are secreted by plasma cells, especially by long-lived plasma cells in the bone marrow, whereas MBCs do not secrete Abs but participate in secondary responses following reexposure. In fact, recent clinical trials with avian IIVs revealed little serum Ab response following primary vaccination but a strong response after subsequent boosting at a late time point, suggesting primary vaccination induced MBCs independent of a detectable serum Ab response [20].
The HA of H3N2v is more closely related to that of seasonal H3N2 viruses that circulated in the 1990s. Studies using animal antisera that cross-react with human H3N2 epidemic influenza virus strains used in recent seasonal influenza vaccines exhibited no cross-reactivity with the H3N2v strain. Furthermore, limited serologic studies indicate that young children have little to no preexisting Abs to H3N2v and that immunization of young children with seasonal trivalent IIV (IIV3) elicits minimal to no cross-reactive Abs. In one earlier study, younger adults had evidence of cross-reactive Ab (approximately one third of adults 18–49 years of age had a titer of ≥40 before seasonal IIV3 immunization), but few developed Ab responses to H3N2v after immunization with seasonal IIV3 (50% with a postvaccination titer of ≥40 against H3N2v) [9]. Older adults were shown to have a lower frequency of Ab to the variant before immunization with seasonal IIV3 than younger adults (17%), and 40% had putative protective titers after vaccination with seasonal IIV3 [9]. Low levels of cross-reactive Ab against H3N2v has been observed in several other studies, particularly among children and older adults [10, 21], and IIV3 failed to increase seroprotection rates substantially in one of these [10]. IIVs made using current and recently circulating seasonal H3N2 strains from humans are therefore unlikely to confer significant protection against H3N2v infections. In the current study, both younger and older adults developed significant serum Ab responses following a single 15-μg dose of H3N2v vaccine. Responses among the younger subjects were greater than those among the older subjects. Higher preexisting Ab levels were positively correlated with higher postimmunization Ab levels, and the frequencies of significant titer rises were lower among those with higher preexisting Ab titers, as observed previously [22]. The geometric mean fold increase from day 8 to day 21 among older subjects was greater than that among younger subjects, consistent with a delayed Ab response, which has been observed by others [23].
While 1 dose of vaccine containing 15 μg of H3N2v HA was immunogenic in adults, children and persons with underlying medical conditions that could impair their immune responses may need different immunization regimens. For the A(H1N1)pdm09 vaccine, 2 doses containing the age-appropriate HA dose were needed for children <9 years of age [24], and while vaccines containing 15 μg of HA were immunogenic in persons ≥9 years old, high-dose vaccines containing 60 μg of HA elicited superior Ab responses among adults infected with human immunodeficiency virus [25]. High-dose seasonal IIV is available for use in persons who are ≥65 years old and stimulates Ab responses that are superior to those stimulated by standard-dose IIV [26]. High-dose vaccines have been shown to be more efficacious than standard-dose vaccines in elderly individuals [27]. Additional studies will be needed to define immunization needs for these and other vulnerable populations.
STUDY GROUP MEMBERS
Additional members of the H3N2v Study Work Group are as follows: Robert L. Atmar and Hana M. El Sahly (Baylor College of Medicine), Ali H. Ellebedy and Colleen Kelley (Emory University School of Medicine), and K. M. Edwards and J. E. Crowe Jr (Vanderbilt University).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants who made this study possible, participating faculty and staff (Nanette Bond, Kathy Bosworth, Janet Brown, Jess Banay, Coni Cheesman, Tracey Lanford, Flor Munoz, Janet Wells, and the BCM Vaccine Research Center staff [Baylor College of Medicine]; Barb Carste, Maya Dunstan, Angel Mathis, Mihir Parikh, C. Hallie Phillips, Alyssa Spingola, Pat Starkovich, and Janice Suyehira [Group Health Research Institute]; Allison Beck, Karen Mask, Mary Bower, Eileen Osinski, Nayoka Rimann, Pamela Turner, DongLi Wang, and the Hope Clinic staff [Emory University School of Medicine]; Jack Stapleton, Regina Won, Nancy Wagner, Geraldine Dull, Necole Gerot, Mary Reidy, Dan Zhao, and Ellen Segar [University of Iowa and Iowa VA Health System]; James C. Slaughter [Vanderbilt University]; and Megan McDonough and Fenhua He [EMMES Corporation]), members of the safety monitoring committee (Robert Salata, Cody Meissner, Jeanne Sheffield, and Michael Spigarelli), the independent safety monitors (Stephen Greenberg and Anoop Agrawal [Baylor College of Medicine], Sascha Dublin and David Arterburn [Group Health Research Institute], David Rimland and Bruce Ribner [Emory University School of Medicine], and Jeff Meier [University of Iowa and Iowa VA Health System], and our colleagues at Division of Microbiology and Infectious Diseases; National Institutes of Health (NIH) (Wendy Buchanan, Soju Chang, Linda Lambert, Suzanne Murray, Valerie Riddle, and David Spiro).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (contracts HHSN272200800002C [to Baylor College of Medicine], HHSN272200800004C [to Group Health Research Institute], HHSN272200800005C [to Emory University School of Medicine], HHSN272200800008C [to the University of Iowa and Iowa VA Health System], HHSN272200800006C [to the University of Cincinnati], HHSN272200800007C [to Vanderbilt University], and HHSN272200800013C [to the EMMES Corporation]) and the National Center for Advancing Translational Sciences (award UL1TR000454), NIH; and the Georgia Research Alliance.
