Abstract
Background. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthase (dhps), in combination with the quintuple mutant involving mutations in both dhps and the gene encoding dihydrofolate reductase (dhfr), the so-called sextuple mutant, has been associated with increased placental inflammation and decreased infant birth weight among women receiving intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP) during pregnancy.
Methods. Between 2009 and 2011, delivering women without human immunodeficiency virus infection were enrolled in an observational study of IPTp-SP effectiveness in Malawi. Parasites were detected by polymerase chain reaction (PCR); positive samples were sequenced to genotype the dhfr and dhps loci. The presence of K540E in dhps was used as a marker for the quintuple mutant.
Results. Samples from 1809 women were analyzed by PCR; 220 (12%) were positive for P. falciparum. A total of 202 specimens were genotyped at codon 581 of dhps; 17 (8.4%) harbored the sextuple mutant. The sextuple mutant was associated with higher risks of patent infection in peripheral blood (adjusted prevalence ratio [aPR], 2.76; 95% confidence interval [CI], 1.82–4.18) and placental blood (aPR 3.28; 95% CI, 1.88–5.78) and higher parasite densities. Recent SP use was not associated with increased parasite densities or placental pathology overall and among women with parasites carrying dhps A581G.
Conclusions. IPTp-SP failed to inhibit parasite growth but did not exacerbate pathology among women infected with sextuple-mutant parasites. New interventions to prevent malaria during pregnancy are needed urgently.
Keywords: malaria, Plasmodium falciparum, pregnancy, sulfadoxine-pyrimethamine, intermittent preventive therapy, Malawi, dihydropteroate synthase
Approximately 32 million women living in malaria-endemic areas of Africa become pregnant each year [1]. Malaria during pregnancy is a major, preventable cause of maternal morbidity, mortality, and poor birth outcomes in sub-Saharan Africa, particularly in the first and second pregnancies [2, 3]. Approximately 20% of deliveries of low-birth-weight infants and up to 200 000 newborn deaths each year occur as a result of malaria during pregnancy [3].
To prevent malaria during pregnancy in areas with stable moderate-to-high malaria transmission, the World Health Organization currently recommends administration of intermittent preventive treatment during pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) at each scheduled antenatal care visit, starting in the second trimester [4, 5]. Increasing parasite drug resistance threatens the effectiveness of IPTp-SP. SP resistance results from the accumulation of mutations in the Plasmodium falciparum genes encoding dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps); resistance increases with the number of mutant alleles. The K540E mutation in dhps is a reliable marker for parasites bearing the following 5 mutant alleles (the so-called dhfr/dhps quintuple mutant): the dhfr substitutions N51I, C59R, and S108N and the dhps substitutions A437G and K540E [6]). Although quintuple mutants are rare in West Africa, they are increasingly found at a high frequency in eastern and southern Africa [7]. IPTp-SP appears to retain a level of protective efficacy even in areas with a high frequency of dhps K540E, despite the fact that SP is no longer effective for treatment of acute malaria [8–10]. However, quintuple mutants with an additional dhps mutation, A581G (so-called sextuple mutants), have been associated with failure of IPTp-SP to improve birth weight [11] and increased placental inflammation and parasite growth in the presence of SP in an earlier study from northern Tanzania, which found one of the highest recorded prevalence of dhps A581G in Africa to date [12]. This latter finding, if broadly documented, would suggest that SP potentiates placental pathology in areas of high-grade SP-resistant parasites, possibly owing to interstrain competition in complex, polyclonal infections in which highly resistant parasites that harbor the additional mutation at codon 581 have a survival advantage under drug pressure over less resistant parasites and may outcompete them, ultimately leading to better growth of these parasites than in the absence of SP [12]. This phenomenon was not confirmed in the second study [11].
Malaria control programs require a better understanding of whether SP is simply ineffective in the face of the highly resistant sextuple mutant, thereby allowing these parasites to grow unchecked, or whether under these conditions SP actually promotes the growth of parasites bearing these mutations and therefore potentially harms pregnant women. Therefore, we compared parasitologic and morbidity end points at delivery among parasitemic IPTp-SP recipients in southern Malawi to investigate whether the sextuple mutant results in a reduced effectiveness of IPTp-SP to clear or suppress parasite densities or is potentially fuelling parasite growth and exacerbation of placental pathology in the presence of SP. In these study sites, >95% of P. falciparum parasites harbor dhps K540E, but the additional mutation at codon 581 has only recently been detected [13–15]. These sites therefore reflect many other African settings in East and Southern Africa and offer an opportunity to characterize the parasitologic and clinical consequences of this emerging drug resistance mutation [16].
METHODS
Study Sites
Women were enrolled from 4 sites in Malawi: Machinga District Hospital in Liwonde (enrollment period, March through August 2010) and the Blantyre sites, comprising Queen Elizabeth Central Hospital in Blantyre and facilities in the neighboring villages of Madziabango and Mpemba (enrollment period, November 2009–January 2011). Transmission of malaria in these areas of Malawi is stable throughout the year, with a peak between February and March, shortly after the rainy season. According to the 2010 Demographic and Health Survey, antenatal clinic attendance in Malawi is high: approximately 98% of women reported at least 1 visit, and 45.5% reported ≥4 visits [17]. The uptake of 2 doses of IPTp-SP, at least one of which had been administered at the antenatal clinic, was 53.2% in 2012 [18].
