Abstract
The CAPRISA 004 preexposure prophylaxis (PrEP) randomized trial demonstrated that women who used a vaginal gel containing the antiretroviral drug tenofovir (TFV) had a 39% lower risk of acquiring human immunodeficiency virus (HIV). It is not known whether topical TFV alters the antibody response to breakthrough HIV infection. In this study, antibody maturation was evaluated using 3 serologic assays: the BED capture enzyme immunoassay (CEIA), the Bio-Plex (Luminex) assay, and the Bio-Rad avidity assay. Tests were performed using serum samples collected 3, 6, 9, 12, 24, 36, 48, and >48 months after seroconversion from 95 women in the CAPRISA 004 trial (35 in the TFV gel arm and 60 in the placebo arm). For the BED CEIA and Luminex assay, linear mixed effects models were used to examine test results by study arm. Cox proportional hazard analysis was used to examine time to avidity cutoff. Anti-HIV antibody titers did not differ between study arms. Women assigned to TFV gel demonstrated slower antibody avidity maturation, as determined by the Bio-Rad (P = .04) and gp120 Bio-Plex (P = .028) assays. Women who were assigned to receive topical TFV but became infected had slower antibody avidity maturation, with potential implications for diagnosis and antibody-based incidence assays as access to antiretroviral therapy–based PrEP is increased.
Keywords: HIV, preexposure prophylaxis, antibody maturation, incidence assay
The use of tenofovir (TFV) or TFV/emtricitabine (TFV/FTC) for preexposure prophylaxis (PrEP) in human immunodeficiency virus (HIV)–uninfected individuals has been found to be effective in reducing HIV incidence [1–3]. The first study to demonstrate the effectiveness of TFV-based PrEP was the CAPRISA 004 trial. In that trial, women were randomized to receive 1% TFV vaginal gel or placebo gel, and women in the TFV arm were found to be 39% less likely to acquire HIV infection during the study [1]. Other studies that have evaluated use of TFV gel for PrEP have had mixed results, in part because of low adherence to PrEP regimens in some studies [4].
Previous studies have shown that the titer and avidity of anti-HIV antibody responses are diminished in individuals who start antiretroviral therapy (ART) during the acute phase of HIV infection [5]. Because antiretroviral drugs inhibit viral replication, the serologic response to HIV infection may also be different in individuals who acquire HIV infection while taking PrEP, compared with those who acquire HIV infection in the absence of antiretroviral drug exposure. A previous study in rhesus macaques demonstrated that TFV/FTC PrEP suppressed the simian immunodeficiency virus load and antibody avidity maturation in breakthrough infections but that the overall antibody titer and neutralizing antibody titer were not affected [6].
In this report, we evaluated the anti-HIV antibody response in women who acquired HIV infection in the TFV and placebo arms of the CAPRISA 004 trial [1] and were subsequently followed in a seroconverter cohort study conducted at the same study sites (CAPRISA 002) [7]. The samples were analyzed using a variety serologic assays that were developed for cross-sectional estimation of the incidence of HIV infection. These assays measure characteristics of the humoral immune response to HIV infection that usually evolve during the course of HIV infection. In particular, they measure the proportion of immunoglobulin G (IgG) that is HIV specific, antibody titer, and antibody avidity [8–10].
METHODS
Ethics Statement
All of the samples analyzed in this report were obtained in a previous study (CAPRISA 002/CAPRISA 004). During the study, written informed consent was obtained from study participants for the use of their samples in future investigations. The study was reviewed and approved by the University of KwaZulu Natal Biomedical Research Ethics Committee (reference E013/04). Only stored samples from individuals who consented to inclusion of their samples in future research were used in this investigation. No new samples were obtained for this study. The research described in this report was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. The research was conducted according to the principles expressed in the Declaration of Helsinki.
Study Population
Plasma samples were obtained from the CAPRISA 004 trial [1] and CAPRISA 002 Seroconverter Cohort Study [11]. HIV-uninfected women were enrolled in the CAPRISA 004 trial. During the CAPRISA 004 trial, women were requested to use the study product (vaginal gel) in relation to coitus, with insertion of 1 dose of gel within 12 hours before sex and a second dose of gel as soon as possible within 12 hours after sex and no more than 2 doses of gel in a 24-hour period (BAT 24). Gel adherence was assessed each month by self-report and via counts of returned applicators. Study product was discontinued if women in either study arm acquired HIV infection; HIV-infected women were enrolled in the CAPRISA 002 Seroconvertor Cohort Study. The date of HIV seroconversion was estimated as the midpoint between last visit when a woman had negative results of HIV antibody and HIV RNA tests and the first visit when she had a positive result of an HIV antibody test [12]; for women with a positive HIV RNA test result and a negative HIV antibody test result during a visit, the date of HIV seroconversion was estimated as 13 days after that visit. Samples were requested from the following study visits: <3, 3–6, 6–9, 9–12, 12–24, 24–36, 36–48, and >48 months after the estimated date of seroconversion. Women were only included if samples were available from at least 2 of these visits.
