Abstract
Hia is a major adhesin of nontypeable Haemophilus influenzae (NTHi) and has long been investigated as a vaccine candidate. Here we show that Hia phase variation is controlled by changes in the length of a polythymidine tract located in the hia promoter. Studies of an invasive clinical isolate (strain R2866) show that strains expressing high Hia levels are more efficiently killed by opsonophagocytosis. An opsonophagocytic assay was used to select for a subpopulation of variants that expressed a low level of Hia, which facilitated their escape from killing by anti-Hia antisera. Conversely, a subpopulation of variants expressing a high level of Hia was selected for during passaging through Chang cells. In both cases, phase variation of Hia expression corresponded directly with discrete modal changes in polythymidine tract length. In the chinchilla model of NTHi infection, we observed consistent selection for high Hia expression upon nasopharyngeal colonization, confirming the key role of phase-variable expression of Hia within a specific niche in vivo.
Keywords: Haemophilus, phase variation, adhesion, colonization
Nontypeable Haemophilus influenzae (NTHi) is a human-adapted pathogen and major cause of otitis media [1], community-acquired pneumonia [2], and chronic obstructive pulmonary disease (COPD) exacerbations [3]. Every year, cases of chronic secretory otitis media occur in 65–330 million children, 60% of whom experience associated hearing loss. In the United States alone, each year there are approximately 25 million episodes of acute otitis media and >13 million antibiotic prescriptions, with public health costs estimated to be $3–$5 billion [4, 5]. According to World Health Organization estimates, 65 million people have moderate to severe COPD. Over 3 million people died of COPD in 2005, which corresponded to 5% of all deaths globally [6]. There is currently no effective vaccine against NTHi.
Several major NTHi outer-membrane proteins are virulence factors that belong to the autotransporter superfamily [7]. Autotransporter superfamily proteins are characterized by a large, barrel-like C-terminal domain that inserts into the outer-membrane, forming a pore or channel through which the functional part of the protein passes to reach the extracellular environment [8, 9]. Many autotransporters expressed by gram-negative bacteria are being investigated as vaccine candidates [10, 11]. Several autotransporters of NTHi are currently being investigated as potential vaccine antigens, including IgA protease [12] and the adhesins Hsf [13], Hap [14], and Hia [15–17]. Hia is expressed by approximately 25% of all NTHi strains [18]. Strains that do not produce Hia express the adhesin HMW [19].
Many NTHi surface proteins and glycans are subject to high-frequency reversible switching of gene expression, called “phase variation”. This process occurs for many key bacterial virulence factors, including iron-acquisition genes [20, 21], pili [22], and LOS biosynthetic genes [23, 24]. In most cases, this switching is mediated by slipped-strand mispairing across simple-tandem DNA repeats during replication, altering the number of repeats. If these DNA repeats are in an open reading frame or a key promoter region, these changes alter gene expression. Our analysis of the hia promoter shows that a large polythymidine (poly-T) tract is present that could potentially result in a phase-variable mode of gene regulation.
In this study, the phase-variable expression of Hia is investigated by studying changes in the poly-T tract located in the hia promoter. The roles that phase-variable expression of Hia plays in the interaction between NTHi and the host are investigated using in vitro and in vivo model systems.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
NTHi strain R2866 was originally isolated from a child with sepsis [25] and was the first NTHi strain shown to contain the modA10 allele [26]. NTHi was grown on brain heart infusion agar (BHI; oxoid) supplemented with hemin (1%) and NAD (2 µg/mL; sBHI) at 37°C in an atmosphere containing 5% (v/v) CO2. Derivatives of NTHi strain R2866 expressing high and low levels of Hia were identified by Western blotting and confirmed by iTRAQ 1D mass spectrometry (APAF, Sydney; Atack and Jennings, unpublished data). The ON/OFF status of modA10 was assessed using the primer pair 6 carboxyfluorescein (FAM)–labeled Him1 and Him3 in GoTaq polymerase chain reactions (PCRs) according to manufacturer's instructions, and fragment length was analyzed as described previously [26].
RNA Isolation and Quantitative Reverse Transcription–PCR (RT-qPCR)
Triplicate cultures of NTHi R2866 HiaON and HiaOFF were grown in sBHI to mid-log phase, pooled, and harvested, and RNA was isolated using the Qiagen RNEasy kit, with contaminating DNA (cDNA) removed using NEB RNAse-free DNAse I and RNA cleaned using the RNEasy kit, all according to manufacturer's instructions. Complementary DNA was synthesized using NEB Protoscript II and random hexamers (Invitrogen; 50 ng/µL) according to manufacturer's instructions. Reverse transcriptase reactions lacking Protoscript II were performed as a negative control. qPCR was performed using LuminoCT SYBR green 2× master mix with 5 µL of cDNA diluted 1:10 as a template, according to the manufacturer's instructions (Sigma-Aldrich), and with primers HiaRT-F and HiaRT-R (for the hia transcript) and 16S-F and 16S-R (for the 16S ribosomal RNA [rRNA] transcript). A Biorad CFX96 Touch Real-Time PCR Detection System was used to perform reactions (initial denaturation at 95°C for 2 minutes, followed by 40 cycles at 95°C for 30 seconds and 60°C for 1 minute; SYBR green was quantified after each cycle). The amount of hia RNA in the HiaON strain versus that in the HiaOFF strain was quantified using relative quantification against the 16S rRNA gene by means of the 2ΔΔCT relative quantification method.
