Abstract
Background. Despite vaccination, residents of long-term-care facilities (LTCFs) remain at high risk of influenza-related morbidity and mortality. More-effective vaccine options for this population are needed.
Methods. We conducted a single-blinded, randomized, controlled trial comparing high-dose (HD) to standard-dose (SD) inactivated influenza vaccine (IIV) in 205 frail, elderly residents of LTCFs during the 2011–2012 and 2012–2013 influenza seasons. Hemagglutination inhibition (HI) antibody titers were measured at baseline and 30 and 180 days following vaccination.
Results. A total of 187 subjects (91%) completed the study. The mean age was 86.7 years. Geometric mean titers (GMTs) were significantly higher (P < .05) at day 30 for HD recipients, compared with SD recipients, for all comparisons except influenza A(H1N1) during 2012–2013 (the HD formulation was noninferior to the SD formulation for influenza A[H1N1] during 2012–2013). GMTs for HD and SD recipients during 2011–2012 were as follows: influenza A(H1N1), 78 (95% confidence interval [CI], 45–136) and 27 (95% CI, 17–44), respectively; influenza A(H3N2), 26 (95% CI, 17–40) and 10 (95% CI, 7–15), respectively; and influenza B, 26 (95% CI, 19–35) and 14 (95% CI, 11–18), respectively. During 2012–2013, GMTs for HD and SD recipients were as follows: influenza A(H1N1), 46 (95% CI, 33–63) and 50 (95% CI, 37–67); influenza A(H3N2), 23 (95% CI, 18–31) and 14 (95% CI, 11–18), respectively; and influenza B, 26 (95% CI, 21–32) and 17 (95% CI, 14–22), respectively. GMTs were significantly higher at day 180 for HD recipients, compared with SD recipients, for influenza A(H3N2) in both years (P < .001).
Conclusions. Among frail, elderly residents of LTCFs, HD influenza vaccine produced superior responses for all strains except influenza A(H1N1) in 2012–2013.
Clinical Trials Registration. NCT01654224.
Keywords: inactivated influenza vaccine, high-dose influenza vaccine, long-term care
(See the editorial commentary by Lindley and Bridges on pages 1860–1.)
Each year in the United States, 3000–49 000 influenza-associated deaths occur, with over 90% reported among older adults aged 65 years or older [1]. Influenza also results in an estimated 226 000 hospitalizations, with hospitalization rates among older adults increasing over the past 2 decades. Mortality is 16-fold higher among adults aged ≥85 years, compared with those aged 65–69 years [2]. The estimated average total cost (±SD) per case of influenza in long-term care facilities (LTCFs) in 2013 was $1886 ± $2899, with ≥80% of the cost due to hospitalization [3].
The clinical efficacy of inactivated influenza vaccine (IIV) is reduced in the elderly population, ranging from 17% to 60%, depending on the end point [4, 5]. The reduced efficacy could be caused by the decreased function of the immune system with age [6]. Decreased functionality of the adaptive immune system is reflected by decreased production of antibodies by antigen-naive T and B cells, decreased diversity of antibody responses, and decreased ability to respond to new and emerging pathogens [7]. The T and B cells produced by elderly individuals are phenotypically different from those produced in young adults and are less effective at responding to infections [6].
Elderly residents of LTCFs have higher risks of influenza exposure, decreased immune defenses, and higher mortality than elderly individuals residing elsewhere. Therefore, more-effective vaccination options are needed for frail older adults [8]. Various strategies have been developed to improve vaccines to elicit a stronger immune reaction among elderly individuals. One approach to improving the immune response to vaccines has been to increase the antigen dose in vaccines to create a high-dose (HD) vaccine. In December 2009, the Food and Drug Administration licensed a HD trivalent IIV (Fluzone High-Dose, Sanofi Pasteur) specifically designed for adults aged ≥65 years. The vaccine contains 180 µg (60 µg of each strain) of influenza virus hemagglutinin per 0.5-mL dose, compared with 45 µg (15 µg of each strain) in the standard-dose (SD) vaccine [9]. The HD vaccine was approved by the Food and Drug Administration via an accelerated process that allows for quick delivery of safe products to the marketplace that may prevent serious or life-threatening diseases.
Several studies have demonstrated that HD vaccines can produce an enhanced immunologic response with no major safety concerns [10–18]. However, no study has examined the issue by using this preparation among LTCF residents. An older dose-ranging study of another preparation found that higher doses increased the response to some but not all antigens in the LTCF setting [19]. Given the rapid growth of the older adult population in the United States, it is critical to examine whether HD IIV can produce an enhanced immunologic response among frail residents of LTCFs. Such an analysis can yield valuable data for future effectiveness trials and, eventually, cost-effectiveness analyses. This clinical trial, conducted during the 2011–2012 and 2012–2013 influenza seasons, assessed the noninferiority and superiority of HD IIV versus SD IIV among LTCF residents.
METHODS
Study Design
This study was conducted to compare the immunogenicity of SD IIV to that of HD IIV in frail, elderly residents of LTCFs 30 and 180 days after vaccination, using hemagglutination inhibition (HI) antibody titers. The single-blinded, randomized, controlled trial was conducted during 2 influenza vaccination seasons (September 2011–March 2012 and August 2012–March 2013). The start of the study was delayed 1 month in the first season while awaiting tandem approval from the Pennsylvania Department of Health and University of Pittsburgh institutional review boards (IRBs). The study was approved by the IRBs of the University of Pittsburgh (PRO100110247) and the Pennsylvania Departments of Health, and registered at ClinicalTrials.gov as NCT01654224. Written informed consent was obtained from all participants or, for those without decisional capacity, from their legal healthcare proxy. Human experimentation guidelines of the Department of Health and Human Services and those of the authors’ institutions were followed in the conduct of this research study.