Enrollment and Study Procedures
Consenting women with a singleton pregnancy were enrolled at delivery; women with documented human immunodeficiency virus infection were excluded. Participant antenatal clinic cards were examined to obtain the number and timing of IPTp-SP doses received during pregnancy.
A blood sample was collected prior to delivery for peripheral smear analysis and measurement of hemoglobin levels, using a Hemocue Hb 201+ Analyzer (Hemocue, Cypress, California). Maternal anemia was defined as a hemoglobin level of <11 g/dL, and severe anemia was defined as a hemoglobin level of <8 g/dL [19]. At delivery, placental blood, placental tissue, and cord blood samples were collected and infant birth weight was measured. Blood smears were stained with Field stains A and B (azure dye and eosin) (at Machinga District Hospital) or Giemsa stain (at the Blantyre sites). Parasite densities were calculated by counting the number of asexual-stage parasites per 200 white blood cells (at Machinga District Hospital) and the number per 300 white blood cells (at the Blantyre sites), assuming 8000 white blood cells per dL of blood. Blood smears were considered negative for Plasmodium species if no parasites were found after counting 1000 (at Machinga District Hospital) or 500 fields (at the Blantyre sites). All slides were read in duplicate, and discordant results resolved by a third reader.
Full-thickness placental biopsy specimens were obtained from a healthy pericentric area and placed into 10% neutral buffered formalin at delivery [20]. Biopsy samples were stored at room temperature until processing and were embedded in paraffin wax by standard techniques. Paraffin sections were stained with hematoxylin–eosin and Giemsa stain. Placental tissue samples were examined for parasites and pigment, using the 5-point scale described by Rogerson et al [21]. Active infection was defined as acute or chronic infections detected by histologic analysis.
Low birth weight was defined as a birth weight of <2500 g. Gestational age was assessed by the Ballard examination within 24 hours of delivery, by trained study nurses. The Ballard score was used to define both preterm delivery (gestational age, <37 weeks) and to determine infants who were small for gestational age [22].
Molecular Testing
Parasites were detected by either nested polymerase chain reaction (PCR) [23] or real-time PCR [24]; positive samples were sequenced to genotype the dhfr and dhps loci [13, 25]. Not all specimens could be amplified at all loci; thus, the presence of dhps K540E was used as a marker for the quintuple mutant.
Statistical Analysis
Statistical analysis was done using SAS, version 9.3 (SAS Institute, Cary, North Carolina). The analytical population to determine the associations between parasite genotype and parasitologic and clinical parameters was restricted to 202 PCR-positive women for whom dhps codon 581 genotyping data were available. The probability of patent infection (defined as a PCR-positive and microscopy-positive infection), parasite densities, and a range of morbidity outcomes were compared among women infected with sextuple-mutant parasites and those infected with parasites bearing the wild-type (WT) dhps codon 581 (99% of which were quintuple mutants). Because a previous study demonstrated a strong association between parasitologic outcomes and timing of SP dose, irrespective of genotype [12], a separate comparison was conducted to compare the same end points among women who had received SP in the last 4 weeks (the maximum anticipated duration of posttreatment prophylaxis from a single course of SP in this area of highly prevalent SP resistance among parasites) versus those who received SP earlier in pregnancy or never [26, 27]. In addition, we investigated the interaction between genotype and the timing of the last SP dose.
Univariable models (unadjusted for potential confounders) and multivariable models (adjusted for study site and gravidity) were conducted. In univariable models, groups were compared using the χ2 test or Fisher exact test, for categorical variables, and the Student t test, for continuous variables. Poisson regression models with robust standard errors were used to compare discrete outcomes and linear regression, using Proc Mixed fit for continuous outcomes. Results are expressed as crude and adjusted prevalence ratios (aPRs) or mean differences (or as the ratio of the mean difference, for log-transformed parasite counts). A 2-sided P value of <.05 was considered statistically significant. For PCR-positive, smear-negative samples, we assumed limits of detection of 40 parasites/µL for microscopy and 5 parasites/µL for PCR. To obtain estimates of the geometric mean parasite densities (GMPDs), smear-negative women were treated as interval censored in the analyses, accounting for the limit of detection of microscopy [28].
Ethics
Data from 2 studies that used similar protocols are presented here. Both studies were approved by the ethical review boards of the University of Malawi College of Medicine (Blantyre, Malawi). The study at the Blantyre sites was also approved by the Liverpool School of Tropical Medicine (Liverpool, United Kingdom); the study in Machinga District Hospital was approved by the Centers for Disease Control and Prevention (Atlanta, Georgia). Written informed consent was obtained from all participating women.
