Abstract
Background. The meningococcal vaccine antigen, factor H (FH)–binding protein (FHbp), binds human complement FH. In human FH transgenic mice, binding decreased protective antibody responses.
Methods. To investigate the effect of primate FH binding, we immunized rhesus macaques with a 4-component serogroup B vaccine (4CMenB). Serum FH in 6 animals bound strongly to FHbp (FHbp-FHhigh) and, in 6 animals, bound weakly to FHbp (FHbp-FHlow).
Results. There were no significant differences between the respective serum bactericidal responses of the 2 groups against meningococcal strains susceptible to antibody to the NadA or PorA vaccine antigens. In contrast, anti-FHbp bactericidal titers were 2-fold lower in FHbp-FHhigh macaques against a strain with an exact FHbp match to the vaccine (P = .08) and were ≥4-fold lower against 4 mutants with other FHbp sequence variants (P ≤ .005, compared with FHbp-FHlow macaques). Unexpectedly, postimmunization sera from all 12 macaques enhanced FH binding to meningococci. In contrast, serum anti-FHbp antibodies elicited by 4CMenB in mice whose mouse FH did not bind to the vaccine antigen inhibited FH binding.
Conclusions. Binding of FH to FHbp decreases protective anti-FHbp antibody responses of macaques to 4CMenB. Even low levels of FH binding skew the antibody repertoire to FHbp epitopes outside of the FH-binding site, which enhance FH binding.
Keywords: factor H binding protein, FH, nonhuman primate animal model, Bexsero, 4CMenB
Factor H (FH)–binding protein (FHbp) is a lipoprotein expressed by nearly all serogroup B meningococcal strains [1–3]. The protein acts as a virulence factor by recruiting FH to the bacterial surface. The bound FH downregulates the alternative complement pathway and permits the organism to survive in human serum [4]. FHbp is a protective antigen in 2 meningococcal serogroup B vaccines. Trumenba (Pfizer Vaccines, Pearl River, New York) contains 2 FHbp sequence variants [5]. Bexsero (Novartis Vaccines, Siena, Italy) is a 4-component serogroup B vaccine (4CMenB) comprising 1 FHbp sequence variant and 3 antigens capable of eliciting serum bactericidal activity [6]. 4CMenB is licensed in Europe, Canada, and Australia for infants, children, and adults [7], and both vaccines are licensed in the United States for the individuals aged 10–25 years.
FHbp was discovered by 2 groups of investigators, using genome mining [8] or more-traditional membrane-fractionation methods [9]. The protein, originally called genome-derived neisserial antigen 1870 [8] or lipoprotein 2086 [9], was subsequently found to bind human FH [4, 10], which resulted in change of the name to FHbp. When humans are vaccinated, the antigen is expected to form a complex with human FH. In human FH transgenic mice, binding of FH to FHbp decreased serum bactericidal antibody responses [11–13]. Immunized humans develop complement-mediated serum bactericidal antibody responses [14–16], but little is known about the effect of FH binding to the vaccine antigen on the human anti-FHbp antibody repertoire or antibody functional activity. This question has been difficult to address in clinical trials because FH binding is specific for human FH [10] and because, to date, all of the FHbp vaccines tested in humans bound human FH. In the present study, we investigated the effect of FH binding on the immunogenicity of the U.S.- and European-licensed 4CMenB in a nonhuman primate model. Because of an amino acid polymorphism in FH domain 6, there is heterogeneity in macaque FH binding to FHbp [17]. Therefore, some immunized animals had low FH binding to the FHbp vaccine antigen, while others had high binding with similar affinity as human FH.
MATERIALS AND METHODS
Rhesus Macaques
The animals were born and housed at the California National Primate Research Center (Davis) in accordance with American Association for Accreditation of Laboratory Animal Care standards. They were maintained in outdoor social housing with their dams and extended families. The colony's founders and genetic relationships [18, 19] and the presence of a FH polymorphism associated with strong or weak FH binding to FHbp have been described elsewhere [17]. We strictly adhered to the Guide for the Care and Use of Laboratory Animals [20]. The study was approved by the Institutional Animal Care and Use Committee of the University of California–Davis.
At ages 2–3 months, we screened sera from 25 animals for binding of macaque FH to FHbp ID 1 by enzyme-linked immunosorbent assay (ELISA), as previously described [17]. Six animals with strong binding (FHbp-FHhigh) and 6 with weak FH binding (FHbp-FHlow) were selected for vaccination. An additional monkey with FHbp-FHhigh and 2 with FHbp-FHlow were followed as negative unvaccinated controls. The respective macaque FH-binding phenotypes were confirmed by a flow cytometric assay with live meningococci.
Immunogenicity
A human 4CMenB dose (0.5 mL) contains 50 µg each of 3 recombinant proteins, which are combined with 25 µg of detergent-treated outer-membrane vesicles [6, 21]. The 4 components are adsorbed with aluminum hydroxide (0.5 mg Al3+ per human dose) [14, 22]. At ages 3–4 months, the animals were vaccinated intramuscularly with 1 human dose divided into two 0.25-mL aliquots, which were given as separate injections in each leg. A second dose was given 1 month later. Blood samples were obtained 3 weeks after the second dose.