Sites and Participants
Frail adults aged ≥65 years were recruited from 15 community-based LTCFs (4 nursing facilities, 3 assisted-living or personal-care homes, and 8 independent-living facilities) with 42–178 beds in western Pennsylvania. Recruitment was conducted throughout the entire influenza season (1 September 2011–31 March 2012 and 1 August 2012–31 March 2013). A letter introducing the study was sent to potential subjects by the facility administration, and recruitment was performed using informational flyers, social gatherings, and facility staff referrals. Potential participants were eligible if they met the following criteria: (1) residence at one of the participating sites; (2) age of ≥65 years; and (3) need for full or partial assistance in at least 2 instrumental activities of daily living and/or at least 1 activity of daily living [20]. Clinical staff with direct knowledge of the subject's functional abilities and/or medical provider confirmed the requirement for assistance. Exclusion criteria included (1) life expectancy of <6 months; (2) history of allergic reaction to influenza vaccine, its components, or eggs; severe allergic reaction to latex, which was in the syringe stopper; (3) history of Guillain-Barré syndrome; (4) immunosuppression, including active chemotherapy or radiation therapy, serious current or expected immunosuppression in the next 6 months, use of prednisone (or another systemic steroid) at prednisone-equivalent dosages of ≥10 mg/day within the past 14 days; and (5) any condition that, in the opinion of the investigator, interfered with the evaluation of study objectives. Conditions in which potential subjects could have been excluded by the investigator included inability to reach the proxy decision maker for subjects with impaired decisional capacity and subjects deemed to have decisional capacity but whose judgment was impaired from a psychiatric condition to the extent they would not be able to comply with the study protocol. There were no exclusions granted as a result of criterion 5.
Randomization and Blinding
At enrollment, participants were randomized to receive either SD IIV (control) or HD IIV (intervention). Randomization was computer generated and conducted by a 1:1 allocation to the SD group or the HD group. A coordinator assigned randomly generated study identification numbers to subjects at enrollment. The subjects and the investigator performing the laboratory measurements were blinded. Study staff administering the vaccines and completing the clinical assessments were not blinded.
Study Interventions
All study interventions were conducted on site. Following provision of informed consent, subjects completed a baseline evaluation that documented their medical condition, medications, immunization history, functional status, and temperature. Frailty was measured using both functional status scales, as well as gait speed. Functional status scales included those for standard activities of daily living and instrumental activities of daily living. Both scales contain 7 items, each scored from 0 to 2, for a maximum score of 14 per scale. Higher scores indicate higher functional status. Gait speed was measured using a timed 4-m walk with a 2-m run-in and cool-down phase. Normal scores are ≥1 m/second, and scores of ≤0.8 m/second indicate significant frailty and increased mortality risk [21, 22]. Data were collected and managed using Research Electronic Data Capture, an Internet-based electronic data-capture tool hosted at the University of Pittsburgh.
Following collection of baseline blood samples, subjects were administered 0.5 mL of the assigned IIV, using a 23-gauge, 2.54-cm (1-inch) needle, in the deltoid. Vaccines used the World Health Organization–recommended influenza virus strains for each season: A/California/7/2009(H1N1), A/Victoria/210/2009(H3N2), and B/Brisbane/60/2008 for 2011–2012 and A/California/7/2009(H1N1), A/Victoria/361/2011(H3N2), and B/Texas/6/2011 for 2012–2013. The SD IIV and HD IIV contained 15 µg and 60 µg, respectively, of hemagglutinin for each strain. Subjects were observed for 15 minutes following vaccination. Follow-up visits were conducted at 30 and 180 days (±14 days) after vaccination, to assess for vaccine-related serious adverse events and to obtain blood samples. Blood samples were labeled using a coded identifier, refrigerated at 4°C, and transported each day to the laboratory for processing. The flow of enrollment to the primary 30-day analysis appears in Figure 1.
Figure 1.