Laboratory Testing
HIV load and CD4+ T-cell counts were measured as previously described [1]. Three serologic assays were performed in this study. The first assay was the BED capture enzyme immunoassay (CEIA; Calypte Biomedical Corporation, Lake Oswego, OR), which measures the proportion of IgG that is specific to an immunodominant region of HIV-1 gp41 [8]. This assay was performed according to the manufacturer's instructions, with the following modification: all samples were run in duplicate, and the average normalized optical density (OD-n) was used for analysis; samples with an OD-n of <0.8 were characterized as assay positive. The second assay, Bio-Rad avidity assay, is a modified version of the Genetic Systems 1/2+O ELISA (Bio-Rad, Hercules, California) [10]. This assay measures antibody avidity; the target antigens are recombinant gp160 and p24 proteins. Samples were diluted 1:10 in duplicate and incubated at 4°C for 30 minutes (the initial antibody-binding step). Samples were then incubated for 30 minutes at 37°C (the antibody-disassociation step), with or without the chaotropic agent diethylamine (DEA; diluted in deionized water). For each sample, the avidity index (AI) was calculated as [optical density of the diethylamine-treated well]/[optical density of the nontreated well] × 100. Samples with AIs of <80% were characterized as having positive results. The third assay, the Bio-Plex (Luminex) assay, was used to measure both anti-HIV IgG titer and antibody avidity. This assay was performed as previously described, with the exception that magnetic COOH beads (Bio-Rad Laboratories, Hercules, California) were used for the antigen-coupling procedure, as opposed to polystyrene beads, which were used in earlier studies [11]. The beads were coupled with recombinant HIV-1 gp120, gp160, and 2 forms of gp41 proteins (gp41 intact and gp41 truncated; Immunodiagnostics, Woburn, Massachusetts, the truncated peptide was produced by the CDC Core Facility). All plasma samples were tested in duplicate. A normalized mean fluorescent intensity (MFI) was calculated for each sample and was compared to the MFI of a calibrator; the AI was determined from these 2 values, as previously described [11].
Statistical Methods
Kaplan–Meier plots, log-rank tests, and Cox proportional hazard models were used to compare the time to the cutoff for each assay (duration that samples were assay positive) in the TFV and placebo study arms, adjusting for viral load and CD4+ T-cell count, when appropriate. Time points were censored from the analysis when the subject initiated antiviral treatment. The rate of increase of antibody titer or avidity, measured using the Luminex assay, was compared in the 2 study arms for each of the 4 target antigens; differences in antibody avidity were examined using linear mixed effects. Frequencies were compared using the Fisher exact test or the χ2 test, when appropriate.
RESULTS
Anti-HIV antibody maturation was evaluated in 553 samples from 95 women who acquired HIV infection in the CAPRISA 004 trial; 35 were in the TFV arm, and 60 were in the placebo arm (Table 1). While TFV exposure at the time of HIV infection could not be assessed, median gel adherence in the 35 seroconverters assigned to receive TFV gel was 55%, based on an average of 7 returned empty applicators per month. The mean number of samples tested per woman and the mean age at the time of HIV acquisition were the same for the 2 study arms. The mean CD4+ T-cell count at the first HIV-positive visit and the median log10 HIV load during follow-up (after infection) were similar for women in the 2 study arms. Seventeen women initiated ART during the study, and 3 women died during follow-up (Table 1).
Table 1.