Poly-T Tract Fluorescent PCR and Fragment Analysis
PCR over the poly-T tract was performed using the primers poly-T–F(FAM) and poly-T–R, with the former primer labeled with FAM to allow quantification and sizing using Genescan fragment analysis methods. GoTaq reactions (30 cycles of annealing at 50°C for 30 seconds and extension at 72°C for 20 seconds). Primers are listed in Supplementary Table 1.
Western Blotting
Blots were performed as described [15], or with slight modifications. Briefly, bacteria were recovered from 1-day old sBHI plates, and OD600 normalized to 1.0 in 1× phosphate-buffered saline (pH 7.5). Samples were run on 4%–12% Bis-Tris gels (Novex). Protein was transferred to nitrocellulose membrane (BioRad) at 30V for 70 minutes, and blocked in 5% (w/v) skim milk in 1× Tris-buffered saline with Tween 20 at 4°C overnight with shaking. Blots were probed with primary anti-Hia antibody 1F4 [15] at a dilution of 1:1000. Secondary goat anti-mouse-alkaline phosphatase conjugate antibody (Sigma Aldrich) was added at 1:2500.
Whole-Cell Enzyme-Linked Immunosorbent Assays (ELISAs)
ELISAs were performed using standard protocols [27] in 96-well Maxisorb plates (NUNC; Thermo Scientific). Cells were diluted to an OD600 of 0.2 (approximately 2 × 108 colony-forming units [CFU]/mL), with 50 µL added per well. All strains were assayed in triplicate. Primary antibody 1F4 was used at a starting concentration of 1:10 000. Secondary antibody (goat anti-mouse horseradish peroxidase conjugate; Sigma Aldrich) was used at a concentration of 1:10 000.
Opsonophagocytic Killing Assays
Bacterial growth, HL-60 growth and differentiation, and opsonophagocytic killing assays were performed as described elsewhere [17]. Percentage killing at each serum dilution was calculated by determining the ratio of the bacterial colony count at each dilution to that of the complement-only control.
Chang Passage of Bacterial Strains
NTHi R2866 strains were serially passaged in Chang cells as described previously [28], with samples collected at appropriate points for Western blotting and poly-T tract fragment analysis, as described above.
Chinchilla Model of NTHi-Induced Otitis Media
Adult chinchillas (Chinchilla lanigera) weighing 500–700 g were acclimated in the vivarium for 7–10 days prior to beginning the study. Two separate studies were performed. The first cohort included 3 chinchillas per cohort. The second study included 5 chinchillas per cohort. Chinchillas were established and then challenged intranasally with NTHi strain R2866 HiaON or NTHi strain R2866 HiaOFF at a challenge dose of approximately 1 × 108 CFU. Delivered doses were confirmed by dilution plate counts of inocula on chocolate agar. The animals were then monitored daily via video otoscopy for 21 days.
On days 2, 4, 7, 10, 14, 18, and 21 after challenge, nasopharyngeal lavage was performed as described previously [29] for the collection of samples. Ten-microliter aliquots of samples were placed in a sterile tube on ice. These aliquots were used for evaluation of the CFU of NTHi/mL sample. Remaining samples were immediately centrifuged (at 4°C for 3 minutes), supernatants were carefully removed, and the pellets were snap frozen in liquid nitrogen and stored at −80°C. PCR analysis of the poly-T tract was performed as described above with all samples containing at least 1 × 103 CFU/mL.
As regards animal treatment and handling, all protocols were approved by the Nationwide Children's Hospital Institutional Animal Care and Use Committee, in accordance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals.
RESULTS
Hia Expression Is Independent of the modA10 Phasevarion and Controlled by a Poly-T Tract in the Promoter
The phasevarion (derived from “phase-variable regulon”) is an epigenetic regulatory system first described in H. influenzae [30] and subsequently described in several human adapted pathogens [31–33]. In these systems, the random switching of DNA methyltransferases leads to differential genome-wide methylation patterns, causing global changes in gene expression. There are many distinct phasevarions in H. influenzae [26, 34]. Investigations into the modA10 phasevarion of NTHi strain R2866, an invasive disease isolate [25] showed that the adhesin Hia was 11.5 fold more abundant in modA10ON vs OFF strains (Supplementary Figure 1; Atack and Jennings, unpublished data). To confirm that hia expression was part of the modA10 phasevarion, strains in the alternate methylation states were reisolated from the wild-type stock of R2866. These modA10ON and modA10OFF strains showed no difference in hia expression levels (Supplementary Figure 1), indicating that an independent system was controlling Hia phase variation.
The hia coding region was examined in more detail, and as previously noted [35] a long poly-T tract was present in the intergenic regions to the 5′ end of the hia gene (Figure 1A). The genome of NTHi strain R2866 (accession number CP002277) indicates that the strain expressing high levels of Hia protein (HiaON) contained 34 T residues in the poly-T tract. Resequencing and further analysis revealed that the strain that expressed very low levels of Hia (HiaOFF) contained 31 T residues in this poly-T tract. Whole-cell ELISA showed a 32-fold difference in titer between HiaON (titer 1:640 000) and HiaOFF (titer, 1:20 000). To determine whether the changes in Hia protein expression corresponded with decreased expression of hia at the level of transcription, RT-qPCR analysis was performed using RNA isolated from HiaON and HiaOFF strains. This analysis showed a mean fold-increase (±SD) of 13.98 ± 3.38 in the number of hia transcripts in HiaON, compared with the number in HiaOFF. We therefore conclude that hia phase variation is controlled at the level of transcription and that the observed variation in length of the hia poly-T tract impacts the efficiency of transcription initiation at the hia promoter.
Figure 1.