RESULTS
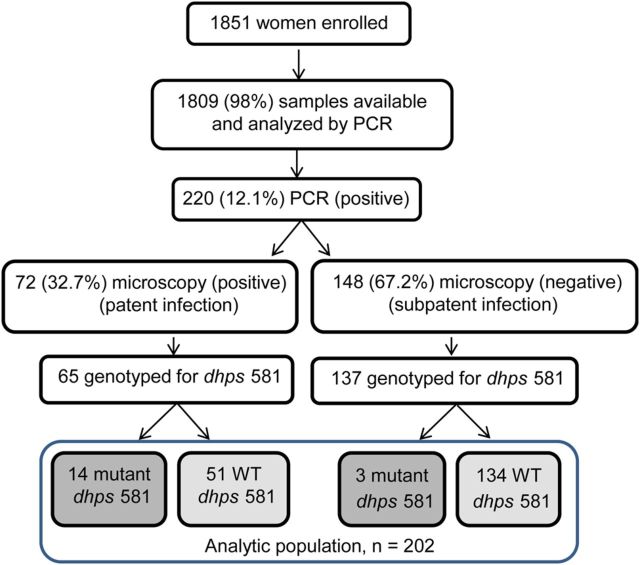
A total of 1851 women were enrolled: 710 from Machinga District Hospital and 1141 from Blantyre. Overall, 117 (6.3%) were smear positive. Samples from 1809 women (98%) were available and analyzed by PCR. Of these, 220 (12.1%) had P. falciparum detected by PCR; 72 of 220 (32.7%) were microscopy positive (indicating patent infection) and 148 of 220 (67.2%) were microscopy negative (indicating subpatent infection; Figure 1). Genotypes at dhps codon 581 were obtained for parasites from 202 women (91.8%); these constituted the evaluable population for all analyses. A total of 66.3% were primigravidae (G1) or secundigravidae (G2), and 39.6% reported using an insecticide-treated bed net (ITN) during the preceding night; these findings were comparable across the 2 groups carrying parasites with each genotype.
Figure 1.
Flow of selection of women with infection due to Plasmodium falciparum with and without the A581G mutation in the gene encoding dihydropteroate synthase (dhps). Abbreviations: PCR, polymerase chain reaction; WT, wild type.
Of the 202 women, 17 harbored parasites bearing the sextuple mutant (8.4%), and 185 (91.6%) were infected with parasites bearing the WT allele at codon 581. Overall, 200 samples (99%) were mutated at dhps codon 540, indicating the presence of the quintuple mutant, including all 17 samples with dhps A581G (Table 1). Among the 202 women, 4 (2%) had not received IPTp-SP, 29 (14%) had received 1 dose, 124 (61%) had received 2 doses, 43 (21%) had received 3 doses, and 2 (1%) had received 4 doses; 49 women received SP within 4 weeks of delivery.
Table 1.
Baseline Characteristics of 202 Pregnant Women, Overall and by Codon 581 Status of the Gene Encoding Plasmodium falciparum Dihydropteroate Synthase (dhps)
| Characteristic | Overall |
dhps A581G |
WT dhps 581 |
Prevalence Ratio (95% CI) | P Values |
|---|---|---|---|---|---|
| 202 | N = 17 | N = 185 | |||
| Blantyre study sitea | 121 (59.9) | 7 (41.2) | 114 (61.6) | 0.67 (.37–1.19) | .17 |
| Primigravidae or secundigravidae | 134 (66.3) | 12 (70.6) | 122 (65.9) | 1.07 (.77–1.48) | .68 |
| Used an ITN last night | 80 (39.6) | 6 (35.3) | 74 (40.0) | 0.88 (.45–1.72) | .71 |
| No. of IPTp-SP doses received | |||||
| 0 | 4 (2.0) | 0 (0.0) | 4 (2.2) | … | .25 |
| 1 | 29 (14.4) | 1 (5.9) | 28 (15.1) | … | |
| 2 | 124 (61.4) | 15 (88.2) | 109 (58.9) | … | |
| 3 | 43 (21.3) | 1 (5.9) | 42 (22.7) | … | |
| 4 | 2 (1.0) | 0 (0.0) | 2 (1.1) | … | |
| SP receipt within 4 wks of delivery | 49 (24.3) | 5 (29.4) | 44 (23.8) | 1.13 (.52–2.46) | .75 |
| dhps K540E | 200 (99.0) | 17 (100.0) | 183 (98.9) | 1.01 (1.00–1.03) | .16 |
Abbreviations: CI, confidence interval; IPTp-SP, intermittent preventive treatment with sulfadoxine-pyrimethamine; ITN, insecticide-treated bed net; SP, sulfadoxine-pyrimethamine; WT, wild type.
a All 3 sites in the Blantyre region, described in “Methods“ section, are pooled as the Blantyre study site. All remaining women were recruited at Machinga District Hospital.