Characteristics of Study Participants, by Intervention Status
| Characteristic | Intervention (n=35) | Placebo (n=60) | P Value |
|---|---|---|---|
| Samples, no. | 197 | 356 | .81 |
| Samples/woman, no. | |||
| Mean ± SD | 6 ± 1 | 6 ± 1 | |
| Range | 3–7 | 4–7 | .43 |
| Age at infection, y | |||
| Mean ± SD | 24 ± 5 | 24 ± 4 | .59 |
| Range | 19–37 | 19–38 | |
| CD4+ T-cell count at first HIV-positive visit, cells/μL, mean (95% CI) | 530 (459–601) | 593 (537–650) | .09 |
| HIV load during follow-up,a log10 copies/mL, median (95% CI) | 4.33 (4.08–4.58) | 4.00 (3.75–4.25) | .96 |
| Receiving ART during follow-up, no. | 8 | 9 | .24 |
| Died during follow-up, no. | 2 | 1 | .31 |
Abbreviations: ART, antiretroviral treatment; CI, confidence interval; HIV, human immunodeficiency virus; SD, standard deviation.
a Defined as study visits after receipt of a diagnosis of HIV infection.
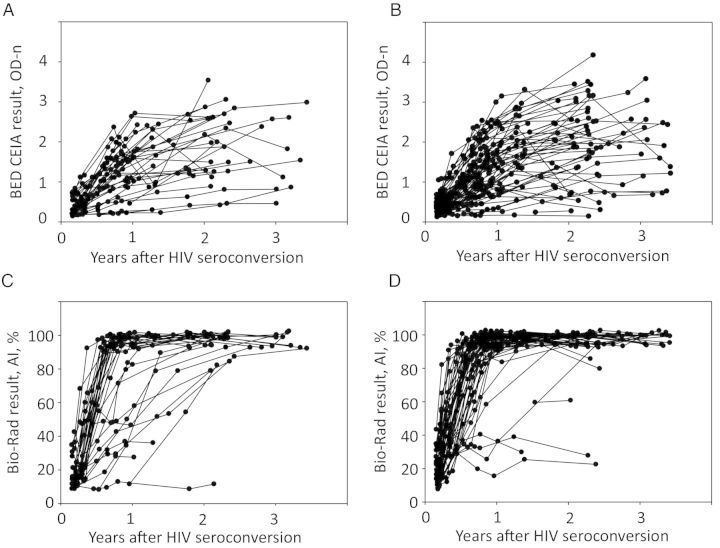
Results obtained using the BED CEIA and Bio-Rad avidity assay are shown in Figure 1. The failure to obtain an assay-positive result indicates delayed or absent antibody maturation. Time trends for the proportion of IgG that is HIV specific after HIV seroconversion were similar for the 2 study arms. The proportion of women who had an assay-positive result throughout the follow-up period was also similar in the 2 study arms (3 of 35 in the TFV arm vs 4 of 60 in the placebo arm [P = .71]; Figure 1A and 1B, respectively). The proportion of women in the 2 arms who had an assay-positive result for the Bio-Rad avidity assay throughout the follow-up period was also similar in the 2 study arms (4 of 35 in the TFV arm vs 3 of 60 in the placebo arm [P = .42]; Figure 1C and 1D, respectively).
Figure 1.
Results obtained using the BED capture enzyme immunoassay (CEIA; top panels) and the Bio-Rad avidity assay (bottom panels) for women in the tenofovir (TFV) study arm (A and C) and those in the placebo study arm (B and D). Results for the BED CEIA are reported as normalized optical density units (OD-n); results for the Bio-Rad avidity assay are reported as avidity indexes (AIs; see “Methods” section). Abbreviation: HIV, human immunodeficiency virus.
Kaplan–Meier analyses were performed to compare the proportion of women with assay-positive test results as a function of time after HIV seroconversion (Figure 2). For the BED CEIA (Figure 2A), there was no significant difference in the results obtained for women in the TFV and placebo arms (P = .58). In contrast, women in the TFV arm had positive results for the Bio-Rad avidity assay for a longer time after seroconversion than women in the placebo arm (P = .036; Figure 2B). The mean delay in achieving an AI of 80% in the TFV arm, compared with the placebo arm, was 22 days.
Figure 2.