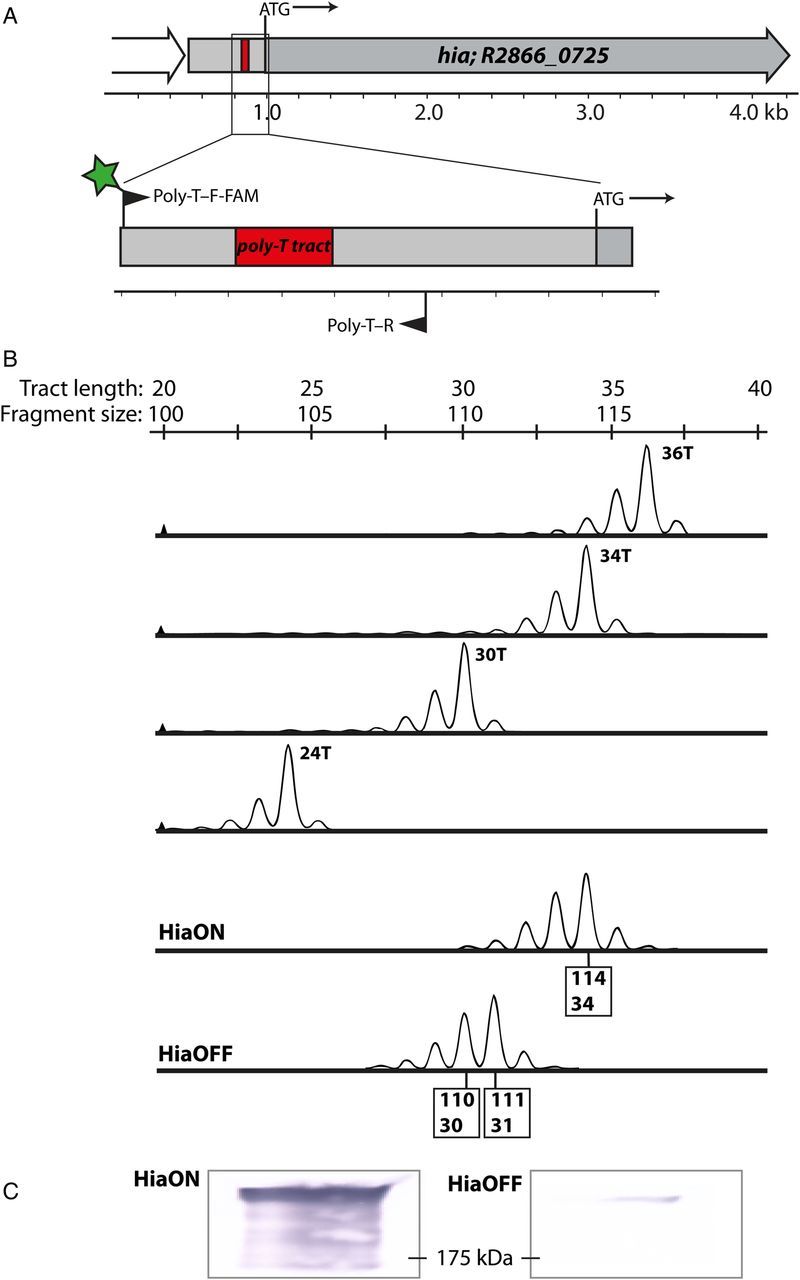
Identification of poly-T tract in the hia promoter and design and validation of a fluorescent polymerase chain reaction (PCR) technique to monitor changes. A, Shown are a large (34 T in HiaON) poly-T tract in the promoter region of the hia gene and the location of primers designed to map this tract (6 carboxyfluorescein [FAM]–labeled poly-T–F and poly-T–R). B, Results of FAM-labeled PCR and fragment analysis, using purified standards containing a known number of T residues in the tract to validate this PCR method. The sequence of the standards matches the sequence of the promoter containing the poly-T tract amplified by primer pair poly-T–F-FAM and poly-T–R, with each containing the specified number of T residues. The bottom 2 panels show results of mapping the poly-T tract in nontypeable Haemophilus influenzae strains R2866 HiaON and HiaOFF, with sizes of major fragments corresponding to the number of T residues. C, Western blots from samples used to make the genomic DNA for HiaON and HiaOFF samples in panel B, confirming that changes in tract length match differences in Hia protein levels. The molecular weight size marker is shown at 175 kDa.
The Poly-T Tract Length Can Be Measured Using a Fluorescent PCR Method
As levels of hia transcription and the amount of Hia protein appeared to be influenced by the length of the poly-T tract, a rapid method for quantifying the length of this poly-T tract was developed so that the precise relationship between Hia expression levels and poly-T tract length could be established. It is not possible to directly sequence over such long homopolymeric tracts and accurately quantify the number of residues present from the resulting sequencing data, owing to polymerase slippage [36]. To solve this problem, a fluorescently labeled primer pair was used to amplify the poly-T tract region and the product analyzed to determine the size of the tract, using fragment-length analysis. The method was validated using synthetic oligonucleotide standards, each containing a different number of T residues in the poly-T tract, because, as noted above, polymerases are error prone when replicating long homopolymeric tracts [36, 37]. PCR was performed over this tract using a set of synthetic oligonucleotide templates containing 24, 30, 34, and 36 T residues each (oligonucleotides poly-T24, poly-T30, poly-T34, and poly-T36, respectively; Supplementary Table 1). Analysis of the PCR products revealed evidence of slippage during PCR cycles, with each length showing a bias towards error of n − 1 (where n is the number of T residues in the poly-T tract). However, the major product for each synthetic oligonucleotide corresponded to the template (Figure 1B). To further validate this method, purified genomic DNA samples were extracted from the HiaON and HiaOFF strains, as well as from independently isolated modA10ON and OFF strains (Supplementary Figure 1). This analysis showed that HiaON contained 34 T residues and that the HiaOFF strain contained 31 T residues, confirming the sequencing data (Figure 1B). This difference resulted in a large difference in Hia protein levels (Supplementary Figure 1 and Figure 1C), confirming that protein levels of Hia correlated with poly-T tract length and that this method is applicable to monitoring poly-T tract length irrespective of the slight error introduced during PCR amplification (Figure 1).