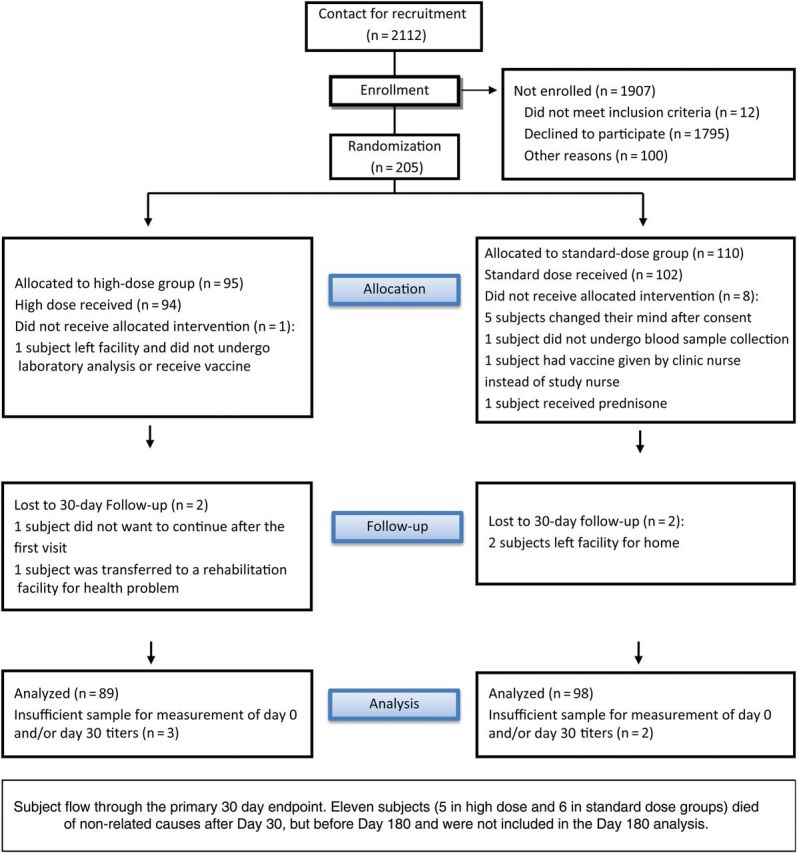
Consolidation Standards of Reporting Trials diagram of recruitment, enrollment, randomization, follow-up, and analyses.
Serum Sample Processing and Immunogenicity Testing
Each serum sample was tested in HI assays against the 3 respective vaccine strains for each season. The HI assay protocol was adapted from the Centers for Disease Control and Prevention laboratory-based influenza surveillance manual [23] and assessed for antibodies that blocked hemagglutinin receptor binding and inhibited agglutination of turkey erythrocytes. To inactivate nonspecific inhibitors, sera were treated with receptor-destroying enzyme before being tested [24–28]. The HI titer was determined in single assays by the reciprocal dilution of the last well that contained nonagglutinated red blood cells. Positive and negative serum controls were included for each plate.
Sample Size
To achieve a statistical power of 0.8 and an α of 0.025, the required number of subjects would depend on following elements [29]: (1) the noninferiority margin, which was set to log2[1.5]; (2) the within-group standard deviation of the log-transformed immunogenicity values, which was set to the standard deviation of the log2 HI titer; and (3) the difference between the arithmetic means of the probability distributions underlying the log-transformed immunogenicity values for the HD and SD groups, which was set to 0. By use of the historical standard deviation of 1.47, 101 subjects per group would be required to achieve statistical power of 80%. On the basis of our observational data with standard deviations ranging from 1.27 to 1.74, a sample size of 75–147 subjects per group would be needed.
Statistical Analyses
Descriptive analyses were performed for select demographic characteristics of patients. χ2 and t tests were used to examine whether participants' characteristics differed between the HD and SD groups. In western Pennsylvania, the primary racial group is white; therefore, race was reported as white and nonwhite.
All outcome measures were analyzed by randomized group. The distribution of titers are generally skewed; therefore, log-transformed titers were used in analyses. Geometric mean titers (GMTs), which are presented with 95% confidence intervals (CIs), were the primary outcome. GMTs on days 0, 30s and 180 were compared between the HD and SD groups, using t tests. Reverse cumulative distribution (RCD) curves were developed. We conducted a log-rank (Mantel–Cox) test for equality of distributions for log2 HI titers between randomized groups. Noninferiority and superiority tests were performed separately for the 2011–2012 and 2012–2013 vaccine formulations because the vaccines contained different influenza A(H3N2) and influenza B strains. An upper bound >0.67 of the 2-sided 95% CI of the ratio of postvaccination GMTHD to GMTSD indicated noninferiority. A lower bound of ≥1.0 of the 2-sided 95% CI of the ratio of GMTHD to GMTSD indicated superiority.
The secondary outcomes measured included (1) the percentage of individuals who were seroprotected (defined as a titer of ≥40) at days 0, 30, and 180; (2) the percentage who seroconverted between day 0 and day 30; and (3) the GMT ratios between day 30 and day 180. Seroconversion was defined as a day 30 HI titer of ≥40 and a 4-fold increase from the day 0 HI titer. Seroprotection and seroconversion were compared for the HD and SD groups by using χ2 tests. McNemar tests were used to compare the difference in seroprotection between day 0 and day 30, as well as between day 0 and day 180, within each group.
We performed linear regression analyses on outcome variables, using randomized group indicators (HD vs SD) and patient characteristics, based on variables significant at P values of ≤.10 in univariate analyses. The regressions were performed separately for the 2011–2012 and 2012–2013 vaccine formulations. The statistical significance of 2-sided tests was set at an α of <0.05.
RESULTS
Baseline Characteristics and Success of Randomization
The completion rate was 91% (187 of 205 participants) at 30 days, including 64 participants in 2011–2012 and 123 in 2012–2013; 32 subjects participated both years. The mean age of participants was 86.7 years; 71% were aged ≥85 years. The majority (99%) were white, and 68% were female (Table 1). Demographic profiles of both groups were similar. Compared with individuals in the HD group, a higher proportion of individuals in the SD group were overweight or obese (61% vs 45%; P < .05) and had a higher body mass index (BMI; defined as the weight in kilograms divided by the height in meters squared). Self-reported health status, functional status, and gait speed did not differ between the SD and HD groups. Functional status and gait speed scores confirmed the frail nature of the study population (Table 1). GMTs before influenza vaccination were not different between the SD and HD groups for all strains during both influenza seasons (Table 2). During each season, vaccines were administered from study start until December of 2011 or November 2012. A total of 97% and 100% of day 30 visits were completed by 31 December 2011 and 31 December 2012, respectively, before the onset of local seasonal influenza activity. Day 30 visits for 2 subjects during 2011–2012 were completed in January 2012. All day 180 visits were completed by June 2012 for the 2011–2012 season and by March 2013 for the 2012–2013 season.