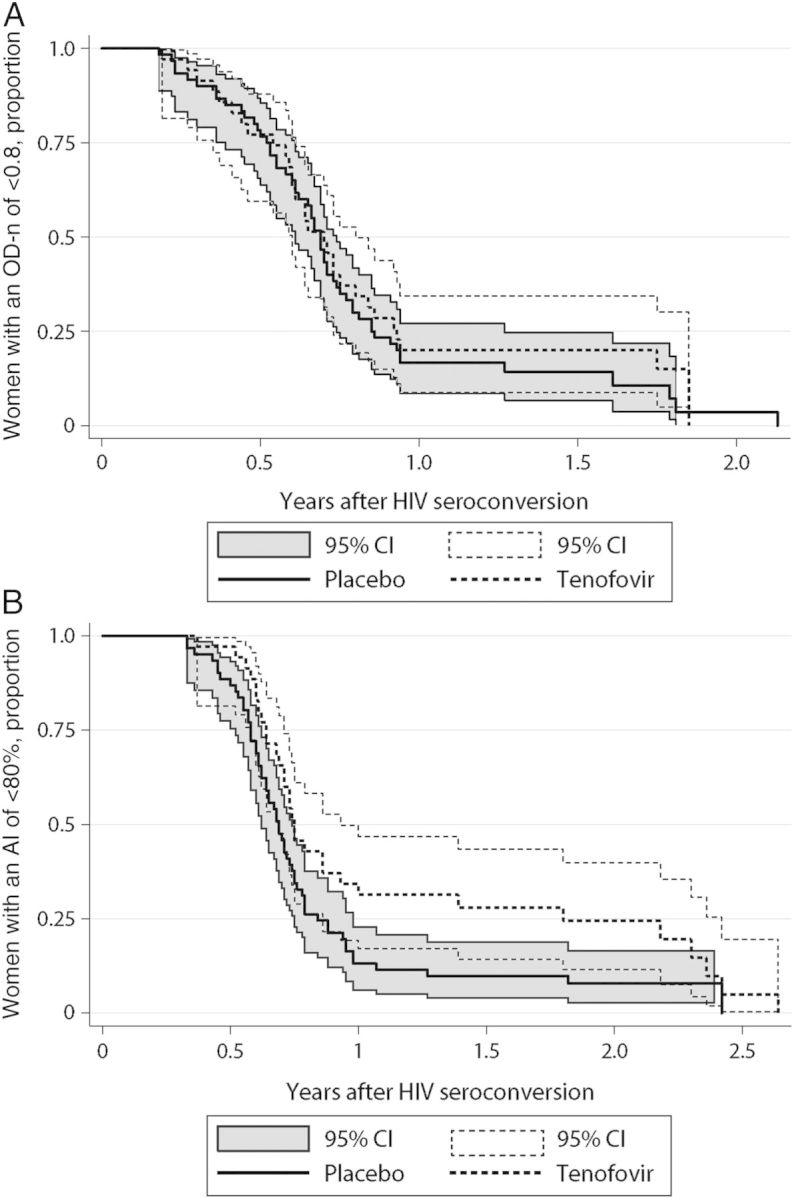
Kaplan–Meier analysis showing the proportion of women crossing the assay cutoff, by time after human immunodeficiency virus (HIV) seroconversion. A, Time to reach a BED capture enzyme immunoassay result of 0.8 normalized optical density units (OD-n). B, Time to reach a Bio-Rad avidity index (AI) of 80%. Results for the TFV arm are shown with a dashed line; results from the placebo arm are shown with a solid line and dark shading. Abbreviations: CI, confidence interval; TFV, tenofovir.
In a univariate Cox proportional hazard model, the time to obtaining an AI result of >80% was significantly longer in the TFV arm than that the placebo arm (hazard ratio [HR], 0.62; 95% confidence interval [CI], .39–.97; P = .035). In a multivariate model, the association between study arm and antibody maturation was independent of CD4+ T-cell count and set point HIV load at the time of HIV seroconversion (HR, 0.52; 95% CI, .32–.84; P = .008).
Antibody titer and antibody avidity were also assessed using the Luminex assay. There was no significant difference in antibody titer for any of the 4 Luminex target antigens for women in the TFV arm versus those in the placebo arm (Supplementary Figure 1). However, after seroconversion, there was significantly lower rate of increase in antibody avidity to the gp120 target antigen (P = .028) and a trend of a lower rate of increase in antibody avidity to the gp160 target antigen (P = .056), using linear mixed effects models (Supplementary Figure 2). These differences were not observed for either of the 2 gp41 target antigens (P = .759 for gp41 intact; P = .224 for gp41 truncated).
