Abstract
Background. Bacterial vaginosis (BV) is a common polymicrobial disease associated with numerous negative reproductive health outcomes, including an increased risk of human immunodeficiency virus acquisition. BV is treatable with antibiotics, but relapse is common. A more detailed understanding of bacterial dynamics during antibiotic therapy for BV could identify conditions that favor establishment, maintenance, and eradication of BV-associated bacterial species, thereby improving treatment outcomes.
Methods. We used mathematical models to analyze daily quantitative measurements of 11 key bacterial species during metronidazole treatment for 15 cases of BV.
Results. We identified complete reorganization of vaginal bacterial composition within a day of initiating therapy. Although baseline bacterial levels predicted a longer time to clearance, all anaerobic species were eliminated rapidly within a median of 3 days. However, reemergence of BV-associated species was common following treatment cessation. Gardnerella vaginalis, a facultative anaerobe, was cleared more slowly than anaerobic BV-associated species, and levels of G. vaginalis often rebounded during treatment. We observed gradual Lactobacillus species growth, indicating that untargeted microbes fill the transient vacuum formed during treatment.
Conclusions. Under antibiotic pressure, the human microbiome can undergo rapid shifts on a scale of hours. When treatment is stopped, BV-associated bacteria quickly reemerge, suggesting a possible role for intermittent prophylactic treatment.
Keywords: bacterial vaginosis, vaginal microbiota, mathematical modeling, metronidazole, Gardnerella vaginalis, Lactobacillus, qPCR
The human microbiome plays a key role in human health. Diverse microbial metabolic activity maintains ecosystems in anatomic niches throughout the body [1], and deviations in microbial composition may result in a dysbiotic disease state [2]. While therapeutic antibiotics or probiotics recalibrate bacterial communities, the effects of these treatments at the individual species level are less well understood. A clearer understanding of polymicrobial dynamics is needed to optimize microbiome-targeted treatments.
Bacterial vaginosis (BV) is a highly prevalent dysbiotic condition in women [3] that is associated with increased risk for preterm birth [4, 5], pelvic inflammatory disease (PID) [6–8], herpes simplex virus shedding [9], and acquisition of sexually transmitted infections [10–12], including human immunodeficiency virus (HIV) infection [12, 13]. BV is characterized by a depletion of specific Lactobacillus species and increased quantities of numerous anaerobic species, many of which have been described only recently, using molecular techniques [14].
Successful treatment of BV may depend on both elimination of BV-associated anaerobes and stabilization of species associated with a healthy vaginal microbiota [15]. However, the composition of the vaginal microbiota is dynamic [16–19], and temporal analyses are necessary to characterize a healthy state. Previous studies observed compositional changes over weeks [16–18], but individual species' turnover rates over shorter time frames remain unexplored. This information may provide important insights for designing BV treatment strategies.
Here, we develop mathematical models to quantify changes in bacteria concentrations during antibiotic treatment for BV. Similar models have been used to better understand the pathogenesis and treatment of chronic viral infections, such as those due to HIV [20–23], hepatitis C virus [24], and herpes simplex virus [25, 26]. Our models demonstrate rapid changes in most prevalent anaerobic bacteria species. Metronidazole treatment results in a transient microbial vacuum that is often filled by growth of Lactobacillus iners. When treatment is stopped, BV-associated bacteria quickly reemerge, suggesting a possible role for intermittent prophylactic treatment. Our findings highlight that the vaginal microbiota can undergo dramatic changes over extremely narrow intervals.
METHODS
Ethics Statement
Vaginal samples were collected using protocol 1789, which was approved by the institutional review board (IRB) at the Fred Hutchinson Cancer Research Center (approval no.: 5485). All participants provided written informed consent prior to study enrollment. Consent forms were approved by the IRB as part of protocol 1789.
Study Population
The study population comprised 45 women enrolled in a longitudinal study of BV at the Public Health–Seattle and King County Sexually Transmitted Diseases Clinic between March 2007 and March 2010. At enrollment, participants were evaluated for BV by means of the Amsel criteria [27]. They then returned for follow-up evaluation at 1 month. In the intervening period, women obtained vaginal swab specimens daily for 7 days and then on days 14, 21, and 28 (protocol 1), or they performed vaginal swabs daily for 30 days (protocol 2). Diagnosis, sample collection, storage, and processing of swabs are described elsewhere [19].
Among the study population, 12 women were diagnosed with BV by means of the Amsel criteria. Eleven received metronidazole treatment and were therefore included in this study. Among these women, 7 followed protocol 1 and 4 followed protocol 2. In protocol 1, 1 woman was enrolled 3 times and 2 were enrolled twice. We analyzed each episode independently, for a total of 15 enrollments. Because including repeated enrollments might introduce bias, we also performed analyses without repeat enrollments.
Thirteen episodes were treated with topical metronidazole gel (5 g with 37.5 mg of metronidazole) nightly for 5 days [28]. Two participants were treated with oral metronidazole (500 mg) twice daily for 7 days [28]; both were included because sensitivity analysis indicated similar clearance between oral treatment and topical treatment.
DNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
Concentrations of bacterial DNA were measured using qPCR assays targeting 11 key vaginal bacteria: Atopobium vaginae, BV-associated bacterium 1 (BVAB1), BVAB2, BVAB3, Gardnerella vaginalis, Lactobacillus crispatus, Lactobacillus jensenii, L. iners, Leptotrichia/Sneathia species, Megasphaera species, and Mobiluncus species [19, 29]. Hereafter, we classify the non-Lactobacillus groups as “BVAB” for this article because all are significantly associated with BV [29].
Bacterial Suppression and Reemergence
We defined bacterial suppression when the treatment resulted in a decrease of a particular bacteria species below the threshold of qPCR detection during treatment. On the basis of laboratory protocol and bacterial qPCR assay, 4 threshold values were used to establish eradication, depending on the species: counts of 375, 750, 1500, and 3000 16S ribosomal RNA (rRNA) gene copies per swab. We defined reemergence as at least 2 positive qPCR-based detections or 1 high qPCR concentration (>6 log measurement) of a given bacterium any time after suppression occurred (including the remaining treatment period and the 3 observed weeks following treatment cessation).
To determine potential predictors of reemergence, we used logistic regression with reemergence of the specific BV-associated bacteria as the outcome. Only species that were present at baseline and suppressed were included in the analysis. To control for within subject correlation, we used a generalized linear mixed model with random intercepts.
Mathematical and Statistical Modeling of Bacterial Dynamics
Analyses were conducted in R (R Foundation for Statistical Computing, Vienna, Austria) using the intraclass correlation coefficient (ICC), lme4, lmerTest, and plyr add-on packages. Figures were made using the ggplot2 add-on package.
To calculate the effect of metronidazole on BV-associated bacteria, we modeled bacterial DNA concentrations across the treatment period. For each enrollment, we used a monophasic exponential model to describe clearance of BV-associated bacteria DNA over time. To estimate clearance rates, we conducted a linear regression of the natural log of the number of bacterial 16S rRNA gene copies per swab on treatment days. For Lactobacillus species, which exhibited nonlinear patterns of change over treatment time, we fit loess splines to establish general trends.
Total clearance time was defined on the basis of when the BV-associated bacteria were suppressed. If suppression was not reached, the clearance window ended the day after the final day of treatment (day 6 for topical treatment or day 8 for oral treatment) or when a large bacterial rebound occurred (>1 log; see the Supplementary Materials for a discussion on selection of clearance end points). Regression was not performed when suppression occurred within 2 days, owing to the inability to establish a trend.
For each fitted exponential model, the estimated clearance rate was converted into its corresponding log10 estimate and half-life for interpretability. We assessed model fit by calculating r2.
To calculate correlation between clearance rate and initial values, we used the Pearson correlation coefficient to measure overall correlation and the Spearman rank correlation coefficient to measure species-specific correlations.
We compared women who experienced emergence of L. crispatus or L. jensenii to women who did not across several continuous predictors, using the Kolmogorov–Smirnov 2-sample test. We classified emergence as a positive swab sample obtained any time after baseline. Episodes positive for a species at baseline (2 women with L. crispatus and 1 woman with both) were excluded.
Analysis of Clearance Rates
To assess the difference in clearance rates among bacterial groups, we regressed our subject-specific clearance rates on bacterial group, using a linear mixed model with a random intercept to control for within subject variability (see the Supplementary Materials for more details). To evaluate clustering by subjects and bacteria, we calculated an ICC, which describes the proportion of the total variance of an outcome attributable to the between-group (within-variable) variance.
Summed Bacteria Calculation
As a crude estimate of total bacteria, we summed the total bacterial DNA concentrations for Lactobacillus species and BVAB levels for each participant on each treatment day.
RESULTS
Study Participants
We documented bacterial dynamics during metronidazole treatment for 15 treatment episodes in 11 women with BV diagnosed by means of clinical criteria [27] and confirmed by means of the Nugent score [30]. A separate analysis that excluded repeated enrollments is presented in the Supplementary Materials and revealed similar findings overall. Topical metronidazole was used for 13 treatment episodes, and oral metronidazole was used for 2 episodes.
Pretreatment Microbial Characteristics Differ Among Species
Atopobium vaginae, G. vaginalis, and L. iners were the most prevalent species in women before treatment and were present at the onset of each treatment course (Figure 1). The least common bacteria at baseline were L. crispatus and L. jensenii. A mean of 6 BVAB (range, 2–8 BVAB) were present in each episode.