Table 1.
Descriptive Statistics, by Randomized Group of Frail, Elderly Residents of Long-term Care Facilities
| Characteristic | Overall (n = 187) |
Randomized Group |
P valuea | ||||
|---|---|---|---|---|---|---|---|
| Standard Dose (n = 98) |
High Dose (n = 89) |
||||||
| Subjects, No. | Value | Subjects, No. | Value | Subjects, No. | Value | ||
| Female sex, % | 128 | 68 | 71 | 72 | 57 | 64 | .22 |
| White, non-Hispanic, % | 185 | 99 | 96 | 98 | 89 | 100 | .18 |
| Age | |||||||
| Overall, y, mean ± SD | 187 | 87 ± 6 | 98 | 86 ± 6 | 89 | 87 ± 6 | .37 |
| ≥85 y, % | 133 | 71 | 68 | 69 | 65 | 73 | |
| BMIb | |||||||
| Overall, mean ± SD | 187 | 26 ± 5 | 98 | 27 ± 5 | 89 | 25 ± 5 | .01 |
| ≥ 25 | 100 | 54 | 60 | 61 | 40 | 45 | <.001 |
| Health status | .77 | ||||||
| Excellent/good, % | 122 | 65 | 63 | 64 | 59 | 66 | |
| Fair/poor/cannot answer, % | 65 | 35 | 35 | 36 | 30 | 34 | |
| Gait speed, m/sec, mean ± SDc | 158 | 0.7 ± 0.3 | 82 | 0.7 ± 0.3 | 76 | 0.7 ± 0.3 | .83 |
| ADL score, mean ± SD | 187 | 11.4 ± 3.7 | 98 | 11.4 ± 3.7 | 89 | 11.5 ± 3.8 | .77 |
| IADL score, mean ± SD | 187 | 7.9 ± 4.2 | 98 | 7.8 ± 4.3 | 89 | 7.9 ± 4.1 | .88 |
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; SD, standard deviation.
a By the χ2 or t test.
b Body mass index (BMI) is defined as the weight in kilograms divided by the height in meters squared. A BMI of ≥ 25 was considered overweight or obese.
c Data were missing for 29 subjects.
Table 2.
Comparison of Geometric Mean Titers (GMTs) From Hemagglutination Inhibition Assays at Days 0, 30, and 180 for Recipients of Standard-Dose and High-Dose Influenza Vaccines, by Influenza Season and Vaccine Strain
| Season, Strain | Day 0, GMT (95% CI) |
Day 30, GMT (95% CI) |
Day 180,a GMT (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard Dose | High Dose | P valueb | Standard Dose | High Dose | P valueb | Standard Dose | High Dose | P valueb | |
| 2011–2012 | n = 33 | n = 31 | n = 33 | n = 31 | n = 24 | n = 26 | |||
| A/California/07/2009(H1N1) | 16.6 (10.3–26.7) | 17.1 (11.3–25.9) | .917 | 27.4 (17–44.3) | 78.2 (45.1–135.7) | .005 | 28.3 (15.3–52.4) | 59.7 (33.5–106.3) | .074 |
| A/Victoria/210/2009(H3N2) | 7.3 (5.3–10.0) | 8.9 (6.5–12.3) | .360 | 10.2 (7.0–14.8) | 26.2 (17.1–40.0) | .001 | 9.4 (6–14.8) | 22.3 (14.5–34.3) | .006 |
| B/Brisbane/60/2008 | 11.6 (8.6–15.5) | 15.3 (10.3–22.6) | .248 | 14.3 (11.1–18.4) | 25.6 (18.7–34.9) | .004 | 15.4 (11.8–20.2) | 22.9 (16.3–32) | .069 |
| 2012–2013 | n = 65 | n = 58 | n = 65 | n = 58 | n = 59 | n = 53 | |||
| A/California/07/2009(H1N1) | 32.3 (23.8–43.9) | 23.6 (16.7–33.4) | .178 | 50.0 (37.4–67) | 45.6 (32.9–63.2) | .672 | 51.8 (37.8–71.1) | 46.8 (33.2–65.9) | .663 |
| A/Victoria/361/2011(H3N2) | 6.2 (5.4–7.1) | 7.2 (6.1–8.3) | .152 | 14.2 (11.0–18.4) | 23.4 (17.6–31) | .011 | 13.4 (10.3–17.5) | 24.7 (18.3–33.2) | .003 |
| B/Texas/6/2011 | 9.1 (7.5–11) | 7.9 (6.5–9.5) | .285 | 17.4 (13.9–21.9) | 26.0 (21.2–31.9) | .010 | 18.9 (14.9–23.9) | 25.3 (20.8–30.9) | .063 |
Abbreviation: CI, confidence interval.
a Six participants in the standard-dose group and 5 in the high-dose group died before collection of blood samples at day 180.
b By the t test.