DISCUSSION
We observed a delay in antibody maturation following HIV infection in women who were using topical TFV gel for PrEP. This association was observed using 2 independent methods for assessing antibody avidity (the Bio-Rad avidity assay, which includes gp160 and p24 target antigens, and the Luminex assay, for the gp120 target antigen). In contrast, there was no significant difference in antibody maturation using an assay that measures the proportion of IgG that is HIV-specific (BED CEIA) or using the Luminex assay to measure antibody titer (for any of the 4 target antigens). The association of delayed evolution of antibody avidity and TFV gel use was independent of HIV load and CD4+ T-cell count measured at the time of HIV seroconversion. The lack of association with HIV load indicates that differences in antibody maturation are not related to viral suppression by TFV; in this setting, viral suppression would have been transient, since study drug was stopped as soon as HIV infection was detected or diagnosed. The delayed or absent increases in antibody avidity in women using TFV gel appear to be selective; these differences were observed for antibodies that bind the Bio-Rad avidity assay target antigens (gp160 and p24) and for some but not all of the Luminex antigens (for gp120, but not for gp41 or gp160).
We compared our findings to results from a study of oral TFV PrEP in rhesus macaques [6]. In both studies, TFV PrEP was associated with delayed/diminished antibody avidity, with no impact on antibody titer. In the macaque model, altered evolution of antibody avidity was observed for antibodies directed toward gp41; this was not observed in our study. Also, in the macaque study, peak simian immunodeficiency virus load and viral load set point were >1 log lower in animals that received oral TFV PrEP [6]. In contrast, women in the TFV arm, who received topical gel PrEP in the CAPRISA 004 study, did not have lower HIV loads than women in the placebo arm at the time of HIV seroconversion [9]. It is notable that the viral load set point was higher among women in the TFV arm, compared with those in the placebo arm [12]. Levels of neutralizing antibodies were reduced following infection in macaques that received oral TFV PrEP [6]; we did not analyze neutralizing antibodies in this study. Species differences and other factors may be responsible for differences observed in antibody maturation and viral load responses in our study and the macaque study.
ART administered during the acute phase of infection has also been associated with a diminished antibody avidity and titer to HIV [13]. When ART is discontinued and these individuals no longer had a suppressed viral load, their antibody responses quickly to rose to levels similar to those of individuals who did not receive ART during acute infection [5]. Additionally, ART-induced viral suppression in individuals with chronic HIV infection is associated with a reduction in antibody titer, and viral breakthrough during ART in these individuals is associated with an increase in antibody titer [14–16]. In contrast, antibody avidity does not typically decline during ART-induced viral load suppression [15, 17, 18].
This report indicates that topical TFV PrEP use influences the maturation of antibody avidity in individuals who become infected with HIV. This decreased antibody avidity maturation may affect population-level incidence estimates calculated using cross-sectional methods that solely rely on antibody avidity to differentiate recent from nonrecent infection. The slower avidity maturation would increase the window period for the assay, which, if unaccounted for, would result in overestimation of the population-level incidence. Use of a combination of serologic assays that measure both antibody titer and avidity should rectify this problem. Further research is needed to identify the mechanisms involved and to determine whether similar associations are seen in individuals receiving oral TFV PrEP (rather than TFV gel). Further research is also needed to determine whether TFV PrEP use is associated with alternations in viral diversity/diversification following infection, which could influence antibody maturation; an initial report did not find altered diversity in this setting [19]. Finally, it is not known whether the size or composition of the latently infected viral reservoir is altered in individuals who are infected while taking PrEP. Given the increase in PrEP use following Food and Drug Administration approval of oral TFV PrEP, it is important to understand how PrEP use influences the immune response to HIV infection. Alterations in antibody maturation may influence the performance of assays used for HIV diagnosis, as well as assays (such as the Bio-Rad avidity assay) that are used for cross-sectional estimation of the incidence of HIV infection. These issues should be considered in surveillance and other studies when TFV is used for HIV prevention.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all the CAPRISA 002 Acute Infection Study participants, who are continuing to make an important personal contribution to human immunodeficiency virus (HIV) research through their support and participation in our studies; the CAPRISA 004 and CAPRISA 002 study and protocol teams, for their scientific and supportive roles; and Allison Kirkpatrick and Kelly Curtis, for their technical support.
Disclaimer. The views expressed by the authors do not necessarily reflect the views of the US Agency for International Development, Gilead Sciences, Eastern Virginia Medical School, CONRAD, or the National Institute of Allergy and Infectious Diseases (NIAID).