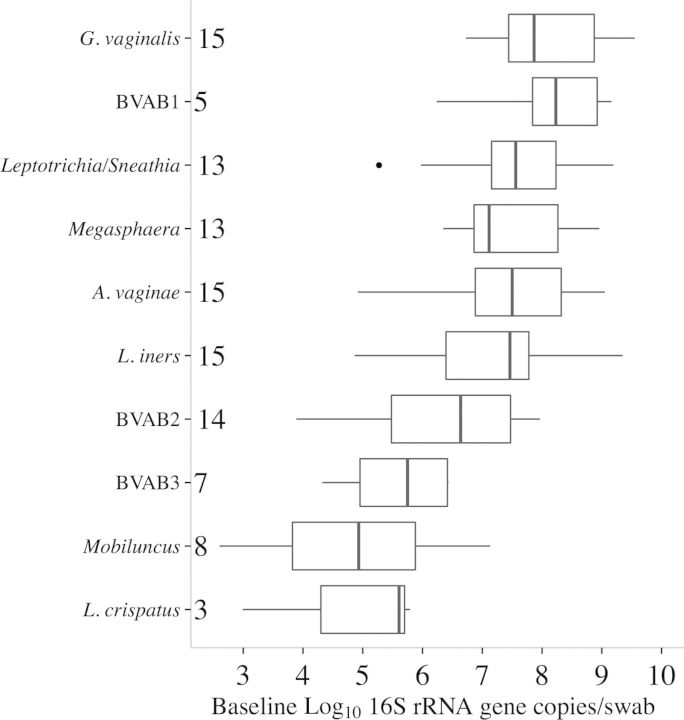
Figure 1.
Pretreatment ranges for bacterial concentration during bacterial vaginosis. Distributions of pretreatment log1016S ribosomal RNA (rRNA) gene copies/swab for each measured bacterium, ordered by increasing mean. Boxes represent the interquartile range (IQR) of the data, the whiskers extend to cover all data within 1.5 times the IQR of the first or third quartile, and solid dots represent outliers. Sample sizes at baseline are given for each species (out of 15 total episodes). Lactobacillus jensenii was removed because of small sample size (n = 1 with a baseline log10 16S rRNA count of 6.40 gene copies/swab). Abbreviations: A. vaginae, Atopobium vaginae; BVAB, BV-associated bacterium; G. vaginalis, Gardnerella vaginalis.
Mean pretreatment concentrations of individual bacteria ranged from 103 to 109 16S rRNA gene copies/swab (Figure 1). Within a given bacterium, baseline value ranges were narrower, generally ranging over 1–2 log units. BVAB1, when present, had the highest mean baseline value. Gardnerella vaginalis levels were uniformly high. L. crispatus had the lowest overall mean and maximum level, while L. iners had high pretreatment levels (Figure 1).
BVAB Suppression Was Rapid and Associated With Baseline Bacterial Levels
With the exception of Leptotrichia/Sneathia species and G. vaginalis, BVAB DNA levels were uniformly driven to levels below the qPCR detection threshold during treatment (Table 1). Leptotrichia/Sneathia species were not eliminated in 2 of 13 treatments; however, in 1 episode, Leptotrichia/Sneathia species were undetectable 2 days after treatment cessation. Gardnerella vaginalis levels reached the threshold of PCR detection during only 3 of 15 treatment sessions.
Table 1.
Effect of Metronidazole Therapy on Bacterial Vaginosis–Associated Bacteria (BVAB) During and After Treatment
| Bacterial Group, Species, or Genus | Clearance |
Reemergence,a Treatments, No. (%) |
||
|---|---|---|---|---|
| Total Treatments, No. (%) | Time, d, Mean | During Treatmentb | Overallc | |
| A. vaginae | 15 (100) | 3.47 | 3 (20) | 9 (60) |
| BVAB1 | 5 (100) | 4.00 | 0 (0) | 1 (20) |
| BVAB2 | 14 (100) | 2.93 | 1 (7.14) | 6 (42.86) |
| BVAB3 | 7 (100) | 1.57 | 0 (0) | 0 (0) |
| G. vaginalis | 3 (20.00) | 4.00 | 1 (33.33) | 3 (100) |
| Leptotrichia/Sneathia | 11 (84.62) | 3.36 | 1 (9.09) | 5 (45.45) |
| Megasphaera | 13 (100) | 3.15 | 0 (0) | 5 (23.08) |
| Mobiluncus | 8 (100) | 2.12 | 1 (12.5) | 2 (25) |
Abbreviations: A. vaginae, Atopobium vaginae; G. vaginalis, Gardnerella vaginalis.
a At least 2 positive results of quantitative polymerase chain reaction (qPCR) or 1 high qPCR-based concentration (>6 log) after suppression occurred.
b Reemergence criteria met before 5-day treatment ended (or, for 2 episodes, before 7-day oral metronidazole treatment ended).
c Reemergence during 4 weeks of observation including treatment.