Antibody Responses at Day 30
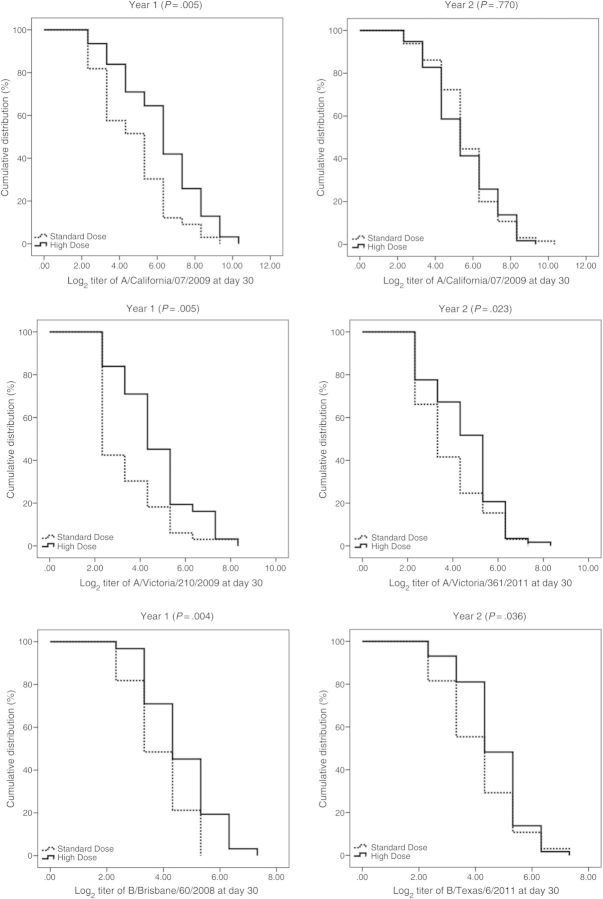
GMTs were significantly higher at day 30 for HD recipients, compared with SD recipients, for all comparisons except influenza A(H1N1) in 2012–2013 (Table 2). HD vaccine induced superior antibody responses to all 3 strains in 2011–2012 and to the influenza A(H3N2) and influenza B strains in 2012–2013 (Supplementary Table 1). HD induced a noninferior antibody response to influenza A(H1N1) in 2012–2013. Figure 2 shows comparisons of RCD curves, by randomized group.
Figure 2.
Reverse cumulative distribution of hemagglutination inhibition titers, by year and by strain, comparing high-dose and standard-dose influenza vaccines.
The rates of seroconversion at day 30 were higher in the HD group for all strains in both seasons (P < .05; Table 3). The rates of seroprotection were similar between the SD and HD groups for all strains at day 0, whereas they were significantly higher for influenza A(H3N2) and influenza B at day 30 in the HD group during both influenza seasons. Within-group comparisons over time showed that day 30 titers were higher than day 0 titers for both groups (P < .01, for each by the McNemar test; data not shown).
Table 3.
Seroprotection and Seroconversion, Based on Hemagglutination Inhibition (HI) Findings at Days 0, 30, and 180, for Recipients of Standard-Dose and High-Dose Influenza Vaccines, by Influenza Season and Vaccine Strain
| Season, Serostatus and Time(s), Strain | Overall, Subjects, No. (%) | Randomized Group, Subjects, No. (%) |
P valuea | |
|---|---|---|---|---|
| Standard Dose | High Dose | |||
| 2011–2012 | ||||
| Seroprotection, day 0 | n = 64 | n = 33 | n = 31 | |
| A/California/07/2009(H1N1) | 22 (34.4) | 12 (36.4) | 10 (32.3) | .73 |
| A/Victoria/210/2009(H3N2) | 4 (6.3) | 2 (6.1) | 2 (6.5) | .95 |
| B/Brisbane/60/2008 | 13 (20.3) | 5 (15.2) | 8 (25.8) | .29 |
| Seroprotection, day 30 | n = 64 | n = 33 | n = 31 | |
| A/California/07/2009(H1N1) | 39 (60.9) | 17 (51.5) | 22 (71.0) | .11 |
| A/Victoria/210/2009(H3N2) | 20 (31.3) | 6 (18.2) | 14 (45.2) | .02 |
| B/Brisbane/60/2008 | 21 (32.8) | 7 (21.2) | 14 (45.2) | .04 |
| Seroprotection, day 180b | n = 50 | n = 24 | n = 26 | |
| A/California/07/2009(H1N1) | 31 (62.0) | 11 (45.8) | 20 (76.9) | .02 |
| A/Victoria/210/2009(H3N2) | 14 (28.0) | 3 (12.5) | 11 (42.3) | .02 |
| B/Brisbane/60/2008 | 7 (14.0) | 1 (4.2) | 6 (23.1) | .10 |
| Seroconversion, day 30/day 0 | n = 64 | n = 33 | n = 31 | |
| A/California/07/2009(H1N1) | 25 (39.1) | 7 (21.2) | 18 (58.1) | <.01 |
| A/Victoria/210/2009(H3N2) | 12 (18.8) | 3 (9.1) | 9 (29.0) | .04 |
| B/Brisbane/60/2008 | 5 (7.8) | 0 (0.0) | 5 (16.1) | .02 |
| 2012–2013 | ||||
| Seroprotection, day 0 | n = 123 | n = 65 | n = 58 | |
| A/California/07/2009(H1N1) | 61 (49.6) | 35 (53.8) | 26 (44.8) | .32 |
| A/Victoria/361/2011(H3N2) | 5 (4.1) | 3 (4.6) | 2 (3.4) | .74 |
| B/Texas/6/2011 | 9 (7.3) | 4 (6.2) | 5 (8.6) | .60 |
| Seroprotection, day 30 | n = 123 | n = 65 | n = 58 | |
| A/California/07/2009(H1N1) | 81 (65.9) | 47 (72.3) | 34 (58.6) | .11 |
| A/Victoria/361/2011(H3N2) | 46 (37.4) | 16 (24.6) | 30 (51.7) | <.01 |
| B/Texas/6/2011 | 47 (38.2) | 19 (29.2) | 28 (48.3) | .03 |
| Seroprotection day 180b | n = 112 | n = 59 | n = 53 | |
| A/California/07/2009(H1N1) | 56 (50.0) | 31 (52.5) | 25 (47.2) | .57 |
| A/Victoria/361/2011(H3N2) | 51 (45.5) | 23 (39.0) | 28 (52.8) | .14 |
| B/Texas/6/2011 | 37 (33.0) | 20 (33.9) | 17 (32.1) | .84 |