Financial support. This work was supported by the NIAID, National Institutes of Health (NIH; grant AI51794); the National Research Foundation (grant 67385), the Columbia University–Southern African Fogarty AIDS International Training and Research Programme, funded by the Fogarty International Center, NIH (grant D43TW00231); and a training grant from LifeLab, a biotechnology centre of the South African Government Department of Science and Technology. The parent trial (CAPRISA 004) was supported by the US Agency for International Development (USAID), FHI360 (USAID cooperative agreement GPO-A-00-05-00022-00; contract 132119), and the Technology Innovation Agency (LifeLab). Tenofovir was provided by Gilead Sciences and the gel was manufactured and supplied for the CAPRISA 004 trial by CONRAD. The current studies are part of the CAPRISA TRAPS (Tenofovir Gel Research for AIDS Prevention Science) Program, which is funded by CONRAD, Eastern Virginia Medical School (USAID cooperative grant GP00-08-00005-00; subproject agreement PPA-09-046). The research was also supported by the HIV Prevention Trials Network, sponsored by NIAID, the National Institute on Drug Abuse, the National Institute of Mental Health, and the Office of AIDS Research, NIH (UM1AI068613); and by the NIAID (R01 AI095068). Additional support was provided in part by the Division of Intramural Research, NIAID, NIH.
Potential conflicts of interest. S. S. A. K. and Q. A. K. are coinventors with scientists from Gilead Sciences of 2 pending patents (61/354.050 and 61/357.892) of tenofovir gel against herpes simplex virus types 1 and 2. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS 2012; 26:F13–9. [DOI] [PubMed] [Google Scholar]
- 5.Killian MS, Norris PJ, Rawal BD, et al. The effects of early antiretroviral therapy and its discontinuation on the HIV-specific antibody response. AIDS Res Hum Retroviruses 2006; 22:640–7. [DOI] [PubMed] [Google Scholar]
- 6.Curtis KA, Kennedy MS, Luckay A, et al. Delayed maturation of antibody avidity but not seroconversion in rhesus macaques infected with simian HIV during oral pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2011; 57:355–62. [DOI] [PubMed] [Google Scholar]
- 7.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One 2008; 3:e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobbs T, Kennedy S, Pau CP, McDougal JS, Parekh BS. Performance characteristics of the immunoglobulin G-capture BED-enzyme immunoassay, an assay to detect recent human immunodeficiency virus type 1 seroconversion. J Clin Microbiol 2004; 42:2623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis KA, Kennedy MS, Charurat M, et al. Development and characterization of a bead-based, multiplex assay for estimation of recent HIV type 1 infection. AIDS Res Hum Retroviruses 2012; 28:188–97. [DOI] [PubMed] [Google Scholar]
- 10.Masciotra S, Dobbs T, Candal D, et al. Antibody avidity-based assay for identifying recent HIV-1 infections based on genetic systems TM 1 / 2 plus O EIA [abstract 937]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, California, 16–19 February 2010. [Google Scholar]
- 11.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One 2008; 16:e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett NJ, Werner L, Naicker N, et al. HIV disease progression in seroconvertors from the CAPRISA 004 tenofovir gel preexposure prophylaxis trial. J Acquir Immune Defic Syndr 2015; 68:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassutto S, Johnston MN, Rosenberg ES. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin Infect Dis 2005; 40:868–73. [DOI] [PubMed] [Google Scholar]
- 14.Marinda ET, Hargrove J, Preiser W, et al. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J Acquir Immune Defic Syndr 2010; 53:496–9. [DOI] [PubMed] [Google Scholar]
- 15.Wendel SK, Mullis CE, Eshleman SH, et al. Effect of natural and ARV-induced viral suppression and viral breakthrough on anti-HIV antibody proportion and avidity in patients with HIV-1 subtype B infection. PLoS One 2013; 8:e55525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimerman S, Sucupira MC, Lewi DS, Diaz RS. Less sensitive HIV-1 enzyme immunoassay as an adjuvant method for monitoring patients receiving antiretroviral therapy. AIDS Patient Care STDS 2007; 21:100–5. [DOI] [PubMed] [Google Scholar]
- 17.Longosz AF, Mehta SH, Kirk GD, et al. Incorrect identification of recent HIV infection in adults in the United States using a limiting-antigen avidity assay. AIDS 2014; 28:1227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating SM, Hanson D, Lebedeva M, et al. Lower-sensitivity and avidity modifications of the vitros anti-HIV 1+2 assay for detection of recent HIV infections and incidence estimation. J Clin Microbiol 2012; 50:3968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valley-Omar Z, Sibeko S, Anderson J, et al. CAPRISA 004 tenofovir microbicide trial: no impact of tenofovir gel on the HIV transmission bottleneck. J Infect Dis 2012; 206:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.