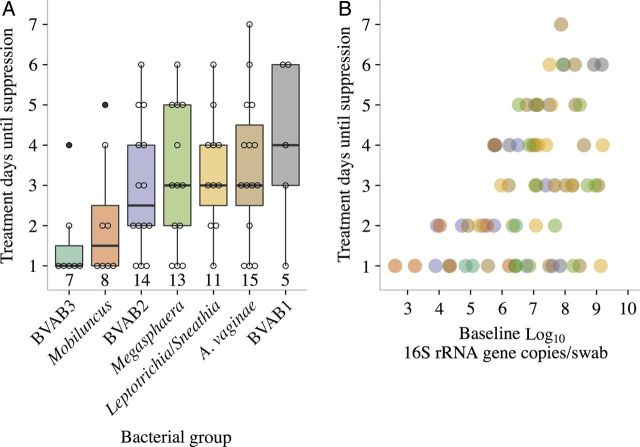
Time until suppression was similar across species (Table 1 and Figure 2A), with the exception of BVAB3. Most suppression occurred rapidly (median time to suppression, ≤4 days). Each species had cases that were cleared in ≤2 days (Figure 2A). However, responses were variable, and some times to suppression exceeded 5 days. Longer times to suppression were associated with higher baseline bacterial loads (Figure 2B). Each log increase in initial bacterial load increased mean clearance time by 0.50 days (95% confidence interval [CI], .19–.81).
Figure 2.
Time until suppression for bacterial vaginosis (BV)–associated bacteria during metronidazole therapy according to pretreatment bacterial concentration. A, Distributions of total treatment days until suppression for each BV-associated bacterium (BVAB). Bacteria are listed in order of increasing pretherapy mean. Boxes represent the interquartile range (IQR) of the data, the whiskers extend to cover all data within 1.5 times the IQR of the first or third quartile, and circles represent raw data. Sample sizes are given below the box plots. B, Scatterplot of log10 baseline counts versus treatment days until bacterial suppression. Gardnerella vaginalis is not included because of low numbers of participants with complete suppression (3 total on days 1, 3, and 8). Colors of dots correspond to colors of box plots in panel A. Abbreviations: A. vaginae, Atopobium vaginae; rRNA, ribosomal RNA.
Reemergence of BVAB Was Common Following Treatment
After treatment cessation, we observed examples of reemergence (detection after suppression within the 28 day observation period) in all species but BVAB3 (Table 1). While reemergence sometimes occurred prior to treatment cessation, it was more frequent after treatment ended. We observed reemergence throughout the 3-week observation period after treatment but most commonly during the first week (Supplementary Figure 1). Gardnerella vaginalis and A. vaginae had the highest reemergence rates. Although G. vaginalis elimination was rare (3 of 15 episodes), all reemerged. Atopobium vaginae was present and eliminated in all 15 episodes, only to reemerge during or after treatment in 10 instances. Excluding G. vaginalis, we observed 5 episodes in which all measured BV-associated bacteria were cleared with no reemergence within 21 days of treatment cessation. Time to suppression, bacterial clearance rates, and presence of Lactobacillus species were not predictive of protection against reemergence (see Supplementary Material).
Exponential Clearance of BVAB Other Than G. vaginalis During Therapy
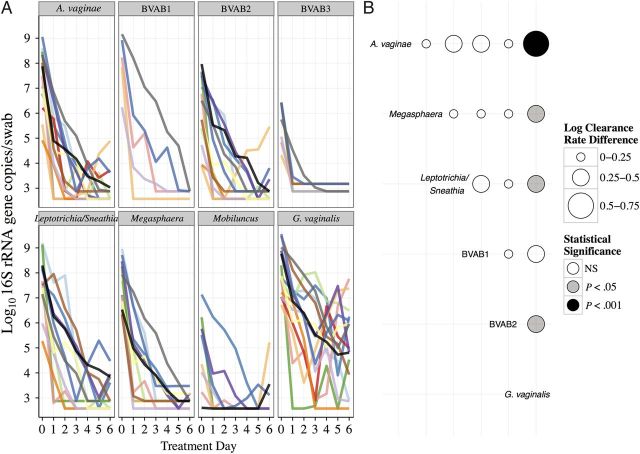
Using an exponential clearance model, we estimated each species' bacterial DNA clearance rate during antibiotic treatment. The mathematical model provided a good fit for all BV-associated bacteria (R2 range, 0.62–0.99; mean R2, >0.80) except G. vaginalis (R2 range, 0.38–0.99; mean R2, 0.77; Table 2). Most BVAB underwent rapid and predictable monotonic clearance (Figure 3A), with mean clearance half-lives ranging from 6 to 14 hours (Table 2). Baseline bacterial levels did not correlate with the clearance rates (Pearson correlation, −0.12; 95% CI, −.37 to .15). Bacterial DNA levels also did not correlate with clearance rates at the single species level. Although at least a 100-fold reduction in G. vaginalis was observed in each episode, responses were more variable than for other species (Figure 3A).
Table 2.