Expression of hia Is Selected Against in Hia-Dependent Opsonophagocytic Killing Assays
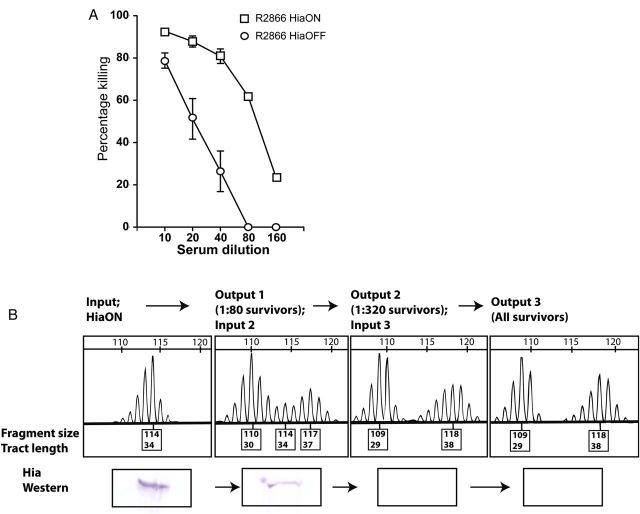
Opsonophagocytic killing has been shown to be important in the immune response to NTHi [38–40]. To determine the selective pressure for Hia, phase-variation assays were conducted to assess the impact of differences in Hia protein on adherence and as a target for immune killing. Previous work has shown that Hia is highly immunogenic and that its expression leads to significant anti-Hia antibody–mediated opsonophagocytosis [15]. The impact of Hia phase variation on the rate of opsonophagocytic killing with anti-Hia sera was examined. Assays using the NTHi R2866 HiaON and HiaOFF strains showed that increased Hia levels led to the killing of HiaON bacteria by Hia antisera at lower titers than those for HiaOFF bacteria (Figure 2A). The HiaON strain was then subjected to multiple rounds of opsonophagocytic killing to select against hia expression. Round 1 used anti-3248 Hia antiserum, with rounds 2 and 3 using the more potent anti-11 Hia antiserum, which leads to higher killing at lower titers [17]. Inputs and survivors from each round were screened using fluorescent PCR and Western blotting (Figure 2B). A clear selection away from tract length permissive to hia expression and toward those nonpermissive for hia expression was observed (Figure 2B and Supplementary Figure 2). No killing or switch to the HiaOFF expression state was evident in assays in which either complement or anti-Hia sera were excluded (Supplementary Figure 2). This is consistent with the hypothesis that poly-T tract length controls hia expression levels and that the natural variants in the population with reduced Hia protein levels can be selected for when a suitable pressure (opsonophagocytic killing) is applied. It appears that tract length can mutate either way (longer or shorter) to reduce hia expression and facilitate escape from opsonophagocytic killing (Figure 2B). Survivors from multiple rounds of opsonophagocytic killing showed a shift from a major peak giving 34 T (input 1; HiaON) to gradually sharper peaks of around 30 and 37 T (output 1; input 2) and around 29 and 38 T (output 2; output 3; Figure 2B). This indicates that the lowest Hia protein levels, and therefore the biggest advantage in escaping killing, correlates with a tract length of 29 or 38 T residues; conversely, maximal Hia protein levels correspond to a poly-T tract length of 34 or 35 T residues. Therefore, a shift from maximal hia expression (34/35 T residues) to minimal hia expression (29 T or 38 T residues) results from the DNA double helix rotating approximately half a turn around the poly-T tract as it shortens or lengthens from the optimal length of 34/35 T residues. This implies that an alignment of elements on either side of the poly-T tract likely occurs to give maximum hia expression.
Figure 2.
Opsonophagocytic killing assays. A, Opsonophagocytic killing of nontypeable Haemophilus influenzae strains R2866 HiaON and HiaOFF. At all dilutions of sera, P ≤ .0001 (by the Student t test). B, Results of multiple rounds of opsonophagocytic killing using R2866 HiaON as the initial input. Survivors from the 1:80 dilution of anti-3248 Hia sera in round 1 were selected as the input for round 2, using anti-11 Hia antisera. The 1:320 survivors from round 2 were then selected as the input for round 3, also using anti-11 Hia antisera. Poly-T tract polymerase chain reactions and Western blots of inputs and survivors were performed as described in “Materials and Methods” section. Full bacterial counts from each round can be located in Supplementary Table 2.
Serial Passage in Chang Cells Selects for Increased Hia Protein Levels
Because Hia is a well-characterized NTHi adhesin [15, 19], we investigated whether the population could be pushed in the reverse direction by selecting for the subpopulation with poly-T tract lengths that are permissive to higher Hia protein levels by using Chang adherence assays. NTHi strain R2866 HiaOFF was serially passaged in Chang cells to select for adherent individuals, which were used as the input for the next round. At each cycle of the process, the Hia expression level and poly-T tract length were assessed. After 3, 6, and 9 serial passages, the tract length had consistently switched to contain a mixture of 32 and 33 T residues (Figure 3A) and matched an increase in Hia protein level in Western blots (Figure 3B). Survivors from 9 serial Chang passages (HiaOFF-Chang) that were subjected to Hia-mediated opsonophagocytic killing showed significantly increased levels of susceptibility to killing at all anti-sera dilutions, compared with HiaOFF (Figure 3C).