Serum IgG Anti-FHbp Antibody Responses
Serum IgG anti-FHbp titers were measured by ELISA, using recombinant FHbp ID 1 as the antigen on the plate [23]. Bound immunoglobulin G (IgG) was measured by alkaline phosphatase–conjugated goat anti-human IgG (Fc specific; Sigma), which cross-reacts with macaque IgG.
Neisseria meningitidis
We measured serum bactericidal antibody responses against 3 invasive serogroup B meningococcal strains: H44/76, 5/99, and SK016. These strains have been previously used in 4CMenB immunogenicity studies to measure antigen-specific serum bactericidal antibody responses to FHbp [14, 22], NadA [14, 22], and PorA P1.4 [12], respectively. Each strain was mismatched for the other 4CMenB antigens known to elicit bactericidal antibody and was killed by human complement only with a mouse antiserum to the matched antigen [12]. We also tested serum anti-FHbp bactericidal antibody responses against 4 mutants of strain H44/76 in which the gene encoding the native FHbp ID 1 that matched the FHbp antigen in 4CMenB had been replaced by either FHbp ID 4, 13, or 15 (subfamily B) or by FHbp ID 22 (subfamily A). The H44/76 mutants were prepared and characterized as previously described for similar mutants from strain NZ98/254 [24].
Bactericidal Assay
Bacteria were grown to mid-log phase in Franz medium supplemented with 4 mM d,l-Lactate (Sigma) and 2 mM cytidine 5′-monophospho-N-acetyl-neuraminic acid (Carbosynth) to enhance sialylation of lipooligosaccharide [25]. Test sera were heated for 30 minutes at 56°C to inactivate complement. The exogenous human complement underwent human serum depletion of IgG with a protein G column (HiTrap Protein G HP 1 mL; GE Healthcare) [11]. Serum titers were assigned by the dilution resulting in 50% survival of the bacteria, compared with the density of bacteria incubated for 60 minutes with negative control sera and complement.
Flow Cytometry With Live N. meningitidis for Detection of Bound FH
The assay was performed as previously described [12, 26]. In brief, 107 colony-forming units (CFU)/mL of bacteria were incubated for 1 hour at room temperature with dilutions of macaque serum. The bacteria were washed with Dulbecco's phosphate buffered saline (Mediatech) containing 1% (w/v) bovine serum albumin (Equitech-Bio) (D-PBS-BSA). Bound macaque FH was detected with a sheep polyclonal antiserum to human FH (Abcam) followed by washing and the addition of donkey anti-sheep IgG antibody (Sigma) conjugated with AlexaFluor 488. After washing and fixation with 0.5% (v/v) formaldehyde in PBS, binding was analyzed by flow cytometry (Fortessa, BD Biosciences), and data were analyzed using FlowJo, version 10.
Inhibition of Binding of Macaque FH
Approximately 107 CFU/mL of bacteria were incubated with a 1:150 dilution of preimmunization or postimmunization macaque sera or of serum from a control unvaccinated macaque with FHbp-FHhigh (as a source of macaque FH) and control antibodies. Bacteria and sera were incubated for 1 hour at room temperature. After washing the cells, bound macaque FH was detected as described above. In some experiments, 2 µg/mL of purified human FH (Complement Technologies) was added with the macaque serum.
C4b Deposition on N. meningitidis
We performed flow cytometry to measure deposition of human C4b on the surface of live meningococci, as previously described [27]. In brief, bacteria were grown as described above and resuspended in D-PBS-BSA to a density of approximately 108 CFU/mL. The bacteria were incubated with human complement (5% IgG-depleted human serum) and a 1:40 dilution of macaque sera. After incubation for 15 minutes at room temperature, bound human C4b was detected with a 1:100 dilution of fluorescein isothiocyanate–conjugated anti-human C4b (Meridian Life Science).
Statistical Analyses
For calculation of geometric mean titers (GMTs), titers below the limit of the detection were assigned half the value of the lowest dilution tested. The Student t test or, where appropriate, the Mann–Whitney U test was used to compare the geometric means between 2 independent test samples. All statistical tests were 2-tailed; P values of ≤.05 were considered statistically significant.
RESULTS
Selection of Macaques
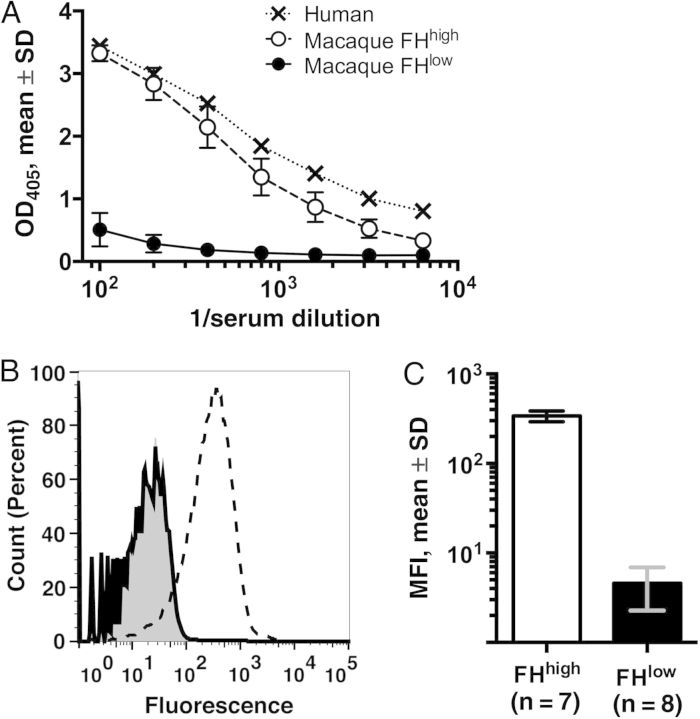
Six animals with FHbp-FHhigh and 6 with FHbp-FHlow were selected for vaccination. An additional macaque with FHbp-FHhigh and 2 with FHbp-FHlow were followed as negative unvaccinated controls. The mean binding of FH to FHbp by ELISA in the 2 groups is shown in Figure 1A. Serum FHbp-FHhigh or FHbp-FHlow was confirmed for each animal by a flow cytometric FH-binding assay, using live bacteria from serogroup B strain H44/76 (representative data for a FHbp-FHhigh animal and a FHbp-FHlow animal are shown in Figure 1B). At a serum dilution of 1:50, the mean fluorescence intensity for the 8 FHbp-FHlow animals differed by 80-fold from that for the 7 FHbp-FHhigh animals (Figure 1C).