Vaginal Bacteria Dynamics During Metronidazole Treatment
| Bacterial Group, Species, or Genus | Treatments, No. | Clearance Rate,a Mean (Range) | R2, Mean (Range) | Half-life, h, Mean |
|---|---|---|---|---|
| A. vaginae | 11 | 1.43 (0.67–2.37) | 0.88 (0.67–0.99) | 5.94 |
| Megasphaera | 8 | 1.23 (0.76–1.85) | 0.88 (0.65–1) | 6.57 |
| Leptotrichia/Sneathia | 10 | 1.17 (0.63–2.21) | 0.87 (0.64–1) | 7.13 |
| BVAB1 | 4 | 1.1 (0.91–1.52) | 0.8 (0.62–0.99) | 6.85 |
| BVAB3 | 1 | 0.92 | 0.97 | 7.86 |
| BVAB2 | 7 | 1.01 (0.72–2) | 0.93 (0.78–1) | 7.94 |
| Mobiluncus | 2 | 0.81 (0.8–0.83) | 0.91 (0.84–0.97) | 8.87 |
| G. vaginalis | 13 | 0.78 (0.18–1.67) | 0.77 (0.38–0.99) | 13.41 |
Abbreviations: A. vaginae, Atopobium vaginae; BVAB, bacterial vaginosis–associated bacterium; G. vaginalis, Gardnerella vaginalis.
a Log10 16S ribosomal RNA gene copies/swab/day.
Figure 3.
Individual clearance curves and clearance rate comparisons for bacterial vaginosis (BV)–associated bacteria (BVAB) during metronidazole treatment. A, Each color denotes a different participant. Only Gardnerella vaginalis is notable for frequent bacterial rebound. B, Each bubble represents the mean differences in clearance rates (log10 16S ribosomal RNA [rRNA] gene copies/swab/day) between the bacterial group listed to the left and the bacterial group listed below. Rate differences and P values were estimated using a linear mixed model. Atopobium vaginae had the fastest mean clearance rate and G. vaginalis the slowest mean clearance rate, compared with the other bacteria. Abbreviation: NS, not significant.
Clearance Rates Were Similar Across BVAB Species
To further investigate differences among bacterial clearance rates, we fit a mixed model of estimated clearance rates by bacterial group. Atopobium vaginae was cleared the most rapidly among BVAB, and the G. vaginalis clearance rate was slower than that for all other BVAB (Figure 3B). Oral metronidazole treatment did not appear to have a different effect than topical therapy (Figure 4).
Figure 4.
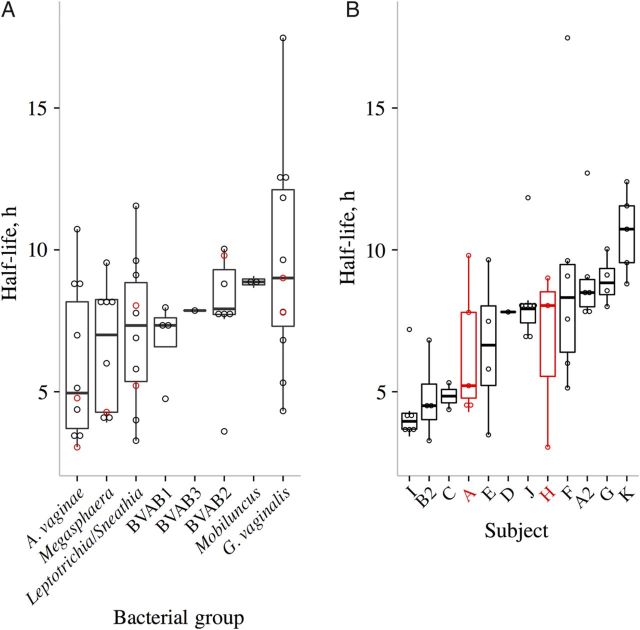
Higher clearance half-life variability within bacterial vaginosis (BV)–associated bacterial (BVAB) species as compared to within study participants. A, Distribution of estimated bacterial clearance half-lives (days) for each BV-associated bacterial group, sorted by increasing median. B, Distributions of half-lives (days) for each participant, arranged by increasing median. Participants who received oral metronidazole treatment are denoted in red. Repeat episodes in the same subject share the same letter. Boxes represent the interquartile range (IQR) of the data, and the whiskers extend to cover all data within 1.5 times the IQR of the first or third quartile. Dots represent each individual clearance half-lives: black denotes topical metronidazole use and red denotes oral metronidazole use. Two Gardnerella vaginalis outliers are not shown (bacterial half-lives of 28 hours for episode G and 41 hours for episode A3). One episode (A3) was removed from panel B because it had only 2 data points, and 2 other episodes (B and C2) were not included owing to a lack of estimated clearance half-lives. Abbreviation: A. vaginae, Atopobium vaginae.
Variability of BVAB Clearance Rates Depend More on Subject-Specific Than Bacteria-Specific Factors
The range of median half-lives was lower among the BVAB (5.13–9.64 hours; Figure 4A) than among the episodes (3.96–10.73 hours; Figure 4B). We calculated ICCs to determine the extent to which clearance rates from the same groups tended to be similar. The ICC is the proportion of overall variance attributable to between-group variance. The ICC for clearance rates was higher across subjects than across species (0.45 vs 0.12), implying that clearance rates cluster more strongly by host. Therefore, although bacterial species explains some variance in clearance, other unmeasured host-specific factors must also be considered.