Figure 3.
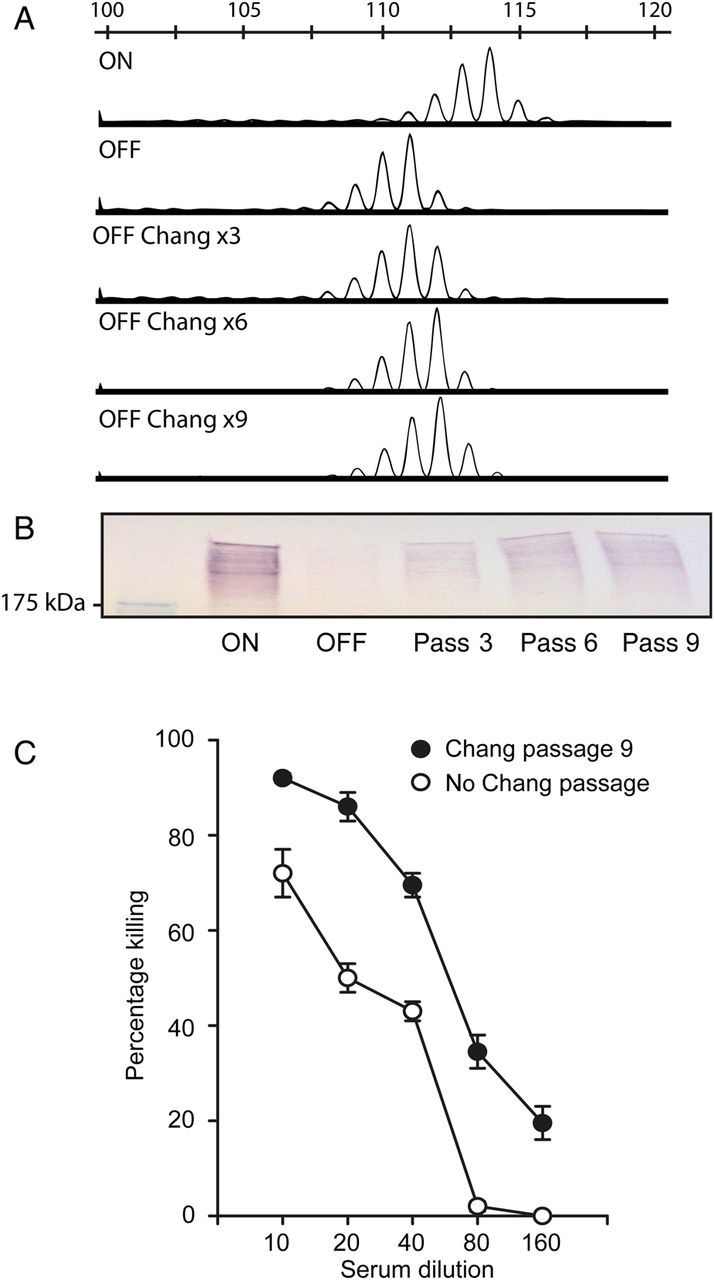
Chang passaging of nontypeable Haemophilus influenzae (NTHi) strain R2866 HiaOFF. A, Poly-T polymerase chain reaction (PCR) fragment analyses of multiple rounds of Chang adherence assays were performed using NTHi strain R2866 HiaOFF, and the size of the poly-T tract and Hia protein levels were probed as described in “Materials and Methods” section. B, Western blots using the 3, 6, and 9 passaged outputs. C, R2866 HiaOFF Chang × 9 passed strain (HiaOFF-Chang) was then used in an opsonophagocytic killing assay with R2866 HiaOFF as a reference. P = .0001, by the Student t test, at all titrations.
Colonization of the Chinchilla Nasopharynx Selects for hia Expression
In vitro evidence presented above suggests that Hia protein levels change depending on the selective pressure applied. To investigate whether Hia expression is selected for in vivo, the well-characterized chinchilla model of NTHi infection [41] was used. Chinchillas were infected intranasally with strains R2866 HiaON and R2866 HiaOFF at inocula of 1 × 108 bacteria, and nasopharyngeal lavage samples were collected 2, 4, 7, 10, 14, 18, and 21 days after infection. The uncultured samples were snap frozen in liquid nitrogen so the bacterial population represented the exact state at the time and site of sampling. Analysis of the poly-T tract length in samples with sufficient CFU to permit amplification showed that animals infected with strain R2866 HiaON retained a poly-T tract length that correlated with high levels of Hia protein in the chinchilla nasopharynx (Figure 4; the full data set for output samples from animals infected with strain HiaON appears is in Supplementary Figure 3). The animals infected with strain R2866 HiaOFF (Figure 4) showed a consistent selection away from a low Hia poly-T tract length to a tract length that corresponded to high levels of Hia protein (Figure 4). This switch was evident from day 2 after initial challenge in all samples analyzed. Therefore, Hia is required for colonization of the chinchilla nasopharynx, confirming its role in adherence and host colonization. There was no correlation between Hia expression levels and CFU plate counts (Supplementary Table 2).
Figure 4.