Figure 1.
Binding of macaque serum factor H (FH). A, Binding of serum FH from 7 macaques with high binding (FHhigh; open circles with dashed line) and 8 macaques with weak binding (FHlow; filled circles with solid line) to FH binding protein (FHbp) as determined by an enzyme-linked immunosorbent assay. Macaque sera were obtained at ages 2–3 months, prior to immunization. For comparison, binding of human FH in pooled serum specimens from 3 healthy human adults is shown (X symbols with dotted line). B, Binding of macaque FH to live Neisseria meningitidis serogroup B strain H44/76 as determined by flow cytometry. Representative data show FH binding in 1:50 dilutions of sera from a macaque with FHhigh (dashed line) or one with FHlow (solid line). The gray region denotes bacteria without macaque serum. Count (percent) describes the percentage of bacteria within a narrow range of fluorescence intensity from a total number tested (80 000). C, Mean median fluorescence intensities (MFIs) ± SD obtained with sera diluted 1:50 (7 macaques with FHhigh and 8 macaques with FHlow to FHbp).
Macaques With the FHbp-FHhigh Phenotype Have Lower Serum Anti-FHbp Bactericidal Antibody Responses Than Macaques With the FHbp-FHlow Phenotype
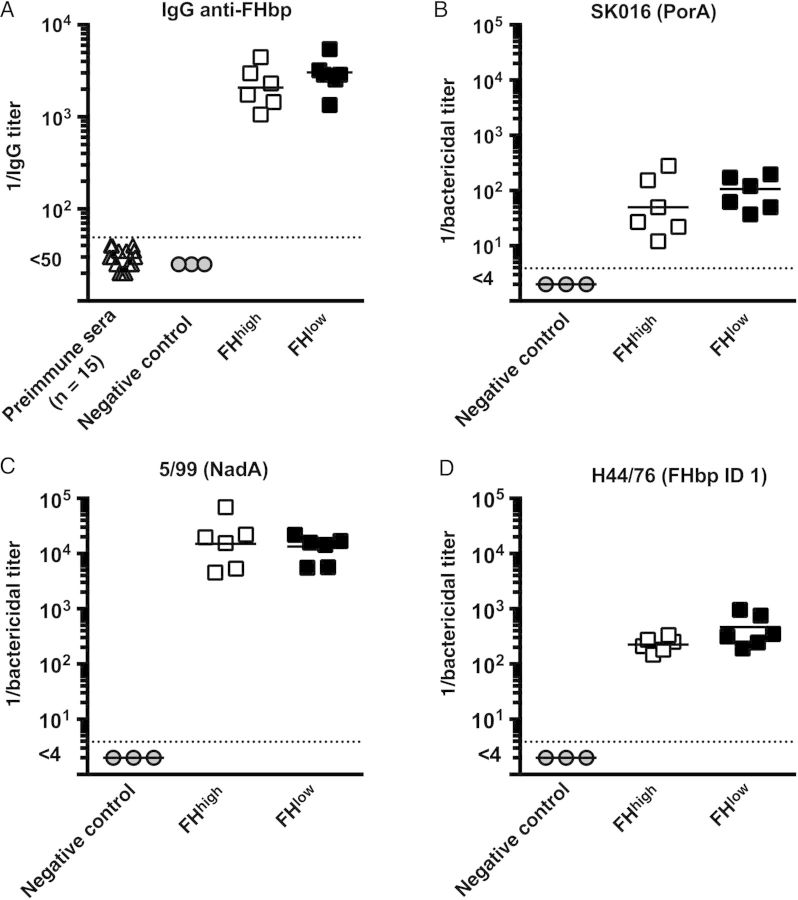
Serum IgG anti-FHbp titers at 2–3 months of age, before vaccination, were <1:50 in all 15 macaques and remained <1:50 at 4–5 months of age for the 3 control unvaccinated animals (Figure 2A). After the second dose of vaccine, there were no significant differences in the respective serum IgG anti-FHbp GMTs of the macaques with FHbp-FHhigh or FHbp-FHlow phenotypes (P = .3).
Figure 2.