Associations With dhps A581G
Microscopy-Determined Parasitemia
The presence of dhps A581G was associated with higher risk of patent infections in both maternal peripheral (aPR, 2.76, 95% confidence interval [CI], 1.82–4.18) and placental blood (aPR, 3.28; 95% CI, 1.88–5.78; Table 2). After accounting for the limit of detection of microscopy, the GMPD in samples with dhps A581G compared, with samples bearing the WT allele at codon 581, was higher in both maternal peripheral blood (ratio of GMPD, 9.98; 95% CI, 2.95–33.74) and placental blood (ratio of GMPD, 4.63; 95% CI, 1.75–12.26). When restricted only to those women who had received 2 antenatal doses of SP, the higher risk of patent infections persisted in both maternal peripheral blood (aPR, 2.48; 95% CI, 1.52–4.05) and placental blood (aPR, 2.28; 95% CI, 1.28–4.05). When restricted only to women with patent infections, dhps A581G was not associated with an increased GMPD, compared with those with WT dhps 581 (Table 3). No dose-response effect of SP on parasite densities was observed among the group as a whole or among women infected with parasites with the WT allele at codon 581 (Table 4). We could not explore a dose-response effect among the women with dhps A581G because only 1 of 17 women had not received SP, and only 1 had received >2 doses.
Table 2.
Effect of the A581G Mutation in the Gene Encoding Plasmodium falciparum Dihydropteroate Synthase (dhps), Compared With Wild-Type dhps Codon 581
| Characteristic | Raw Dataa |
Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|---|---|
| dhps A581G (n = 17) | WT dhps 581 (n = 185) | PR or Mean Difference (95% CI) | P Values | aPR or Adjusted Mean Difference (95% CI)b | P Values | |
| Smear positivity, by specimen | ||||||
| Any | 14/17 (82.4) | 51/185 (27.6) | 2.98 (2.17–4.12) | <.0001 | 2.85 (2.02–4.03) | <.0001 |
| Maternal | 12/17 (70.6) | 46/185 (24.9) | 2.84 (1.91–4.22) | <.0001 | 2.76 (1.82–4.18) | <.0001 |
| Placental | 9/17 (52.9) | 25/185 (13.5) | 3.92 (2.20–6.98) | <.0001 | 3.28 (1.88–5.78) | <.0001 |
| Histologic finding of active infection | 10/16 (62.5) | 121/183 (66.1) | 0.95 (.64–1.40) | .78 | 0.99 (.65–1.49) | .94 |
| Anemia | 6/17 (35.0) | 86/185 (46.5) | 0.76 (.39–1.47) | .41 | 0.72 (.37–1.40) | .33 |
| Hemoglobin level, g/dL | 11.2 ± 1.2 | 11.1 ± 1.6 | 0.14 (−.67 to .95) | .73 | 0.32 (−.47 to 1.11) | .43 |
| Low-birth-weight infant | 1/17 (5.9) | 20/185 (10.8) | 0.54 (.08–3.81) | .54 | 0.68 (.10–4.48) | .69 |
| SGA infant | 8/16 (50.0) | 64/183 (35.0) | 1.43 (.84–2.42) | .18 | 1.39 (.78–2.48) | .27 |
| Preterm delivery | 1/16 (6.3) | 21/183 (11.5) | 0.54 (.07–3.79) | .54 | 0.78 (.11–5.45) | .80 |
| Infant birth weight, g | 3019 ± 595 | 2922 ± 424 | 96.5 (−123.5 to 316.5) | .39 | 51 (−154 to 256) | .63 |
| Infant gestational age, wksc | 40.1 ± 2.1 | 39.5 ± 2.1 | 0.6 (−1.7 to .5) | .28 | −0.04 (−.85 to .77) | .93 |
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; PR, prevalence ratio; SGA, small for gestational age; WT, wild type.
a Data are no. of women with the characteristic/no. evaluated (%) or mean value ± range.
b Analysis adjusted for site, gravidity, and sulfadoxine-pyrimethamine use within 4 weeks of delivery.
c Data are for infants delivered by 16 women with mutant infection and by 183 women with WT infection.
Table 3.