| Seroconversion, day 30/day 0 | n = 123 | n = 65 | n = 58 | |
| A/California/07/2009(H1N1) | 21 (17.1) | 7 (10.8) | 14 (24.1) | <.05 |
| A/Victoria/361/2011(H3N2) | 40 (32.5) | 13 (20.0) | 27 (46.6) | <.01 |
| B/Texas/6/2011 | 35 (28.5) | 11 (16.9) | 24 (41.4) | <.01 |
Seroprotection was defined as a hemagglutination inhibition (HI) titer of ≥40. Seroconversion was defined as a day 30 HI titer of ≥40 and a 4-fold increase from the day 0 HI titer.
a By the χ2 test.
b Six participants in the standard-dose group and 5 in the high-dose group died before collection of blood samples at day 180.
To assess the impact of BMI, which differed at randomization, we conducted regression analyses with log2 HI titers as the dependent variable. The difference in BMI was not found to be significant (Supplementary Table 2). After controlling for BMI, the HD group had significantly higher log2 HI titers for all strains in both seasons, with the exception of influenza A(H1N1) in 2012–2013.
Antibody Responses at Day 180
Six individuals in the SD group and 5 in the HD group died before blood sample collection at day 180. GMTs were significantly higher at day 180 for the HD group, compared with the SD group, for influenza A(H3N2) in both years (P < .01), with increases of borderline significance for influenza A(H1N1) in 2011–2012 and B in both seasons (Table 2). Seroprotection at day 180 was significantly higher for the HD group for influenza A(H1N1) and influenza A(H3N2) during 2011–2012 but did not differ during 2012–2013 (Table 3).
Change in Antibody Titers Between Days 30 and 180
The duration of immunity was analyzed by comparing GMT ratios between days 30 and 180 within the SD and HD groups; all within-group comparisons showed a <1-log2 difference over time (Table 4). Although differences were <1 log2, statistically significant (P < .05) decreases were found for HD and SD influenza B strains in 2011–2012, for HD and SD influenza A(H1N1) strains in 2012–2013, and for the HD influenza B strain in 2012–2013. For influenza A(H3N2), increases over time were found in 2012–2013, which were statistically significant for the SD group but not the HD group; in that season, Pittsburgh experienced an outbreak of influenza A(H3N2) infection clinically in LTCFs.
Table 4.
Comparisons of the Duration of Immunity, Based on Day 30 and Day 180 Hemagglutination Inhibition (HI) Titers, for Recipients of High-Dose and Standard-Dose Inactivated Influenza Vaccines, by Influenza Season and Vaccine Strain
| Season, Strain | Log2 HI Titer, Day 30 − Day 180, Mean ± SD |
|
|---|---|---|
| Standard Dose | High Dose | |
| 2011–2012 | ||
| A/California/07/2009(H1N1) | 0.3 ± 1.0 | 0.1 ± 1.2 |
| A/Victoria/210/2009(H3N2) | 0.0 ± 0.8 | 0.1 ± 1.0 |
| B/Brisbane/60/2008 | 0.7 ± 0.8a | 0.7 ± 1.0a |
| 2012–2013 | ||
| A/California/07/2009(H1N1) | 0.6 ± 0.9a | 0.8 ± 1.2a |
| A/Victoria/361/2011(H3N2) | −0.8 ± 2.2a,b | −0.5 ± 2.2 |
| B/Texas/6/2011 | 0.2 ± 1.5 | 0.5 ± 1.4c |
Abbreviation: SD, standard deviation.
a P < .01, by the paired t test.
b Pittsburgh experienced an outbreak of influenza A(H3N2) infection in 2012–2013, which is a possible explanation for day 180 titers exceeding day 30 titers.
c P < .05, by the paired t test.
Serious Adverse Events
Eleven individuals (6 in the SD group and 5 in the HD group) died after the day 30 visit but before the day 180 visit. These deaths were due to underlying comorbidities and were unrelated to the study interventions. No other serious adverse events occurred.