Serum antibody responses to the 4-component serogroup B meningococcal vaccine (4CMenB). A, Immunoglobulin G (IgG) anti–factor H (FH) binding protein (FHbp) antibody responses as determined by enzyme-linked immunosorbent assay. Preimmune sera were obtained from 15 macaques at 2–3 months of age, before vaccination. Negative control sera were obtained from 3 nonvaccinated control macaques aged 5–6 months, to coincide with ages at which postvaccination sera were collected from immunized animals. Postvaccination sera were obtained 3 weeks after dose 2 from 6 animals with strong FH binding to FHbp (FHhigh; open squares) and from 6 animals with weak FH binding to FHbp (FHlow; closed squares). Postimmunization geometric mean titers (GMTs) are not significantly different (P > .10). B–D, Human complement-mediated serum bactericidal titers against 3 invasive serogroup B strains. Strain SK016 was mismatched for all antigens in 4CMenB except PorA (B). Strain 5/99 was mismatched for all antigens except NadA (C). Strain H44/76 was mismatched for all of the antigens except FHbp ID 1 in subfamily B, which matched the amino acid sequence of FHbp in 4CMenB (D). Respective differences in reciprocal GMTs between animals with FHhigh or FHlow phenotypes are not significantly different (P > .2 for strains 5/99 and SK016 and P = .08 for strain H44/76).
We tested serum bactericidal titers against 3 invasive serogroup B strains, each with only 1 antigen that matched one of the 4 antigens in 4CMenB that are known to elicit serum bactericidal activity [6]. Against strain SK016, which is susceptible to antibodies elicited by PorA subtype P1.4 in the outer-membrane vesicle component [12], and strain 5/99, which is susceptible to antibodies elicited by the recombinant NadA antigen [14, 22], the respective serum bactericidal titers of the macaques with FHbp-FHhigh or FHbp-FHlow phenotype were not significantly different from each other (P > .2; Figure 2B and 2C). Against strain H44/76, which is susceptible to antibodies to the recombinant FHbp antigen [14, 22], there was a trend for lower serum bactericidal antibody responses in the macaques with FHbp-FHhigh (reciprocal GMT, 226 vs 394 in macaques with the FHbp-FHlow phenotype; P = .08; Figure 2D).
The FHbp sequence variant, ID 1, in strain H44/76 matches the FHbp ID 1 antigen in 4CMenB. We also tested serum anti-FHbp bactericidal antibody responses against 4 H44/76 mutants in which the gene encoding FHbp ID 1 had been replaced by either FHbp ID 4, 13, or 15 (subfamily B, with 87%–96% amino acid identity, compared with ID 1), or FHbp ID 22 (subfamily A, with 69% amino acid identity to ID 1; Figure 3A). For all 4 mutants, expression of FHbp was similar to that of the parent wild-type H44/76 strain (Figure 3B), which is known to express relatively high levels of FHbp [30].
Figure 3.
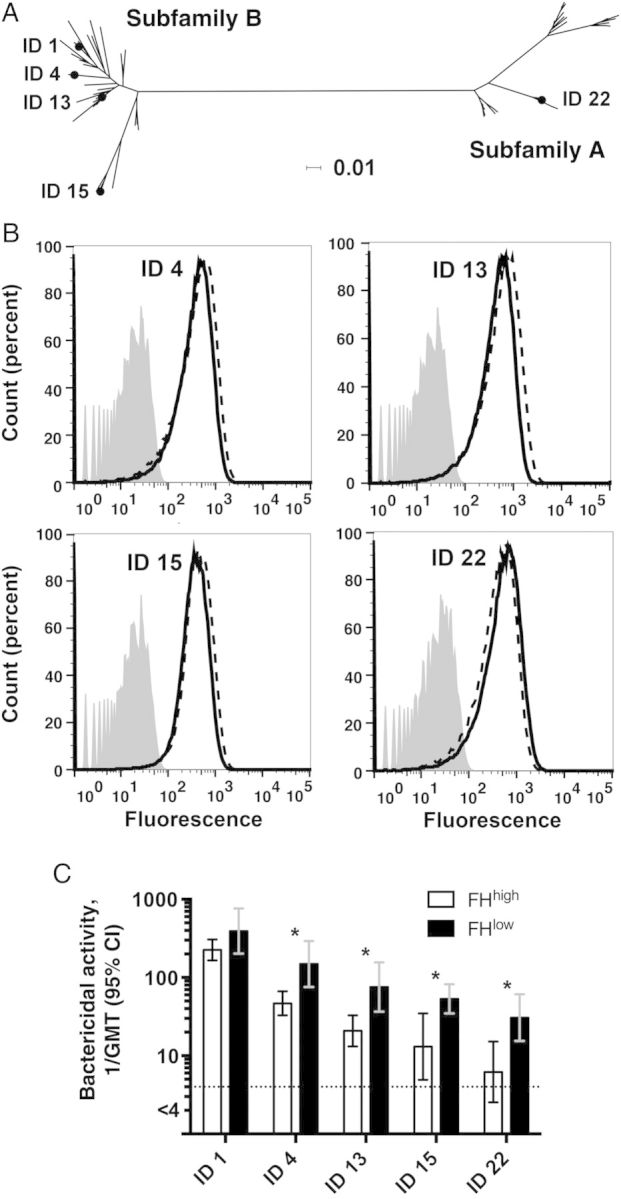
Serum bactericidal titers against 4 factor H (FH) binding protein (FHbp) mutants of H44/76. A, Phylogram (constructed using SplitsTree [28]) showing the relationship between FHbp amino acid sequence variants ID 4, 13, or 15 (subfamily B) or ID 22 (subfamily A) and FHbp ID 1 (sequence variant in the 4-component serogroup B meningococcal vaccine and in wild-type group B strain H44/76). The horizontal bar denotes 1% amino acid sequence divergence. B, Expression of FHbp by different serogroup B strain H44/76 mutants, as measured by flow cytometry with anti-FHbp monoclonal antibody (JAR 41; 10 µg/mL) and live bacteria. The monoclonal antibody recognizes FHbp sequence variants in both subfamily A and B [29]. Solid line, wild-type H44/76 with FHbp ID 1; dashed lines, H44/76 mutants. Counts (percent), see legend to Figure 1B. C, Serum bactericidal antibody responses to wild-type H44/76 with ID 1 and mutants with different FHbp amino acid sequence variants. For each mutant, macaques with strong FH binding to FHbp (FHhigh) had lower reciprocal serum bactericidal antibody geometric mean titers (GMTs) than those with weak FH binding to FHbp (FHlow). *P ≤ .005. Abbreviation: CI, confidence interval.