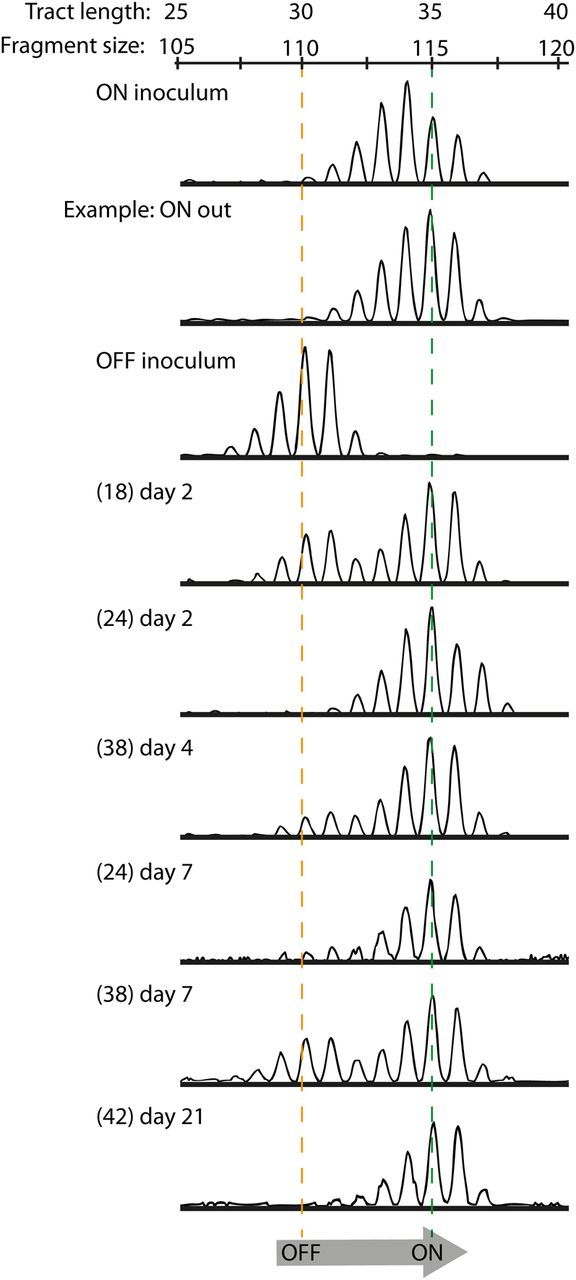
In vivo assay for hia expression levels in a chinchilla model. Nontypeable Haemophilus influenzae (NTHi) strain R2866 HiaON and HiaOFF were used to infect chinchillas at a colony-forming unit dose of approximately 1 × 108, with samples collected from the nasopharynx on days 2, 4, 7, 10, 14, 18, and 21 after infection. Plate counts were enumerated by plating 10 µL of each sample (full plate counts for the 3- and 5-animal cohorts are shown in Supplementary Table 2), with the remaining sample snap frozen so that bacterial cultures represented the state at the time and site of sampling. Polymerase chain reaction analysis of poly-T fragments was performed to characterize phase variation in the poly-T tract length relative to the input strains. All HiaON-infected animals were colonized by strains containing the same number of T residues (34 or 35) that would give high levels of Hia protein (ON inoculum; only a single example [ie, ON out] of an output from an animal infected with HiaON is shown; the full data set is in Supplementary Figure 2). All animals infected with HiaOFF (OFF input) showed a clear switch from 31 T residues in the input to 35 T residues in all output samples, which would correlate with a switch from low to high Hia protein levels. Numbers of animals from which samples could be analyzed from HiaOFF input are shown in brackets, with the day of sample collection shown on each individual trace (the nasopharyngeal lavage label was only used in the Supplementary Figure 3).
DISCUSSION
This work is the first to show that a phase-variable mode of regulation controls hia expression levels in NTHi. The development and validation of a PCR-based method for quantification of the poly-T tract located in the hia promoter allowed us to rapidly analyze tract length changes and to directly correlate these to changes in Hia protein levels. The results of our poly-T tract mapping also showed that, in the population of cells, there exists natural variation in poly-T tract length, with our approach giving an accurate snapshot of the dynamic that exists within the population.
Using a highly specific opsonophagocytic killing assay, we selected against high levels of Hia protein and confirmed a perfect correlation between changes in Hia protein level and changes in the poly-T tract. Multiple rounds of opsonophagocytic killing showed that it is possible to push the bacterial population to a state whereby it is not killed by Hia antiserum because it no longer expresses enough Hia to mediate killing. The loss of Hia protein is directly related to poly-T tract length, confirming that the bacterial population must contain a high level of tract length variation and that appropriate selective pressures will select for individuals best suited to prevailing conditions. We could select for both positive and negative changes in poly-T tract length—we selected for poly-T tract lengths that gave low Hia protein levels, using opsonophagocytic killing; and, conversely, we pushed the poly-T tract to lengths that were permissive to higher hia expression by placing adherence-associated selective pressure on a population of cells. The levels of Hia protein and changes in the tract length achieved using our Chang adherence assay were not as dramatic as that seen with an all-or-nothing killing assay specific for Hia.