DISCUSSION
The results of this randomized clinical trial show that, with the exception of A/H1N1 in 2012–2013, HD IIV produces higher GMTs and seroconversion rates than SD IIV in frail, older residents of LTCFs at day 30. Why HD IIV was superior to SD IIV for influenza A(H1N1) during 2011–2012 but not 2012–2013 is not clear. This may be because 26% of subjects (32 of 123) participated in both seasons, when influenza A(H1N1) strains were identical. Both vaccines were well tolerated, and there were no study-related serious adverse events. These results are important because this is the first study to evaluate the currently licensed HD IIV in the frail LTCF population. Prior studies evaluating HD IIV were conducted in healthy or medically stable community-dwelling older adults and showed an enhanced immunologic response with no major safety concerns [11, 12, 30]. SD IIV has decreased immunogenicity in persons ≥65 years of age [4, 31].
The duration of immunity is a key issue with the availability of substantial vaccine supplies in September and influenza seasons that extend into March or later [32]. One systematic review of 8 studies found no clear evidence that vaccine-induced antibody levels declined more rapidly in elderly individuals [33]. Seroprotection was maintained at least 4 months after vaccination in all 8 studies reporting these data for influenza A(H3N2) and in 5 of the 7 studies reporting data for the influenza A(H1N1) and B components. In evaluating seroprotection at the end of the influenza season in elderly individuals, the primary antibody response appears to be more relevant than antibody decline [33, 34]. Antibody titers decline in elderly individuals by 6 months after vaccination. In our data, antibody titers generally showed little change between 1 and 6 months. Declines that did occur, while statistically significant, were all <1 log2 HI on average and thus are not likely to be clinically significant. Pairwise comparison demonstrated an increase in influenza A(H3N2) titers over time during 2012–2013, probably owing to influenza A(H3N2) infection, which was widespread clinically in these LTCFs during 2012–2013.
This is the only study of HD IIV immunogenicity conducted in LTCFs, where recruitment and retention of frail individuals was particularly challenging. This study has several strengths, including its randomized, controlled design; inclusion of well-matched subjects; recruitment of subjects from multiple LTCFs; inclusion of subjects typical of LTCFs nationally, including those with significant cognitive impairment; and confirmation of frailty status by 2 separate methods, functional status and gait speed. Gait speed is a recognized measure of the frailty phenotype, directly correlated with functional status, and is inversely proportional to mortality [22]. Mean gait speed was only 0.7 m/second, confirming significant frailty. In addition, the study measured antibody levels at 6 months from vaccination.
The study has several limitations. First, the study identified titer response, rather than clinical disease, as the primary outcome. While protection from clinical disease is directly related to serum titer response, it is not possible to specify an absolute titer that confers protection [35]. A recently presented efficacy study comparing HD and SD IIV in community-dwelling older adults showed a correlation between higher titers and lower rates of influenza illness and hospitalization [36]. In that study, HD IIV produced GMTs that were nearly double those in the SD IIV recipients while reducing laboratory-confirmed influenza by 24%. With the exception of influenza A(H1N1) in 2012–2013, we found similarly increased GMTs in the HD IIV group. It is not possible to know whether this will translate into similar reductions in clinical disease among frail HD IIV recipients in LTCFs. We note that the GMTs and seroprotection rates observed in our study were substantially lower than those in this recent study. However, GMTs cannot be directly compared across studies. While intralaboratory measurement of GMT is reliable, reproducibility across laboratories is not. In addition, the validity of using seroprotection as a surrogate marker of vaccine protection remains doubtful [35, 37, 38]. It is unlikely that a clinical efficacy trial will be conducted in LTCFs. The recruitment rate for the present study was 9.7%. While low compared with that for clinical trials in other settings, this rate is typical of recruitment rates in other LTCF-associated trials [39–41]. Recruitment of LTCF residents presents significant challenges owing to the unique characteristics of the setting and IRB protections for this vulnerable population. Moreover, recruitment for a LTCF-associated vaccine trial has an additional challenge of competing with a facility's immunization program. Conducted annually at the start of each influenza season, facility immunization programs typically immunize the entire resident population over a 24–48-hour period. Thus, any vaccine trial in LTCFs must enroll and vaccinate individuals in an exceedingly short time frame—as soon as vaccine becomes available but before the facility begins immunizing. A trial using clinical outcomes would require a much larger sample size for adequate statistical power. These realities reinforce the need for LTCFs to continue focusing on other important influenza prevention measures, such as improving healthcare personnel vaccination rates, active surveillance of respiratory disease, early laboratory detection, and prompt use of antiviral medications, when indicated. A second limitation of this study is that it was only partially blinded. We do not believe this to be an important factor, however, because the study focused on antibody titers and only the research coordinators administering the vaccine were not blinded; all other investigators, including those performing the titer measurements, were blinded. Third, while multiple LTCFs were used, the trial was only conducted in the Pittsburgh metropolitan region, possibly limiting the generalizability of the findings. Fourth, the sample size was modest, although statistical significance was achieved.
In summary, this is the first randomized, controlled trial of the HD IIV immune response among LTCF residents. HD IIV produced superior HI titers for all strains among frail, elderly residents of LTCFs, except for influenza A(H1N1) in 2012–2013 (in which case noninferiority criteria were met), perhaps because 26% of subjects (32 of 123) participated in both seasons, during which influenza A(H1N1) strains were identical.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The sponsor had no role in the conduct, laboratory analysis, statistical analyses, and interpretation of the study. The views expressed herein are those of the authors and not those of the funding agency.