Against the 4 mutants, the respective reciprocal serum bactericidal GMTs of the macaques with the FHbp-FHhigh phenotype were significantly lower than those of the macaques with FHbp-FHlow (P ≤ .005 for each comparison; Figure 3C; titers of the individual animals in each group against each of the mutants are shown in Supplementary Figure 1). In both groups of macaques, there were decreasing serum bactericidal titers with increasing sequence divergence between the FHbp ID 1 in the vaccine and FHbp antigen in the mutant strain (Figure 3A and 3C). This result was expected on the basis of previous data from wild-type mice immunized with different recombinant FHbp vaccines [24]. The data also parallel those from human infants and toddlers immunized with the 3 recombinant proteins in 4CMenB (high serum bactericidal antibody responses against strain H44/76 with FHbp ID 1 that matched the vaccine antigen and lower responses against 3 other strains with different subfamily B FHbp variants [14, 22].
The macaque bactericidal titers were measured with test sera that had been heated to inactivate internal complement. Since the activity of FH is minimally affected by the heat treatment [31], it is possible that the lower anti-FHbp serum bactericidal titers of the macaques with the FHbp-FHhigh phenotype were a result of greater FH complement downregulation by the combination of macaque serum FHbp-FHhigh and human FH in the exogenous human complement, compared with macaque serum FHbp-FHlow and human FH. Therefore, we repeated the bactericidal assay against the H44/76 FHbp ID 15 mutant and added an equal volume (1:1) of negative control serum from an unvaccinated macaque with FHbp-FHhigh to each of the postimmunization sera from macaques with FHbp-FHlow, and vice versa, to equalize the amounts of FHbp-FHhigh or FHbp-FHlow in each reaction. The macaques with the FHbp-FHhigh phenotype still had lower serum bactericidal antibody responses (reciprocal GMT, 12 vs 47 for macaques with the FHbp-FHlow phenotype; P = .02, by the Mann–Whitney U test).
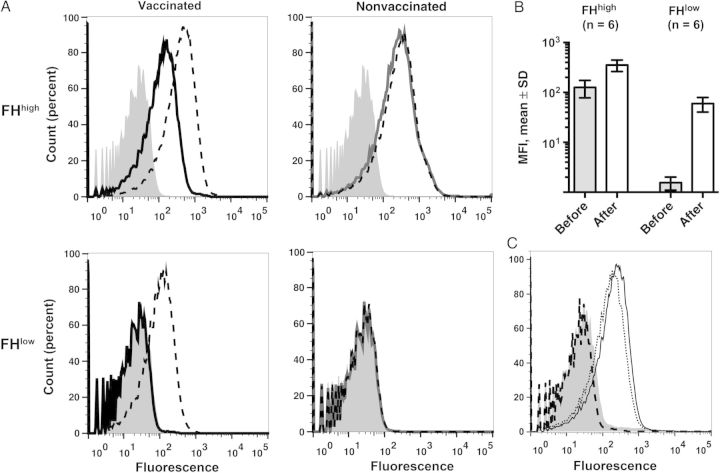
Postimmunization Sera From Macaques That Received 4CMenB Enhance Binding of FH to Meningococci
In previous studies, the serum anti-FHbp antibodies of 4CMenB-vaccinated wild-type mice whose FH did not bind to the FHbp vaccine antigen–inhibited binding of human FH to FHbp, which increased bactericidal activity [12, 24]. We therefore investigated the ability of postimmunization macaque sera to inhibit binding of macaque FH to the wild-type H44/76 strain (Figure 4). The macaque sera were tested at a 1:150 dilution and served as a source of both antibody and macaque FH. The postimmunization sera from vaccinated macaques with the FHbp-FHhigh or FHbp-FHlow phenotypes enhanced macaque FH binding, compared with the respective preimmunization sera (Figure 4A). There were no changes in serum FH binding between 2 and 3 months of age and 5 and 6 months of age in negative control macaques that had not been vaccinated (Figure 4A). When the data from all 12 vaccinated macaques were analyzed, there were significant increases in binding of macaque serum FH after vaccination in both groups (P ≤ .002, by the Mann–Whitney U test; Figure 4B). In contrast, when a serum pool from wild-type mice immunized with 4CMenB was added as a source of antibody to negative control serum from a nonvaccinated macaque (as a source of macaque FHbp-FHhigh), there was complete inhibition of macaque FH binding (Figure 4C). We also investigated the effect of the postimmunization macaque sera on binding of human FH to meningococci and found similar enhancement to that found with binding of macaque FH to the bacteria (Supplementary Figure 2). As expected [12, 24], the sera from control wild-type mice that received 4CMenB inhibited binding of human FH (Supplementary Figure 2C).