Five different episodes were associated with clearance of at least 1 species in a single day despite a baseline level of >6 log genomic copies. Because we could not use regression to estimate clearance rates when clearance occurred in 1 or 2 days, we examined the last detectable DNA concentration before suppression as an estimate for clearance rate. We reasoned that if the last positive values for bacteria cleared within 1–2 days are the same as bacteria cleared in >2 days, then differences in clearance times are attributable to lower baseline values. However, the last detectable DNA concentrations were considerably higher for single-day eradications than multiple-day eradications (Supplementary Figure 2), indicating an extremely rapid treatment effect during a subset of treatment episodes. Two women experienced suppression of all BVAB (one with and one without G. vaginalis) within a single day. Both had concurrent growth of L. crispatus and L. jensenii during treatment but did not have distinct initial bacterial profiles. Rapid clearance did not predict long-term suppression: 100% reemergence (7 of 7 species) was observed in the woman who rapidly cleared G. vaginalis, while 50% reemergence (3 of 6 species) was noted in the other woman.
Lactobacillus Species Expand During Treatment
Metronidazole therapy resulted in complete reorganization of the vaginal microbiota in the majority of treated women, with a massive decrease in BVAB quantities (Figure 3A) and emergence of Lactobacillus species (Figure 5). At baseline, only 3 episodes were positive for either L. crispatus or L. jensenii; however, we observed swabs positive for either species in 7 episodes by the end of the treatment period and in 11 episodes by the end of the observation period (28 days; Supplementary Figure 3). By fitting a spline through the data during treatment, we found that average quantities of L. crispatus changed very little during treatment, whereas levels of L. jensenii increased by several log units among the 6 women with this species (Figure 5A). BVAB clearance rate, time until suppression, baseline L. iners levels, baseline BVAB levels, and total BVAB present at baseline were not predictive of Lactobacillus species emergence (Supplementary Materials).
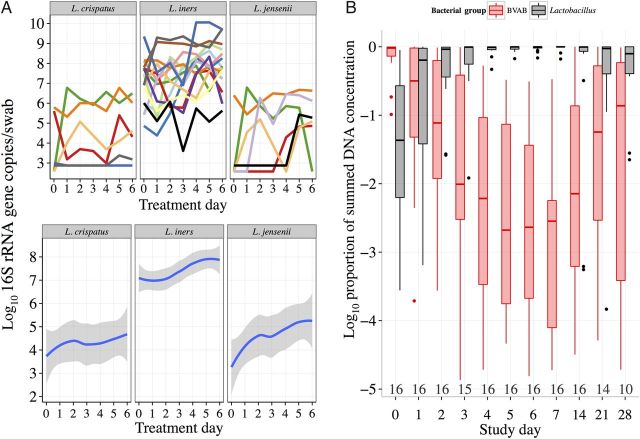
Figure 5.
Lactobacillus species growth during antibiotic therapy. A, Time series plots (top half) for each Lactobacillus species during antibiotic treatment (each color denotes a different participant). Loess splines (bottom half) correspond to the data in the top half of figure. B, Daily distribution of the vaginal microbiota composition (log10 proportion of total bacteria), comparing bacterial vaginosis (BV)–associated bacteria (BVAB; red box plots) to Lactobacillus species (black box plots) during the observation period (sample sizes are depicted below box plots). Boxes represent the interquartile range (IQR) of the data, the whiskers extend to cover all data within 1.5 times the IQR of the first or third quartile, and solid dots represent outliers. Abbreviation: rRNA, ribosomal RNA.
Lactobacillus iners was detected at high levels in all women at baseline (Figure 1) and was present in all episodes throughout the entire observation period. During treatment, population levels of L. iners increased by about a log, but individual dynamics were highly variable (Figure 5A). Although we observed nonmonotonic growth patterns in most women, a spline indicated that L. iners often expanded slowly after 2 days, filling the vacuum created by antibiotic treatment.
To assess the extent of microbiota transition, we measured a sum of all available qPCR levels for each woman, grouped separately by Lactobacillus species and BV-associated species. Owing to variable amplification efficiency among BV-associated species and exclusion of potential key BV-associated species from our qPCR measurements, the summed measures of BVAB are likely underestimates of true values. Therefore, this analysis establishes general trends only. While BVAB dominated at baseline (90% of the total bacteria level), levels dropped below that of Lactobacillus species after 1 day of treatment (Figure 5B). By the end of treatment, BVAB represented only a small proportion of the summed total, compared with Lactobacillus species.
DISCUSSION
Using mathematical models, we demonstrate that the human microbiome is dynamic within a single anatomic niche and can undergo complete reorganization in less than a day during antibiotic treatment. Within 24 hours of metronidazole treatment initiation, most BVAB undergo rapid exponential depletion. Levels of Lactobacillus species, particularly L. iners, often surge to fill the transient microbial vacuum. The microbiome remains unstable when antibiotic therapy is stopped; reemergence of anaerobic species is common, often within a week.