Geometric Mean Parasite Densities (GMPDs) Overall and Among Smear-Positive Women, Stratified by Plasmodium falciparum Dihydropteroate Synthase Gene (dhps) Codon 581 Status and Receipt of Intermittent Preventive Treatment With Sulfadoxine-Pyrimethamine Within 4 Weeks Before Delivery
| Variable | All Women |
Nonrecipients of SP |
Recipients of SP |
P, Nonrecipients vs Recipients | |||
|---|---|---|---|---|---|---|---|
| No. | GMPD (95% CI) | No. | GMPD (95% CI) | No. | GMPD (95% CI) | ||
| Overall | |||||||
| Maternal peripheral parasitemia | |||||||
| Overall | 202 | 33 (23–48) | 153 | 34 (22–51) | 49 | 31 (15–65) | .84 |
| WT dhps 581 | 185 | 27 (19–39) | 141 | 28 (18–43) | 44 | 24 (12–51) | .69 |
| dhps A581G | 17 | 266 (77–920) | 12 | 268 (74–966) | 5 | 263 (15–4691) | .94 |
| P, mutant vs WT | .0002 | .003 | .03 | ||||
| Placental parasitemia | |||||||
| Overall | 202 | 17 (12–23) | 153 | 18 (12–25) | 49 | 14 (8–25) | .36 |
| WT dhps 581 | 185 | 15 (11–20) | 141 | 16 (11–23) | 44 | 12 (7–20) | .25 |
| dhps A581G | 17 | 67 (26–174) | 12 | 66 (20–220) | 5 | 73 (18–295) | .97 |
| P, mutant vs WT | .002 | .02 | .01 | ||||
| Smear positive | |||||||
| Maternal peripheral parasitemia | |||||||
| Overall | 61 | 824 (530–1283) | 47 | 808 (490–1334) | 14 | 882 (344–2260) | .87 |
| WT dhps 581 | 49 | 787 (479–1293) | 38 | 824 (463–1469) | 11 | 672 (261–1731) | .72 |
| dhps A581G | 12 | 995 (378–2619) | 9 | 737 (281–1930) | 3 | 2454 (248–24 244) | .27 |
| P, mutant vs WT | .68 | .86 | .24 | ||||
| Placental parasitemia | |||||||
| Overall | 42 | 274 (153–491) | 33 | 319 (162–627) | 9 | 162 (59–440) | .30 |
| WT dhps 581 | 33 | 255 (120–539) | 27 | 297 (129–683) | 6 | 130 (27–631) | .37 |
| dhps A581G | 9 | 314 (182–542) | 6 | 387 (200–748) | 3 | 206 (94–449) | .25 |
| P, mutant vs WT | .81 | .79 | .73 | ||||
All models controlled for study site and gravidity.
Abbreviations: CI, confidence interval; SP, sulfadoxine-pyrimethamine; WT, wild type.
Table 4.
Geometric Mean Parasite Densities (GMPDs) Overall and Among Women Infected With Plasmodium falciparum Bearing Wild-Type Codon 581 of the Gene Encoding Dihydropteroate Synthase (dhps), by Number of Doses of Intermittent Preventive Treatment With Sulfadoxine-Pyrimethamine Received
| Variable | Doses, No. |
P Values | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | ||
| Overall | |||||
| Women, no. | 4 | 29 | 124 | 45 | |
| Maternal GMPD (95% CI) | 29 (2–344) | 27 (11–69) | 33 (21–51) | 40 (17–94) | .47 |
| Placental GMPD (95% CI) | 36 (2–620) | 19 (8–46) | 18 (12–26) | 12 (7–21) | .12 |
| Infected with wild-type dhps 581 | |||||
| Women, no. | 4 | 28 | 109 | 44 | |
| Maternal GMPD (95% CI) | 29 (2–344) | 24 (9–61) | 26 (16–41) | 34 (15–78) | .52 |
| Placental GMPD (95% CI) | 36 (2–620) | 16 (7–38) | 16 (11–23) | 11 (6–19) | .13 |
Abbreviation: CI, confidence interval.
Histopathologic Findings
dhps A581G was not associated with an increased risk of active infection diagnosed on the basis of histologic analysis, both among all women (aPR, 0.99; 95% CI, .65–1.49) and among those who had received 2 doses of IPTp-SP (aPR, 0.93; 95% CI, .56–1.54). Compared with women who received none or only 1 dose of SP, receipt of at least 2 doses of IPTp-SP was associated with a decreased risk of having active infection diagnosed on the basis of histologic analysis (aPR, 0.76; 95% CI, .62–.92).
Maternal Anemia and Birth Outcomes
There was no difference in mean hemoglobin level (±SD) between women harboring parasites with and those harboring parasites without the dhps 581G allele (11.22 ± 1.2 g/dL and 11.08 ± 1.6 g/dL, respectively; adjusted mean difference, 0.32 g/dL [95% CI], −.47 to 1.11 g/dL). There were no differences in the frequencies of women who had preterm delivery and those who had small-for-gestational-age infants (Table 2). Similarly, there was no difference in the prevalence of anemia (Table 2) or severe anemia (mutant vs WT, 0% vs 2.5%; P = 1.00). Among infants delivered by the 17 women with mutant infections, adjusted mean birth weights were 51 g higher (P = .63), and the prevalence of low birth weight was lower (5.9% vs 10.8%; P = .54).