Figure 4.
Sera from macaques immunized with the 4-component serogroup B meningococcal vaccine (4CMenB) enhance binding of macaque factor H (FH) to meningococci. Flow cytometric detection of binding of macaque serum FH to live wild-type Neisseria meningitidis serogroup B strain H44/76. Gray histograms denote bacteria without macaque serum. All sera were tested at a dilution of 1:150. A, Macaque sera obtained before and 3 weeks after 2 doses of 4CMenB from a representative macaque with strong FH binding to FH binding protein (FHbp; FHhigh) as measured in pre-immunation serum or weak FH binding to FHbp (FHlow; left). For comparison, data are shown from testing sera obtained from unvaccinated macaques with FHhigh or FHlow phenotypes at 2–3 months of age (solid gray lines) or at 5–6 months of age (dashed black lines), to coincide with ages of vaccinated animals (right). Counts (percent), see legend to Figure 1B. B, Histogram of mean median fluorescence intensities (MFIs) ± SDs obtained testing sera obtained before and after vaccination from 6 macaques with FHhigh and 6 with FHlow phenotypes. C, Postimmunization serum pools from wild-type mice that received 4CMenB (dark dashed) or aluminum hydroxide alone (solid line) together with a 1:150 dilution of serum from an unvaccinated control macaque with FHhigh. The dotted line represents FH binding with the macaque serum tested without added mouse antiserum. In contrast to the sera from macaques that received 4CMenB, the mouse antiserum to 4CMenB showed nearly complete inhibition of binding of macaque FH to the bacteria.
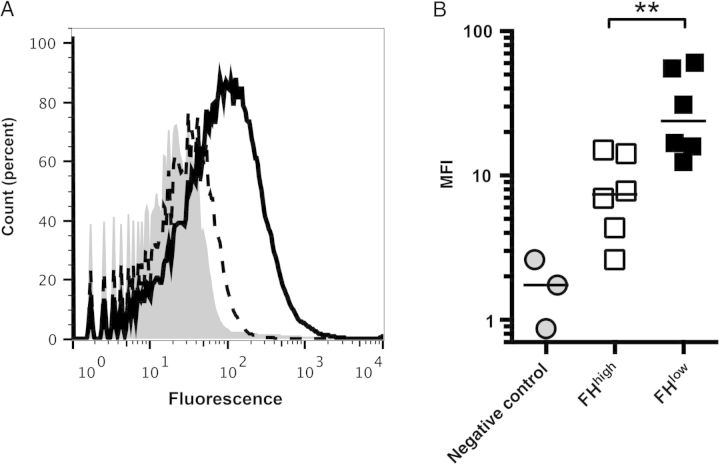
Postvaccination Sera From FHbp-FHhigh Macaques Activate Less C4b Deposition on Meningococci Than That From FHbp-FHlow Macaques
Deposition of human C4b on the surface of live N. meningitidis cells can be used as a marker of lectin and/or classical complement pathway activation [27]. As shown in Figure 5A, there was significantly less C4b deposition elicited by a postimmunization serum sample from a macaque with the FHbp-FHhigh phenotype than a macaque with the FHbp-FHlow phenotype. The geometric mean of the median fluorescence intensity for the 6 macaques with the FHbp-FHhigh phenotype was 4-fold lower than that of the macaques with the FHbp FHlow phenotype (P = .007; Figure 5B). Collectively, these data indicate that the lower serum anti-FHbp bactericidal titers of the macaques with the FHbp-FHhigh phenotype resulted at least in part from less activation of the classical pathway.
Figure 5.
Human C4b deposition elicited by postimmunization sera from immunized macaques. A, C4b deposition on the bacterial surface by representative postimmunization sera from macaques with strong factor H (FH) binding to FH binding protein (FHbp; FHhigh; dashed line) or weak FH binding to FHbp (FHlow; solid line). The gray region denotes bacteria with 5% human complement but no macaque serum. Heat-inactivated macaque sera were tested at 1:40 dilutions in the presence of 5% immunoglobulin G–depleted human serum with the H44/76 mutant with FHbp ID 15. Counts (percent), see legend to Figure 1B. B, Median fluorescence intensities (MFIs) for C4b deposition elicited by macaque sera (3 nonimmunized controls [gray filled circles], 6 with FHhigh [open squares], and 6 with FHlow [closed squares]). The horizontal bars represent geometric means. The difference between the vaccinated groups with FHhigh and those with FHlow was statistically significant. **P = .007.
DISCUSSION
In previous studies, immunization of wild-type mice with different FHbp vaccines elicited serum anti-FHbp antibodies that activated the classical complement pathway directly and also inhibited binding of FH to the bacteria [4, 26, 30]. With less bound FH, the bacteria became more susceptible to complement-mediated killing by the alternative pathway. This dual antibody function was especially important for eliciting bactericidal activity against meningococcal strains with low expression of FHbp [26, 30] or with FHbp amino acid sequences that did not closely match that of the vaccine antigen [24].