Many studies correlate cross-sectional profiles of diversity with disease risk, based on the assumption that the microbiome is a rather stable entity. However, we demonstrate that cross-sectional analyses may be insufficient to capture the dynamic landscape of polymicrobial composition. Other human microbiota niches, such as the gut and mouth, may also undergo frequent rapid shifts akin to those in the vagina. Permanent or transient alterations to bacterial composition can occur both during and after antibiotic use [31–33]. Future studies of the microbiome should incorporate serial, high-frequency quantitative measures of multiple bacterial species.
While the presence of BVAB does not always result in BV, an understanding of bacterial reemergence may be useful to prevent BV recurrence. If reemergence depends on seeding from sources outside of the vagina, such as the gastrointestinal tract or the genital tract of sex partners, then preventive interventions may include risk-modification counseling. Alternatively, levels of metabolically dormant BVAB may persist in anatomic drug sanctuaries or in G. vaginalis biofilms [34–37]. Under these conditions, intermittent suppressive regimens may prevent BVAB from reemerging and causing disease [38].
We demonstrate that metronidazole therapy is not an effective treatment against G. vaginalis. Among the measured BVAB in our study, G. vaginalis had markedly lower clearance rates. The exponential model fit poorly to G. vaginalis levels during treatment, owing to frequent bacterial rebound. The lack of efficacy of metronidazole against G. vaginalis is only partially understood [39, 40]. Rebound may indicate emergent drug resistance or persistence within biofilms [34–37, 41].
For sustained treatment effect, the drivers of successful Lactobacillus expansion and persistence may be of central importance. Lactobacillus reemergence could result from reduction in spatial constraints on growth or diminished competition with BVAB for limited nutritional resources. Thus, metronidazole may indirectly but positively affect Lactobacillus growth. In our study, emergence of either L. crispatus or L. jensenii occurred in 8 of the 12 episodes that had negative tests for these species at baseline. Given that L. crispatus is inversely associated with BVAB [17, 19, 29, 41–47], strategies to ensure its sustained presence in the vaginal microbiota could be beneficial. Lactobacillus iners was the most dominant Lactobacillus species in these women, although its role in protection against BV is uncertain [19, 41, 43–48].
While clearance rates are similar within BV-associated anaerobic species, host-specific features play an even more important role in determining clearance rates. Factors that determine more-rapid clearance rates during certain treatment sessions warrant further study. Aside from species, possible determinants of the bacterial clearance rate include individual variability related to pharmacodynamics, microbiota profile at baseline, treatment adherence, and timing of treatment during the menstrual cycle [17, 19, 47].
Our interpretation of clearance rates is limited because estimates encompass several possible ongoing processes, including, (1) natural bacterial death rate, (2) bactericidal effects of metronidazole, and (3) bacterial fission during treatment. If metronidazole's bactericidal effects [49, 50] predominate in vivo, then our measured clearance rate may exceed the natural death rate of bacteria. Yet, if bacterial fission continues to occur at a meaningful rate during treatment, then our measured clearance rate may be lower than the natural death rate. The estimated clearance rates would approximate natural bacterial death rates if metronidazole is bacteriostatic in vivo and the natural birth and death rates of BVAB exceed the killing rate of metronidazole. The subtle differences in clearance rates between species likely include some differential combination of these processes.
Some limitations arise when estimating bacterial levels by using qPCR. Bacteria may, in theory, die at a faster rate than their DNA degrades. Our estimated daily clearance rates of BVAB DNA exhibited a clear log-linear relationship, and this trend should be confirmed using colony counts of cultivatable bacteria. We were also not able to measure levels of all species in the vaginal niche. It is therefore not possible to estimate a true total bacterial count: our estimates of BVAB levels relative to Lactobacillus levels are imprecise. Nevertheless, there is a clear trend toward complete turnover in microbial composition within a very short time of antibiotic initiation.