The demonstration of selection for poly-T tract lengths that give high Hia levels in an in vivo animal model adds direct evidence of the importance of Hia as a host colonization factor and shows that the selective pressures applied in vitro mirror those experienced in vivo during nasopharyngeal colonization. It is interesting that high levels of Hia were selected for during initial colonization, even though the highly immunogenic Hia protein elicits an immune response [16]. It appears that adherence during initial colonization is more important. Changes in the poly-T tract that give lower Hia protein levels could occur as infection progresses, which would select for individuals who are better equipped to avoid an Hia-primed immune response, such as the one that occurs as bacteria ascend to the middle ear. We speculate that the shortening and lengthening of this repeat tract by removal or addition of T residues causes 2 elements either side of the tract to fall in or out of alignment, leading to positive or negative effects on transcription, similar to a recently described mechanism in Helicobacter pylori that controls expression of a sialic acid–binding adhesin [42]. Changes in a heptanucleotide-repeat tract in the promoter region of the NTHi adhesin HMW have been shown to influence expression levels of this protein [43], although decreasing the tract length was associated increased HMW expression, rather than with the cyclic mode of expression seen here with Hia. Many bacterial pathogens also encode surface exposed or secreted proteins that have polynucleotide-repeat tracts in their promoters [35, 44], with changes in the length of these promoter-located polynucleotide tracts and cyclical levels of gene expression well characterized [45–49].
Recent studies have indicated the importance of targeting an NTHi adhesin, type IV fimbriae, as a key protective antigen for NTHi [29, 50], and Hia has been investigated as an NTHi vaccine candidate for several years [15–17]. Hia expression may phase vary to escape an immune response against NTHi, but our data suggest that it is a key factor in initial colonization of the nasopharynx. Recent data also show in vivo selection for phase variation to express another NTHi adhesin, phosphorylcholine-modified LOS, in human subjects [24]. HMW adhesins are also phase-variably expressed and have a well-established role in adhesion [28]. These phase-variably expressed adhesins are generally ruled out of consideration in vaccine development yet are highly conserved surface antigens. Detailed analysis of NTHi infection to identify key stages of where their expression is essential may support the strategy of a combined adhesin vaccine to prevent colonization, and inclusion of multiple adhesins would make this vaccine broadly effective and highly stable.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was funded by the National Institutes of Health (NIH; grant R01 AI 081887 to S. J. B.); the National Institute on Deafness and Other Communication Disorders, NIH (grant R01 DC003915 to L. O. B.); and the National Health and Medical Research Council (program grant 565526 to M. P. J. and project grant 1034401 to M. P. J. and L. O. B.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Haggard M. Otitis media: prospects for prevention. Vaccine 2008; 26(suppl 7):G20–4. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RH. Community-acquired pneumonia: etiology, diagnosis, and treatment. Clin Ther 1988; 10:568–73. [PubMed] [Google Scholar]
- 3.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–65. [DOI] [PubMed] [Google Scholar]
- 4.A.A.P. Diagnosis and management of acute otitis media. Pediatrics 2004; 113:1451–65. [DOI] [PubMed] [Google Scholar]
- 5.Alsarraf R, Jung CJ, Perkins J, Crowley C, Alsarraf NW, Gates GA. Measuring the indirect and direct costs of acute otitis media. Arch Otolaryngol Head Neck Surg 1999; 125:12–8. [DOI] [PubMed] [Google Scholar]
- 6.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–73. [DOI] [PubMed] [Google Scholar]
- 7.Spahich NA, St Geme JW. Structure and function of the Haemophilus influenzae autotransporters. Front Cell Infect Microbiol 2011; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grijpstra J, Arenas J, Rutten L, Tommassen J. Autotransporter secretion: varying on a theme. Res Microbiol 2013; 164:562–82. [DOI] [PubMed] [Google Scholar]
- 9.Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 2004; 68:692–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comanducci M, Bambini S, Brunelli B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med 2002; 195:1445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier PS, Troller R, Grivea IN, Syrogiannopoulos GA, Aebi C. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine 2002; 20:1754–60. [DOI] [PubMed] [Google Scholar]
- 12.Mistry DV, Stockley RA. The cleavage specificity of an IgA1 protease from Haemophilus influenzae. Virulence 2011; 2:103–10. [DOI] [PubMed] [Google Scholar]
- 13.Watson ME, Jr, Nelson KL, Nguyen V, et al. Adhesin genes and serum resistance in Haemophilus influenzae type f isolates. J Med Microbiol 2013; 62:514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabatabaee A, Siadat SD, Moosavi SF, et al. Overexpression and purification of C-terminal fragment of the passenger domain of hap protein from nontypeable haemophilus influenzae in a highly optimized Escherichia coli expression system. Avicenna J Med Biotechnol 2013; 5:176–85. [PMC free article] [PubMed] [Google Scholar]
- 15.Winter LE, Barenkamp SJ. Antibodies specific for the Hia adhesion proteins of nontypeable Haemophilus influenzae mediate opsonophagocytic activity. Clin Vaccine Immunol 2009; 16:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter LE, Barenkamp SJ. Construction and immunogenicity of recombinant adenovirus vaccines expressing the HMW1, HMW2, or Hia adhesion protein of nontypeable Haemophilus influenzae. Clin Vaccine Immunol 2010; 17:1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter LE, Barenkamp SJ. Antibodies to the HMW1/HMW2 and hia adhesins of nontypeable haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clin Vaccine Immunol 2014; 21:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barenkamp SJ, St Geme JW., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol 1996; 19:1215–23. [DOI] [PubMed] [Google Scholar]