In immunized humans, the FHbp vaccine antigen is expected to form a complex with human FH. Data from a human FH transgenic mouse model indicated that the presence of human FH skewed the serum anti-FHbp antibody repertoire to FHbp epitopes outside of the FH-binding site, which did not inhibit FH binding [12]. For unknown reasons, these anti-FHbp antibodies enhanced FH binding [12]. In contrast, mutant FHbp vaccines with decreased FH binding elicited higher serum bactericidal activity in transgenic mice than control FHbp vaccines that bound human FH [11, 13, 32]. Further, the antibodies elicited by the mutant antigens inhibited binding of human FH to FHbp [11, 12]. Thus, failure of the mouse anti-FHbp antibodies elicited in the human FH transgenic mice to inhibit FH binding was specific for FHbp vaccine antigens that bound human FH [12].
In the present study, we investigated the effect of binding of rhesus macaque FH on the immunogenicity of 4CMenB. As expected, the macaques with the FHbp-FHhigh or FHbp-FHlow phenotypes had similar respective serum bactericidal antibody responses against 2 test strains that each contained only 1 antigen, PorA P1.4 or NadA, that matched the respective antigens in the vaccine. Neither of these antigens is known to bind FH. In contrast, the macaques with the FHbp-FHhigh phenotype had lower serum bactericidal responses against a panel of H44/76 mutants mismatched for all of the antigens in 4CMenB except for FHbp. Since the serum IgG anti-FHbp titers of the 2 groups were similar, the lower C4b deposition elicited by the postvaccination sera from the macaques with the FHbp-FHhigh phenotype suggests that strong binding of macaque FH to the vaccine antigen resulted an anti-FHbp antibody repertoire that was less effective in activating bactericidal activity by the classical complement pathway than the anti-FHbp antibody repertoire of the macaques with the FHbp-FHlow phenotype.
On the basis of previous data from FHbp-vaccinated wild-type mice whose mouse FH does not bind to FHbp and from human FH transgenic mice, we also expected that FHbp-FHlow macaques would generate anti-FHbp antibodies that would inhibit FH binding and that FHbp-FHhigh macaques would generate anti-FHbp antibodies directed at epitopes located outside of the FH-binding site, which would not inhibit FH binding and might enhance FH binding [12]. Instead, neither group of macaques developed antibodies that inhibited FH binding, and both groups developed FH enhancing antibodies. The enhancement appeared to be more dramatic in the macaques with the FHbp-FHlow phenotype because no FH binding was detected in their prevaccination sera (Figure 4A). One difference between FH in the macaques with the FHbp-FHlow phenotype and mouse FH in the wild-type mice is that we cannot detect binding of mouse FH to FHbp by sensitive methods. Thus, in the macaques it seems that even the low-affinity interactions between FH and the vaccine antigen are sufficient to skew the anti-FHbp antibody repertoire to epitopes outside of the FH-binding site. The underlying mechanism responsible for the enhanced FH binding is unknown. Conceivably, the enhancement results from conformation changes in the FHbp molecule after binding of anti-FHbp antibodies to certain FHbp epitopes outside of the FH-binding site [12]. Despite enhanced FH binding, 4CMenB elicited serum anti-FHbp bactericidal antibody responses in both groups of vaccinated macaques. These findings suggest that the increased FH binding was either not functional or that activation of complement by bound anti-FHbp antibody Fc was sufficient for eliciting bactericidal activity despite FH downregulation.
In previous studies, C3b covalently bound to IgG was resistant to degradation by FH [33]. Further, both the amount of C3b and the location where C3b is deposited on the bacterial surface in the presence of bound antibody can affect bactericidal activity [33, 34]. Note also that the ability of anti-FHbp antibodies to inhibit or enhance FH binding was best detected at low human FH concentrations (2 µg/mL). However, typical human serum FH concentrations are approximately 150–500 µg/mL [11]. Under high physiologic serum FH concentrations, the anti-FHbp antibody–induced enhancement of FH binding may not result in functional differences in FH downregulation because FH binding to FHbp is saturated. In a previous study, saturation of FH binding to the bacterial surface explained why isogenic meningococcal mutants with FHbp sequences that differed by >10-fold in binding affinity to FH survived equally well in human plasma or whole blood from nonimmune donors [35]. In the present study, saturation of human FH binding also would explain why the mixing experiment to equalize macaque FH binding in animals with the FHbp-FHhigh or FHbp-FHlow phenotypes did not affect the serum bactericidal titers measured with 20% human complement, compared with testing the macaque sera from the animals with FHbp-FHhigh or FHbp-FHlow phenotypes without mixing.
In summary, macaques with the FHbp-FHhigh phenotype had lower serum bactericidal anti-FHbp antibody responses to 4CMenB than those with the FHbp-FHlow phenotype. Thus, in this primate model, which is likely to be more relevant for predicting human antibody responses than mouse models, stronger binding of a primate host protein, FH, to a vaccine antigen decreased protective anti-FHbp antibody responses. Conceivably, mutant FHbp antigens with low FH binding may be superior immunogens in humans, compared with FHbp antigens that bind FH, and the mutants also may avoid the theoretical safety risk of eliciting autoreactive antibodies to FH [12]. However, given the desirability to elicit serum anti-FHbp antibodies that inhibit FH binding, it may be necessary to design mutant FHbp vaccines that completely eliminate FH binding in order for the antibody repertoire to be directed to FHbp epitopes in the FH-binding site.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Sanjay Ram, University of Massachusetts School of Medicine (Worcester, Massachusetts), for critical review of the manuscript; Patricia Zuno-Mitchell, for expert technical assistance from; and Rolando Pajon, for supervision of creating of the mutant strain panel, and for helpful advice through the conduct of the study.