Financial support. This work was supported by Sanofi Pasteur (investigator-initiated grant) and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (National Institutes of Health grant P30 AG024827 to D. A. N.).
Potential conflicts of interest. R. K. Z. and C. J. L. have received research grants from Pfizer, Merck, and Sanofi Pasteur. C. J. L. consults for MedImmune. T. M. R. is funded by Sanofi Pasteur to develop a universal influenza vaccine. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease C, Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–62. [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the united states. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 3.Carroll N, Delafuente J, McClure K, Weakley D, Khan Z, Cox F. Economic burden of influenza-like illness in long-term-care facilities. Am J Health Syst Pharm 2001; 58:1133–8. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006; 24:1159–69. [DOI] [PubMed] [Google Scholar]
- 5.Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: Support for policies to vaccinate elderly people against influenza. Vaccine 2013; 31:6030–3. [DOI] [PubMed] [Google Scholar]
- 6.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing 2007; 4:1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel J-P, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res 2009; 21:201–9. [DOI] [PubMed] [Google Scholar]
- 8.Nace DA, Drinka P, Mann J, Poland GA. LTC information series: immunization in the long-term care setting. 2nd ed Columbia, MD: AMDA, 2010. [Google Scholar]
- 9.Parodi V, Florentiis D, Martini M, Ansaldi F. Inactivated Influenza Vaccines. Drugs Aging 2011; 28:93–106. [DOI] [PubMed] [Google Scholar]
- 10.Treanor JJ, Schiff GM, Couch RB, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis 2006; 193:1223–8. [DOI] [PubMed] [Google Scholar]
- 11.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–7. [DOI] [PubMed] [Google Scholar]
- 12.Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cate TR, Rayford Y, Niño D, et al. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine 2010; 28:2076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan SJ, Jacobson R, Poland GA. Advances in the vaccination of the elderly against influenza: role of a high-dose vaccine. Expert Rev Vaccines 2010; 9:1127–33. [DOI] [PubMed] [Google Scholar]
- 15.Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine 2011; 29:2865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Cioppa G, Nicolay U, Lindert K, et al. Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59® adjuvant: Results from a dose-finding clinical trial in older adults. Hum Vaccin Immunother 2012; 8:216–27. [DOI] [PubMed] [Google Scholar]
- 17.Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–14. [DOI] [PubMed] [Google Scholar]
- 18.DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: Safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine 2013; 31:861–6. [DOI] [PubMed] [Google Scholar]
- 19.Palache AM, Beyer WEP, Sprenger MJW, et al. Antibody-response after influenza immunization with various vaccine doses—a double-blind, placebo-controlled, multicenter, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9. [DOI] [PubMed] [Google Scholar]
- 20.Gallo JJ, Fulmer T, Paveza GJ, Reichel W. Handbook of geriatric assessment. 3rd ed Gaithersburg, MD: Aspen Publishers, 2000. [Google Scholar]
- 21.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–9. [DOI] [PubMed] [Google Scholar]
- 22.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev 2006; 19:614–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol 2000; 1:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell J. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine 2003; 21:902–14. [DOI] [PubMed] [Google Scholar]
- 26.Bright RA, Ross TM, Subbarao K, Robinson HL, Katz JM. Impact of glycosylation on the immunogenicity of a DNA-based influenza H5 HA vaccine. Virology 2003; 308:270–8. [DOI] [PubMed] [Google Scholar]
- 27.Bright RA, Medina M-J, Xu X, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 2005; 366:1175–81. [DOI] [PubMed] [Google Scholar]
- 28.Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 2006; 295:891–4. [DOI] [PubMed] [Google Scholar]
- 29.Nauta J. Statistics in clinical vaccine trials. Berlin: Springer-Verlag, 2010. [Google Scholar]
- 30.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–80. [DOI] [PubMed] [Google Scholar]
- 31.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine 2005; 23(suppl 1):S10–25. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Seasonal influenza (flu): the flu season, 2013. http://www.cdc.gov/flu/about/season/flu-season.htm Accessed 20 February 2014.
- 33.Skowronski DM, Tweed SA, Tweed SA, De Serres G. Rapid decline of influenza vaccine—induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502. [DOI] [PubMed] [Google Scholar]
- 34.Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine 2010; 28:3929–35. [DOI] [PubMed] [Google Scholar]
- 35.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879–85. [DOI] [PubMed] [Google Scholar]
- 36.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. New Eng J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 37.Wood JM, Gaines-Das RE, Taylor J, Chakraverty P. Comparison of influenza serological techniques by international collaborative study. Vaccine 1994; 12:167–74. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson I, Heath A, Major D, et al. Reproducibility of serologic assays for influenza virus A (H5N1). Emerg Infect Dis 2009; 15:1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenspan S, Nace D, Perera S, et al. Lessons learned from an osteoporosis clinical trial in frail long-term care residents. Clin Trials 2012; 9:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeb M, Carusone SC, Goeree R, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA 2006; 295:2503–10. [DOI] [PubMed] [Google Scholar]
- 41.Kiel DP, Magaziner J, Zimmerman S, et al. Efficacy of a hip protector to prevent hip fracture in nursing home residents: the HIP PRO randomized controlled trial. JAMA 2007; 298:413–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.