- 19.St Geme JW, III, Kumar VV, Cutter D, Barenkamp SJ. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun 1998; 66:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson AR, Stojiljkovic I. HmbR, a hemoglobin-binding outer membrane protein of Neisseria meningitidis, undergoes phase variation. J Bacteriol 1999; 181:2067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Z, Jin H, Whitby PW, Morton DJ, Stull TL. Role of CCAA nucleotide repeats in regulation of hemoglobin and hemoglobin-haptoglobin binding protein genes of Haemophilus influenzae. J Bacteriol 1999; 181:5865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blyn LB, Braaten BA, Low DA. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. Embo J 1990; 9:4045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox KL, Atack JM, Srikhanta YN, et al. Selection for phase variation of LOS biosynthetic genes frequently occurs in progression of non-typeable Haemophilus influenzae infection from the nasopharynx to the middle ear of human patients. PLoS One 2014; 9:e90505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole J, Foster E, Chaloner K, et al. Analysis of nontypeable Haemophilus influenzae phase variable genes during experimental human nasopharyngeal colonization. J Infect Dis 2013; 208:720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nizet V, Colina KF, Almquist JR, Rubens CE, Smith AL. A virulent nonencapsulated Haemophilus influenzae. J Infect Dis 1996; 173:180–6. [DOI] [PubMed] [Google Scholar]
- 26.Fox KL, Dowideit SJ, Erwin AL, Srikhanta YN, Smith AL, Jennings MP. Haemophilus influenzae phasevarions have evolved from type III DNA restriction systems into epigenetic regulators of gene expression. Nucleic Acids Res 2007; 35:5242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed Cold Spring Harbor, NY: CSH Laboratory Press; 1989. [Google Scholar]
- 28.St Geme JW, Falkow S, Barenkamp SJ. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A 1993; 90:2875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novotny LA, Clements JD, Bakaletz LO. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine 2012; 31:3417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srikhanta YN, Maguire TL, Stacey KJ, Grimmond SM, Jennings MP. The phasevarion: A genetic system controlling coordinated, random switching of expression of multiple genes. Proc Natl Acad Sci U S A 2005; 102:5547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srikhanta YN, Dowideit SJ, Edwards JL, et al. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog 2009; 5:e1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srikhanta YN, Gorrell RJ, Steen JA, et al. Phasevarion mediated epigenetic gene regulation in Helicobacter pylori. PLoS One 2011; 6:e27569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakeway LV, Power PM, Jen FE-C, et al. ModM DNA methyltransferase methylome analysis reveals a potential role for Moraxella catarrhalis phasevarions in otitis media. FASEB J 2014; 28:5197–207. [DOI] [PubMed] [Google Scholar]
- 34.Gawthorne JA, Beatson SA, Srikhanta YN, Fox KL, Jennings MP. Origin of the diversity in DNA recognition domains in phasevarion associated modA genes of pathogenic Neisseria and Haemophilus influenzae. PLoS One 2012; 7:e32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power PM, Sweetman WA, Gallacher NJ, et al. Simple sequence repeats in Haemophilus influenzae. Infect Genet Evol 2009; 9:216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennings MP, Hood DW, Peak IRA, Virji M, Moxon ER. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol Microbiol 1995; 18:729–40. [DOI] [PubMed] [Google Scholar]
- 37.Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol 1987; 4:203–21. [DOI] [PubMed] [Google Scholar]
- 38.Musher DM, Hague-Park M, Baughn RE, Wallace RJ, Jr, Cowley B. Opsonizing and bactericidal effects of normal human serum on nontypable Haemophilus influenzae. Infect Immun 1983; 39:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen EJ, Hart DA, McGehee JL, Toews GB. Immune enhancement of pulmonary clearance of nontypable Haemophilus influenzae. Infect Immun 1988; 56:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Chen J, Cheng Z, Robbins JB, Battey JF, Gu XX. Biological activities of antibodies elicited by lipooligosaccharide based-conjugate vaccines of nontypeable Haemophilus influenzae in an otitis media model. Vaccine 2000; 18:1264–72. [DOI] [PubMed] [Google Scholar]
- 41.Bakaletz LO. Chinchilla as a robust, reproducible and polymicrobial model of otitis media and its prevention. Expert Rev Vaccines 2009; 8:1063–82. [DOI] [PubMed] [Google Scholar]
- 42.Åberg A, Gideonsson P, Vallström A, et al. A repetitive DNA element regulates expression of the Helicobacter pylori sialic acid binding adhesin by a rheostat-like mechanism. PLoS Pathog 2014; 10:e1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawid S, Barenkamp SJ, St Geme JW. Variation in expression of the Haemophilus influenzae HMW adhesins: A prokaryotic system reminiscent of eukaryotes. Proc Natl Acad Sci U S A 1999; 96:1077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders NJ, Jeffries AC, Peden JF, et al. Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol Microbiol 2000; 37:207–15. [DOI] [PubMed] [Google Scholar]
- 45.Arhin FF, Moreau F, Coulton JW, Mills EL. Sequencing of porA from clinical isolates of Neisseria meningitidis defines a subtyping scheme and its genetic regulation. Can J Microbiol 1998; 44:56–63. [PubMed] [Google Scholar]
- 46.Cope LD, Lafontaine ER, Slaughter CA, et al. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J Bacteriol 1999; 181:4026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metruccio MME, Pigozzi E, Roncarati D, et al. A novel phase variation mechanism in the meningococcus driven by a ligand-responsive repressor and differential spacing of distal promoter elements. PLoS Pathog 2009; 5:e1000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfau JD, Taylor RK. Genetic footprint on the ToxR-binding site in the promoter for cholera toxin. Mol Microbiol 1996; 20:213–22. [DOI] [PubMed] [Google Scholar]
- 49.Sarkari J, Pandit N, Moxon ER, Achtman M. Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Mol Microbiol 1994; 13:207–17. [DOI] [PubMed] [Google Scholar]
- 50.Novotny LA, Adams LD, Kang DR, et al. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 2009; 28:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.