Influence of Timing of IPTp-SP, Overall and by Genotype
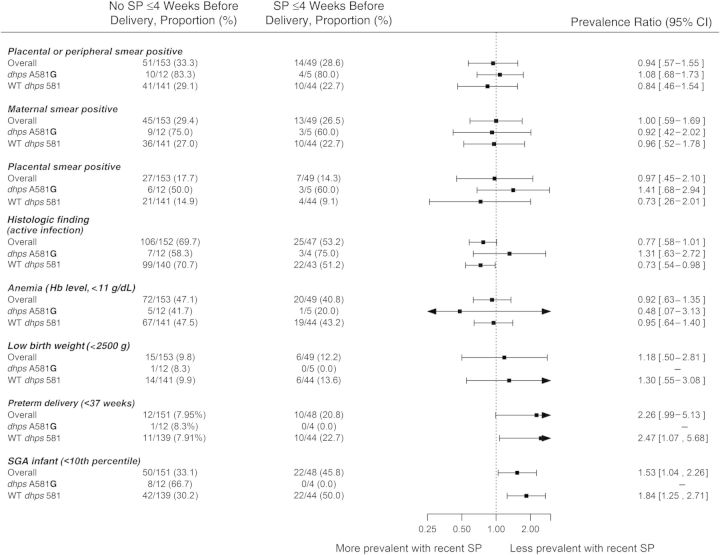
Recent receipt of SP prior to delivery has been hypothesized to exacerbate placental pathology in resistant, multiclonal malaria infections. Therefore, we explored the impact of recent SP use (within 4 weeks of delivery) on outcomes. There was no significant difference in GMPD between women who received SP recently and those who had not (Figure 2A). There was no difference in the risk of patent maternal peripheral or placental infection when comparing women with and without recent SP use overall (aPR, 0.94; 95% CI, .57–1.55), among women with WT infections (aPR, 0.84; 95% CI, .68–1.73), or among the 17 women infected with parasites bearing dhps 581G (aPR, 1.08; 95% CI, .68–1.73).
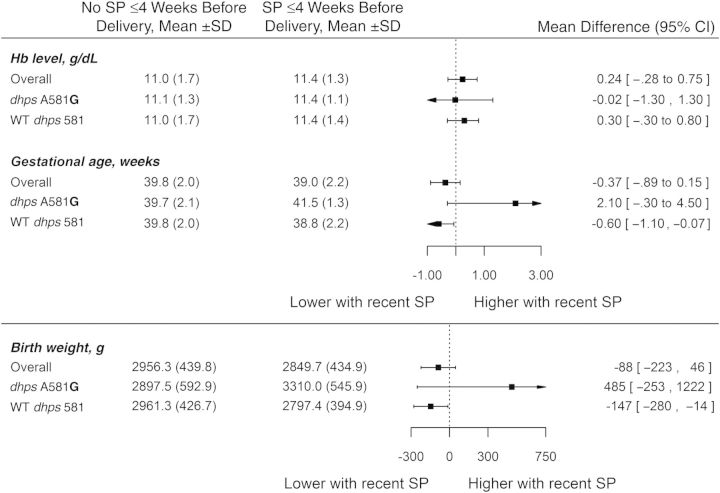
Figure 2.
Associations between binomial variables (A) and continuous variables (B) and parasitologic and morbidity end points, by time since last dose of sulfadoxine-pyrimethamine (SP), among 202 women who tested positive for Plasmodium falciparum by polymerase chain reaction. Abbreviations: CI, confidence interval; dhps, dihydropteroate synthase; Hb, hemoglobin; SD, standard deviation; SGA, small for gestational age; WT, wild type.
Among women who received IPTp-SP and had results of histologic analyses (n = 199), receipt of recent SP (n = 47) was associated with a nonsignificantly lower prevalence of active infection, based histopathologic findings (aPR, 0.77; 95% CI, .58–1.01; P = .06), when controlling for site, gravidity, and presence of the dhps A581G mutation.
Recent receipt of SP was not associated with an increased or decreased risk of anemia or with a significant difference in maternal hemoglobin level, although hemoglobin levels tended to be highest in recent recipients of SP (Figure 2B).
DISCUSSION
In our sites, 8.4% of delivering women with P. falciparum infection harbored sextuple-mutant parasites. The sextuple mutant was associated with a decreased effectiveness of IPTp-SP, but in contrast to a previous observational study from Tanzania [12], there was no indication that use of SP in the last month was associated with higher prevalence or parasite densities, suggesting that, although SP use is of decreased benefit in the presence of the sextuple mutant, there was no evidence that its use was harmful per se.
Compared with women infected with parasites with the wild type allele at dhps codon 581, women infected with P. falciparum bearing dhps A581G had higher parasite densities and thus were more likely to have patent parasitemia. This finding, which has been reported previously, suggests that this additional mutation confers a greater level of resistance than the quintuple mutant alone, resulting in a failure of SP to successfully suppress parasite densities to below the levels of microscopic detection. Despite this, in our study, the presence of this mutant allele was not associated with worsened clinical morbidity, as manifested by lower maternal hemoglobin concentrations at birth, birth weight, or birth size, or with worsened histologic features, in contrast to what has been reported from Tanzania [11, 12], although this may be a result of insufficient power in our study.