Financial support. This work was supported by the Public Health Service (grants R01 AI 046464 and AI 082263 [to D. M. G.], AI 099125 [to P. T. B.], and AI 114701 [to D. M. G. and P. T. B.]) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and by a Pilot Program grant (to D. M. G. and P. T. B.) from grant P51OD011107 from the Office of Research Infrastructure Programs to the California National Primate Research Center. The work at Children's Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant number C06 RR 016226 from the National Center for Research Resources, NIH.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murphy E, Andrew L, Lee KL, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009; 200:379–89. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Cohn A, Comanducci M, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 2011; 29:4739–44. [DOI] [PubMed] [Google Scholar]
- 3.Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin Vaccine Immunol 2011; 18:1002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madico G, Welsch JA, Lewis LA, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 2006; 177:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang HQ, Hoiseth SK, Harris SL, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28:6086–93. [DOI] [PubMed] [Google Scholar]
- 6.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 2006; 103:10834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollard AJ, Riordan A, Ramsay M. Group B meningococcal vaccine: recommendations for UK use. Lancet 2014; 383:1103–4. [DOI] [PubMed] [Google Scholar]
- 8.Masignani V, Comanducci M, Giuliani MM, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 2003; 197:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004; 72:2088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun 2009; 77:764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beernink PT, Shaughnessy J, Braga EM, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 2011; 186:3606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa I, Pajon R, Granoff DM. Human Factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enchance FH binding. MBio 2014; 5:e01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog 2012; 8:e1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snape MD, Dawson T, Oster P, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J 2010; 29:e71–9. [DOI] [PubMed] [Google Scholar]
- 15.Richmond PC, Nissen MD, Marshall HS, et al. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 2012; 30:6163–74. [DOI] [PubMed] [Google Scholar]
- 16.Marshall HS, Richmond PC, Nissen MD, et al. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18–36 months: a phase 1 randomized-controlled clinical trial. Pediatr Infect Dis J 2012; 31:1061–8. [DOI] [PubMed] [Google Scholar]
- 17.Beernink PT, Shaughnessy J, Stefek H, Ram S, Granoff DM. Heterogeneity in rhesus macaque complement Factor H binding to meningococcal Factor H binding protein (FHbp) informs selection of primates to assess immunogenicity of FHbp-based vaccines. Clin Vaccine Immunol 2014; 21:1505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanthaswamy S, Kou A, Smith DG. Population genetic statistics from rhesus macaques (Macaca mulatta) in three different housing configurations at the California National Primate Research Center. J Am Assoc Lab Anim Sci 2010; 49:598–609. [PMC free article] [PubMed] [Google Scholar]
- 19.Kanthaswamy S, Kou A, Satkoski J, et al. Genetic characterization of specific pathogen-free rhesus macaque (Macaca mulatta) populations at the California National Primate Research Center (CNPRC). Am J Primatol 2010; 72:587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed Washington, DC: National Academies Press, 2011. [Google Scholar]
- 21.Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 2005; 23:2191–6. [DOI] [PubMed] [Google Scholar]
- 22.Findlow J, Borrow R, Snape MD, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127–37. [DOI] [PubMed] [Google Scholar]
- 23.Beernink PT, Shaughnessy J, Ram S, Granoff DM. Impaired immunogenicity of a meningococcal factor H-binding protein vaccine engineered to eliminate factor H binding. Clin Vaccine Immunol 2010; 17:1074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konar M, Granoff DM, Beernink PT. Importance of inhibition of binding of complement Factor H for serum bactericidal antibody responses to meningococcal Factor H-binding protein vaccines. J Infect Dis 2013; 208:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandrell RE, Kim JJ, John CM, et al. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol 1991; 173:2823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun 2011; 79:3751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuntini S, Reason DC, Granoff DM. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor H binding protein. Infect Immun 2012; 80:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 2006; 23:254–67. [DOI] [PubMed] [Google Scholar]
- 29.Vu DM, Pajon R, Reason DC, Granoff DM. A broadly cross-reactive monoclonal antibody against an epitope on the N-terminus of meningococcal fHbp. Sci Rep 2012; 2:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis 2008; 197:1053–61. [DOI] [PubMed] [Google Scholar]
- 31.Kask L, Villoutreix BO, Steen M, Ramesh B, Dahlback B, Blom AM. Structural stability and heat-induced conformational change of two complement inhibitors: C4b-binding protein and factor H. Protein Sci 2004; 13:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi R, Granoff DM, Beernink PT. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 2013; 31:5451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joiner KA, Goldman RC, Hammer CH, Leive L, Frank MM. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F(ab’)2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol 1983; 131:2563–9. [PubMed] [Google Scholar]
- 34.Joiner KA, Fries LF, Schmetz MA, Frank MM. IgG bearing covalently bound C3b has enhanced bactericidal activity for Escherichia coli 0111. J Exp Med 1985; 162:877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunphy KY, Beernink PT, Brogioni B, Granoff DM. Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood. Infect Immun 2011; 79:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.