Potential conflicts of interest. J. E. C. is a member of the scientific advisory boards of Enumeral, PaxVax, and Compuvax. K. M. E. is a member of a data and safety monitoring board for Novartis and receives research support from Novartis for an unrelated study. M. J. M. serves on a safety monitoring committee for VaxInnate and receives a personal fee for this service. M. M. M. provides laboratory support for Sanofi Pasteur. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.CDC. Update: influenza A (H3N2)v transmission and guidelines—five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–4. [PubMed] [Google Scholar]
- 2.CDC. Swine-origin influenza A (H3N2) virus infection in two children—Indiana and Pennsylvania, July–August 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1213–5. [PubMed] [Google Scholar]
- 3.CDC. Limited human-to-human transmission of novel influenza A(H3N2) virus—Iowa, November 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1615–7. [PubMed] [Google Scholar]
- 4.Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindstrom S. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Outbreak of influenza A (H3N2) virus among persons and swine at a county fair-Indiana, July 2012. MMWR Morb Mortal Wkly Rep 2012; 61:561. [PubMed] [Google Scholar]
- 7.CDC. CDC reports cases 18–29 of H3N2v virus infection; continues to recommend interim precautions when interacting with pigs. http://www.cdc.gov/flu/spotlights/h3n2v_us_cases.htm Accessed 14 July 2014.
- 8.CDC. Case count: detected U.S. human infections with H3N2v by state since August 2011. http://www.cdc.gov/flu/swineflu/h3n2v-case-count.htm Accessed 16 September 2014.
- 9.CDC. Antibodies cross-reactive to influenza A (H3N2) variant virus and impact of 2010–2011 seasonal influenza vaccine on cross-reactive antibodies-United States. MMWR Morb Mortal Wkly Rep 2012; 61:237–41. [PubMed] [Google Scholar]
- 10.Skowronski D, De Serres G, Janjua N, et al. Cross-reactive antibody to swine influenza A(H3N2) subtype virus in children and adults before and after immunisation with 2010/11 trivalent inactivated influenza vaccine in Canada, August to November 2010. Euro Surveill 2012; 17:20066. [DOI] [PubMed] [Google Scholar]
- 11.Skowronski DM, Janjua NZ, De Serres G, et al. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v). J Infect Dis 2012; 206:1852–61. [DOI] [PubMed] [Google Scholar]
- 12.Chen WH, Winokur PL, Edwards KM, et al. Phase 2 assessment of the safety and immunogenicity of two inactivated pandemic monovalent H1N1 vaccines in adults as a component of the U.S. pandemic preparedness plan in 2009. Vaccine 2012; 30:4240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Jacobson DL, Ashworth LA, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol 2009; 104:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A(H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlaudecker EP, Steinhoff MC, Omer SB, et al. IgA and neutralizing antibodies to influenza A virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One 2013; 8:e7086714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111–22. [DOI] [PubMed] [Google Scholar]
- 17.Crotty S, Feigner P, Davies H, Glidewell J, Villareal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 2003; 171:4969–73. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration (FDA). Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Silver Spring, MD: FDA, 2007. [Google Scholar]
- 19.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol 1983; 37:529–49. [DOI] [PubMed] [Google Scholar]
- 20.Talaat KR, Luke CJ, Khurana S, et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 2014; 209:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waalen K, Kilander A, Dudman SG, Ramos-Ocao R, Hungnes O. Age-dependent prevalence of antibodies cross reactive to the influenza A(H3N2) variant virus in sera collected in Norway in 2011. Euro Surveill 2012; 17:pii:20170. [PubMed] [Google Scholar]
- 22.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess K. Efficacy of repeated annual immunization with inactivated influenza virus vaccine over a five year period. Vaccine 1997; 15:1114–22. [DOI] [PubMed] [Google Scholar]
- 23.Levine M, Beattie BL, McLean DM, Corman D. Characterization of the immune response to trivalent influenza vaccine in elderly men. J Am Geriatr Soc 1987; 35:609–15. [DOI] [PubMed] [Google Scholar]
- 24.Frey SE, Bernstein DI, Gerber MA, et al. Safety and immunogenicity of 2009 H1N1 vaccine in children. J Infect Dis 2012; 15:1–10. [Google Scholar]
- 25.El Sahly HM, Davis C, Kotloff K, et al. Higher antigen content improves the immune response to 2009 H1N1 influenza vaccine in HIV-infected adults: a randomized clinical trial. J Infect Dis 2012; 205:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keitel WA, Atmar RL, Cate TR, et al. Safety of high-doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–7. [DOI] [PubMed] [Google Scholar]
- 27.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.