One of the studies from Tanzania suggested that giving SP was potentially harmful in areas with very high levels of SP resistance [12]. In that study, the receipt of IPTp-SP was associated with a higher mean parasite density, a more-intense placental inflammation, and a greater density of parasites bearing dhps A581G; additionally, a dose-response relationship was evident, in which the highest densities were reported among women who had received SP in the last few weeks before pregnancy or who had received multiple doses of SP. This finding was reported for the overall sample (including infections with WT parasites). It was hypothesized that this resulted from a phenomenon of competitive facilitation. If these findings were confirmed, they would have major implications for malaria control programs during pregnancy, as they would suggest that, in areas where highly resistant sextuple mutants circulate, IPTp-SP may not simply lack efficacy, but rather may exacerbate placental malaria and harm women and their newborns. Neither our study nor the second study in Tanzania, conducted in an area where the prevalence of sextuple-mutant parasites was 43% among infected women with no SP exposure, have confirmed this finding [11]. In our study, recent SP use was not associated with the prevalence or density of parasites. The prevalence of patent peripheral malaria at delivery overall was similar among recent recipients of SP (28.6%), compared with women who had not received SP recently (33.3%), irrespective of parasite genotype. The risk of acute or chronic infections (ie, active infections), as assessed by histologic analysis, was lower in recent recipients of SP, compared with those who received SP >4 weeks prior to delivery (53% vs 70%; aPR, 0.77 [95% CI, .58–1.01]; P = .06). We were not able to explore a dose-response in women bearing parasites carrying dhps A581G because 15 of the 17 women had received 2 doses of SP. Among the 185 women with the WT dhps codon 581 infection, there was no trend toward higher parasite densities with increasing doses of SP, but the number of women receiving no SP (n = 4) was too small to allow for a meaningful analysis of the dose-response relationship. Furthermore, in the overall sample of 1852 women (including the women for whom PCR did not detect parasites at delivery), recent SP use was associated with a lower risk of infection detected by histologic analysis, compared with women who received their last dose of SP earlier in pregnancy (aPR, 0.63; 95% CI, .49–.81; P = .0003; data not shown). Taken together, our findings indicate that, in southern Malawi, SP delivered as IPTp reduces the risk of infection among the majority of women but that this beneficial effect is significantly reduced or absent among women bearing parasites carrying dhps A581G.
It is possible that these differences from the first Tanzanian study [12] regarding recent use of SP prior to delivery are explained by a difference in the level of resistance or other differences in the parasite population. dhps A581G was still rare in southern Malawi (prevalence, 1.46% [95% CI, 1.3%–1.62%] at the first antenatal clinic visit [data not shown] and 8.4% at delivery), compared with northern Tanzania. The very small size of the SP-naive group in our cohort (n = 4 overall) reduced our ability to look at a dose-response relationship and may have hampered our ability to detect any potential harm mediated by SP. An additional possibility is that unidentified mutations in addition to dhps A581G are involved that serve to mediate parasite fitness when exposed to SP, either within the enzymes targeted by the drugs or in other targets; it has recently been shown that these mutant lineages have emerged independently, supporting the premise that other mutations may be present in the Tanzanian parasites [29].
Although the overall sample size is reasonable, the small number of women with mutant infections (n = 17) precluded more-comprehensive analysis of morbidity outcomes. As mentioned above, the vast majority of women with mutations received 2 doses of IPTp-SP; thus, it was not possible to examine the effect of the dose-response relationship for IPTp-SP doses on parasitologic outcomes or to further explore the phenomenon of competitive facilitation. Thus, although no significant association between the presence of dhps A581G and low birth weight or anemia was noted, this may simply reflect a lack of power in this observational study, rather than a true lack of association. Furthermore, only 12 of 17 women with dhps A581G had parasites detectable by microscopy, and this may have further limited our ability to detect changes in birth weight.
This is the third study implicating the sextuple mutant in the failure to prevent the consequences of antenatal malaria, either by increased parasite densities (as in this study and the study by Harrington et al [12]), increased placental inflammation [12], or decreased birth weight [11]. Our data suggest that, while the presence of the sextuple mutant is associated with a higher likelihood of patent parasitemia, this effect seems to be independent of the timing of IPTp-SP use, and there was no evidence that parasite growth was fuelled by the presence of SP. Thus, although the sextuple-mutant haplotype potentiates clinical resistance to SP and attenuates the beneficial impacts of IPTp-SP, antenatal receipt of IPTp-SP among women harboring these highly resistant parasites was not associated with harm to the pregnant women or their offspring in Malawi. Fortunately, at present, these highly resistant sextuple mutants are found in a limited number of places in sub-Saharan Africa [16], although as these mutants spread, the impact of IPTp-SP will diminish. It is critical to continue monitoring for the emergence of sextuple-mutant P. falciparum and to improve our understanding of the influence of this mutation on IPTp-SP effectiveness. Future biochemical or molecular genetic analyses will be useful adjuncts to understanding these geographical and parasitologic differences in the impacts of IPTp-SP reported to date. Finally, our results stress the urgent need for therapeutic alternatives to IPTp-SP, including new drugs or strategies, such as intermittent screening and treatment.
Notes
Acknowledgments. We thank all the participants and healthcare workers who made this study possible.