In summary, we documented extremely rapid turnover of resident vaginal bacteria during and after antibiotic treatment. The vaginal microbiota can be highly dynamic, particularly in response to antibiotics, and future work should investigate the impact of antibiotic and other perturbations on microbial community structure and function.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. S. S., J. M. M., and D. N. F. conceived and designed the experiments. S. S. and T. L. F. performed the experiments. B. T. M. and J. T. S. analyzed the data and wrote the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI061628 [to D. N. F.], K23 AI087206 [to J. T. S.], and U19 AI113173).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Foxman B, Rosenthal M. Implications of the human microbiome project for epidemiology. Am J Epidemiol 2013; 177:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haiser HJ, Turnbaugh PJ. Is it time for a metagenomic basis of therapeutics? Science 2012; 336:1253–5. [DOI] [PubMed] [Google Scholar]
- 3.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 34:864–9. [DOI] [PubMed] [Google Scholar]
- 4.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 1995; 333:1737–42. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Thom E, Moawad AH, Johnson F, Roberts J, Cartis SN. The preterm prediction study: Fetal fibronectin, bacterial vaginosis, and peripartum infection. Obstet Gynecol 1996; 87:656–60. [DOI] [PubMed] [Google Scholar]
- 6.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: Insight into subclinical pelvic inflammatory disease. Obstet Gynecol 2002; 100:456–63. [DOI] [PubMed] [Google Scholar]
- 7.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 2005; 162:585–90. [DOI] [PubMed] [Google Scholar]
- 8.Larsson PG, Platzchristensen JJ, Thejls H, Forsum U, Pahlson C. Incidence of pelvic inflammatory disease after 1st-trimester legal-abortion in women with bacterial vaginosis after treatment with metronidazole - a double-blind, randomized study. Am J Obstet Gynecol 1992; 166:100–3. [DOI] [PubMed] [Google Scholar]
- 9.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis 2005; 40:1422–8. [DOI] [PubMed] [Google Scholar]
- 10.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 11.Watts DH, Fazarri SM, Minkoff H. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection among HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis 2005; 191:1129–39. [DOI] [PubMed] [Google Scholar]
- 12.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 13.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 12:1699–706. [DOI] [PubMed] [Google Scholar]
- 14.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis - 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109:114–20. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Xiao BB, Shang CG, et al. Molecular analysis of the relationship between specific vaginal bacteria and bacterial vaginosis metronidazole therapy failure. Eur J Clin Microbiol Infect Dis 2014; 33:1749–56. [DOI] [PubMed] [Google Scholar]
- 16.Brotman RM, Shardell MD, Gajer P, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 2014; 210:1723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santiago GL, Cools P, Verstraelen H, et al. Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS One 2011; 6:e28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 2010; 5:e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995; 373:123–6. [DOI] [PubMed] [Google Scholar]
- 21.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997; 387:188–91. [DOI] [PubMed] [Google Scholar]
- 22.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 1996; 271:1582–6. [DOI] [PubMed] [Google Scholar]
- 23.Ramratnam B, Bonhoeffer S, Binley J, et al. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet 1999; 354:1782–5. [DOI] [PubMed] [Google Scholar]
- 24.Rong L, Dahari H, Ribeiro RM, Perelson AS. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci Transl Med 2010; 2:30ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffer JT. Mucosal HSV-2 specific CD8+ T-Cells represent containment of prior viral shedding rather than a correlate of future protection. Front Immunol 2013; 4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffer JT, Swan D, Corey L, Wald A. Rapid viral expansion and short drug half-life explain the incomplete effectiveness of current herpes simplex virus-2 directed antiviral agents. Antimicrob Agents Chemother 2013; 57:5820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amsel R, Totten PA, Spiegel CA, Chen KCS, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention; Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006; 55:1–94. [PubMed] [Google Scholar]
- 29.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol 2009; 47:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun 2009; 77:2367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 2010; 5:e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–86. [DOI] [PubMed] [Google Scholar]
- 35.Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005; 106:1013–23. [DOI] [PubMed] [Google Scholar]
- 36.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008; 198:97.e1–6. [DOI] [PubMed] [Google Scholar]
- 37.Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Curr Opin Infect Dis 2013; 26:86–9. [DOI] [PubMed] [Google Scholar]
- 38.Aguin T, Akins RA, Sobel JD. High-dose vaginal maintenance metronidazole for recurrent bacterial vaginosis: a pilot study. Sex Transm Dis 2014; 41:290–1. [DOI] [PubMed] [Google Scholar]
- 39.Edwards DI. Nitroimidazole drugs--action and resistance mechanisms. II. Mechanisms of resistance. J Antimicrob Chemother 1993; 31:201–10. [DOI] [PubMed] [Google Scholar]
- 40.Easmon CS, Ison CA, Kaye CM, Timewell RM, Dawson SG. Pharmacokinetics of metronidazole and its principal metabolites and their activity against Gardnerella vaginalis. Br J Vener Dis 1982; 58:246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial Communities in Women with Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PLoS One 2012; 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danielsson D, Teigen PK, Moi H. The genital econiche: focus on microbiota and bacterial vaginosis. Ann N Y Acad Sci 2011; 1230:48–58. [DOI] [PubMed] [Google Scholar]
- 43.Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol 2010; 48:1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol 2007; 45:1016–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin DH, Zozaya M, Lillis R, Miller J, Ferris MJ. The microbiota of the human genitourinary tract: trying to see the forest through the trees. Trans Am Clin Climatol Assoc 2012; 123:242–56. [PMC free article] [PubMed] [Google Scholar]
- 46.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 2009; 9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 2012; 66:371–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ling Z, Kong J, Liu F, et al. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 2010; 11:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratton CW, Weeks LS, Aldridge KE. Comparison of the bactericidal activity of clindamycin and metronidazole against cefoxitin-susceptible and cefoxitin-resistant isolates of the Bacteroides fragilis group. Diagn Microbiol Infect Dis 1991; 14:377–82. [DOI] [PubMed] [Google Scholar]
- 50.Stratton CW, Weeks LS, Aldridge KE. Inhibitory and bactericidal activity of selected beta-lactam agents alone and in combination with beta-lactamase inhibitors compared with that of cefoxitin and metronidazole against cefoxitin-susceptible and cefoxitin-resistant isolates of the Bacteroides fragilis group. Diagn Microbiol Infect Dis 1992; 15:321–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.