Disclaimer. The findings and conclusions presented in this article are those of the authors and do not necessarily reflect the official position of the US President's Malaria Initiative, the US Agency for International Development, or the Centers for Disease Control and Prevention (CDC). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the US President's Malaria Initiative; the US Agency for International Development, under the terms of an interagency agreement with the CDC and through a cooperative agreement (5 U01 CI000189) between the CDC and the University of Malawi College of Medicine Malaria Alert Centre; the European Developing Countries Clinical Trials Partnership; and the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine (to F. O. t. K.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 2010; 7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 2001; 64:28–35. [DOI] [PubMed] [Google Scholar]
- 3.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). A strategic framework for malaria prevention and control during pregnancy in the African Region. Brazzaville: WHO Regional Office for Africa, 2004. [Google Scholar]
- 5.World Health Organisation. Updated WHO policy recommendation: intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). http://www.who.int/entity/malaria/publications/atoz/who_iptp_sp_policy_recommendation/en/index.html. Accessed 5 June 2014.
- 6.Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis 2002; 185:380–8. [DOI] [PubMed] [Google Scholar]
- 7.Flegg J, Guerin P, White N, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J 2011; 10:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayentao K, Garner P, van Eijk AM, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in africa: Systematic review and meta-analysis. JAMA 2013; 309:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy. JAMA 2007; 297:2603–16. [DOI] [PubMed] [Google Scholar]
- 10.Eisele TP, Larsen DA, Anglewicz PA, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis 2012; 12:942–9. [DOI] [PubMed] [Google Scholar]
- 11.Minja D, Schmiegelow C, Mmbando B, et al. Infections with Plasmodium falciparum sextuple dihydrofolate reductase/dihydropteroate synthetase allelic haplotypes during pregnancy are associated with decreased birth weight in Korogwe, Tanzania. Emerg Inf Dis 2013; 19:1446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington WE, Mutabingwa TK, Muehlenbachs A, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci U S A 2009; 106:9027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor SM, Antonia A, Feng G, et al. Adaptive evolution and fixation of drug-resistant Plasmodium falciparum genotypes in pregnancy-associated malaria: 9-year results from the QuEERPAM study. Infect Genet Evol 2012; 12:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutman J, Mwandama D, Wiegand R, Ali D, Mathanga DP, Skarbinski J. Effectiveness of intermittent preventive treatment with sulfadoxine-pyrimethamine in pregnancy on maternal and infant birth outcomes in Machinga District, Malawi. J Infect Dis 2013; 208:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SM, Antonia A, Chaluluka E, et al. Antenatal receipt of sulfadoxine-pyrimethamine does not exacerbate pregnancy-associated malaria despite the expansion of drug-resistant Plasmodium falciparum: clinical outcomes from the QuEERPAM study. Clin Infect Dis 2012; 55:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegg JA, Patil AP, Venkatesan M, et al. Spatio-temporal mathematical modelling of mutations of the dhps gene in African Plasmodium falciparum. Malar J 2013; 12:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Statistical Office (NSO) and ICF Macro. Malawi Demographic and Health Survey 2010. Zomba, Malawi, and Calverton, MD: NSO and ICF Macro, 2011. [Google Scholar]
- 18.National Malaria Control Programme (NMCP) [Malawi] and ICF International. Malawi Malaria Indicator Survey (MIS) 2012. Lilongwe, Malawi, and Calverton, MD: NMCP and ICF International, 2012. [Google Scholar]
- 19.Parise ME, Ayisi JG, Nahlen BL, et al. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg 1998; 59:813–22. [DOI] [PubMed] [Google Scholar]
- 20.Bulmer JN, Rasheed FN, Morrison L, Francis N, Greenwood BM. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology 1993; 22:219–26. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 2003; 68:115–9. [PubMed] [Google Scholar]
- 22.Landis S, Lokomba V, Ananth CV, et al. Impact of maternal malaria and under-nutrition on intrauterine growth restriction: a prospective ultrasound study in Democratic Republic of Congo. Epidemiol Infect 2009; 137:294–304. [DOI] [PubMed] [Google Scholar]
- 23.Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 1993; 61:315–20. [DOI] [PubMed] [Google Scholar]
- 24.Taylor SM, Juliano JJ, Trottman PA, et al. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol 2010; 48:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeder JC, Rieckmann KH, Genton B, Lorry K, Wines B, Cowman AF. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua New Guinea. Am J Trop Med Hyg 1996; 55:209–13. [DOI] [PubMed] [Google Scholar]
- 26.Karunajeewa H, Salman S, Mueller I, et al. Davis TME pharmacokinetic properties of sulfadoxine-pyrimethamine in pregnant women. Antimicrob Agents Chemother 2009; 53:4368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzinjalamala F, Macheso A, Kublin JG, et al. Association between the pharmacokinetics and in vivo therapeutic efficacy of sulfadoxine-pyrimethamine in Malawian children. Antimicrob Agents Chemother 2005; 49:3601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Hein MJ, Deddens JA, Hines CJ. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection Using SAS. Ann Occup Hyg 2011; 55:97–112. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SM, Antonia AL, Harrington WE, et al. Independent lineages of highly sulfadoxine-resistant Plasmodium falciparum haplotypes, eastern Africa. Emerg Infect Dis 2014; 20:1140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]