Abstract
Background. Inactivated polio vaccine (IPV) is necessary for global polio eradication because oral polio vaccine can rarely cause poliomyelitis as it mutates and may fail to provide adequate immunity in immunocompromised populations. However, IPV is unaffordable for many developing countries. Intradermal IPV shows promise as a means to decrease the effective dose and cost of IPV, but prior studies, all using 20% of the standard dose used in intramuscular IPV, resulted in inferior antibody titers.
Methods. We randomly assigned 231 adults with well-controlled human immunodeficiency virus infection at a ratio of 2:2:2:1 to receive 40% of the standard dose of IPV intradermally, 20% of the standard dose intradermally, the full standard dose intramuscularly, or 40% of the standard dose intramuscularly. Intradermal vaccination was done using the NanoPass MicronJet600 microneedle device.
Results. Baseline immunity was 87%, 90%, and 66% against poliovirus serotypes 1, 2, and 3, respectively. After vaccination, antibody titers increased a median of 64-fold. Vaccine response to 40% of the standard dose administered intradermally was comparable to that of the standard dose of IPV administered intramuscularly and resulted in higher (although not significantly) antibody titers. Intradermal administration had higher a incidence of local side effects (redness and itching) but a similar incidence of systemic side effects and was preferred by study participants over intramuscular administration.
Conclusions. A 60% reduction in the standard IPV dose without reduction in antibody titers is possible through intradermal administration.
Keywords: intradermal, fractional dose, inactivated polio vaccine, HIV, polio, vaccine
Globally, paralytic poliomyelitis rates from wild poliovirus have dropped by >99% since 1988, with 406 cases reported in 2013, and only 3 countries remain with uninterrupted endemic transmission [1]. Much of this success is due to oral polio vaccine (OPV), which is used for polio vaccination in most of the developing world. However, as a live virus, OPV can mutate into forms capable of causing paralytic poliomyelitis, such as vaccine-derived poliovirus (VDPV), which caused 6 outbreaks of paralytic poliomyelitis in 2013 alone [2]. Because of these risks from OPV, the recent Polio Eradication and Endgame Strategic Plan 2013–2018 proposed by the World Health Organization (WHO) includes initiating at least 1 dose of inactivated polio vaccine (IPV) for children in all countries and subsequently phasing out OPV [3]. In addition, 2 recent studies have demonstrated that a booster dose of IPV results in significantly higher humoral and mucosal polio immunity than a booster dose of OPV in children who received OPV as their primary regimen [4, 5]. IPV has also been shown to significantly increase seroconversion rates in children who did not respond to OPV [6]. Consequently, IPV may also have a role as a booster dose in supplementary immunization campaigns to control outbreaks of wild poliovirus or VDPV infection.
One difficulty with these plans is that IPV is currently too expensive for many developing countries, costing approximately 20-fold more than OPV per dose [7]. A potential method to make IPV more affordable is to decrease the dose by using intradermal instead of intramuscular administration. The skin has a particularly high concentration of dendritic cells, and it has been possible to reduce the dose of other vaccines to 20%–60% of the standard intramuscular dose without decreasing immunogenicity through intradermal administration [8]. For influenza vaccines, some studies have shown superior immunogenicity despite using fractional intradermal doses [9, 10]. However, past clinical studies of fractional-dose intradermal IPV have only used 20% of the standard dose and have all resulted in significantly lower antibody titers, compared with full-dose intramuscular IPV [7, 11–15].
To determine whether a booster of intradermal IPV using a fractional dose >20% of the standard dose can be equally effective as the full standard dose of intramuscular IPV, we conducted a randomized, controlled clinical trial comparing booster doses of 40% fractional-dose intradermal IPV, 20% fractional-dose intradermal IPV, full-dose intramuscular IPV, and 40% fractional-dose intramuscular IPV. Because this was a proof-of-concept study and the first time 40% fractional-dose intradermal IPV had been tested in humans, we elected to enroll adult volunteers at our home institution. Because all past studies of intradermal IPV were in healthy children and adults, we chose to limit enrollment to subjects infected with human immunodeficiency virus (HIV).
HIV-infected adults have a reduced immunologic response to most vaccines. Although some of the decreased immunologic response seen in these individuals can be explained by the low number of CD4+ T cells associated with advanced HIV infection, the decreased immunologic response to vaccines persists even in HIV-infected individuals who are receiving antiretroviral therapy and have CD4+ T-cell counts in the normal range [16, 17]. This has been shown for multiple vaccines, including the pneumococcal vaccines [17], hepatitis B vaccines [18, 19], and influenza vaccines [16]. We have previously shown that HIV infection significantly reduces the immunologic response to OPV in Zimbabwean infants [20]. Prior studies evaluating the effect of HIV infection on the immunologic response to IPV were all conducted prior to the development of combined antiretroviral therapy, and all used full IPV doses administered intramuscularly [21–26]. In general, these studies show that advanced HIV infection decreased the response to intramuscular IPV but that HIV-infected subjects with higher CD4+ T-cell counts had similar seroprotection rates, although lower antibody titers, after intramuscular IPV vaccination, compared with uninfected controls. Since the countries with the highest rates of HIV infection primarily use OPV and will need to transition to IPV with the new WHO polio eradication plan, it is important to know whether fractional-dose intradermal IPV would be effective even in immunocompromised populations.
MATERIALS AND METHODS
Study Design and Population
This study was conducted at the Eastern Virginia Medical School (EVMS) HIV clinic in Norfolk, Virginia, and followed the principles of the Declaration of Helsinski and good clinical practice guidelines. The study was approved by the EVMS Institutional Review Board and was registered with ClinicalTrials.gov (NCT01686503). Of note, in the United States, IPV (the older formulation) was licensed and its widespread use began in both infants and older children in 1955 [27]. OPV was licensed in 1961, rapidly replacing IPV, and then enhanced IPV replaced OPV in 2000. Wild poliovirus was prevalent in the United States prior to the introduction of IPV in 1955, but annual cases of paralytic poliomyelitis had dropped to <150 by the early 1960s, and the last case of naturally occurring paralytic poliomyelitis due to wild poliovirus in the United States was in 1979.
We enrolled 231 subjects between 7 September 2012 and 8 July 2013. Inclusion criteria included documented HIV infection, age >18 years, and an HIV load of <400 copies/mL at the most recent measurement. Exclusion criteria included current acute illness, pregnancy, or history of allergic reaction to any component of IPV. Written informed consent was obtained from all subjects.
At the enrollment visit, a blood specimen was collected, and subjects were randomly assigned to one of 4 study groups in a ratio of 2:2:2:1, based on a computer-generated randomization scheme done in 3 blocks of 77 to ensure even distribution over the enrollment period. Group 1 received 40% (0.2 mL) of the standard dose of IPV intradermally (66 subjects), group 2 received 20% (0.1 mL) of the standard dose intradermally (66 subjects), group 3 (the control group) received the full dose (0.5 mL) intramuscularly (66 subjects), and group 4 received 40% (0.2 mL) of the standard dose intramuscularly (33 subjects). The IPV used was IPOL (Sanofi Pasteur), containing 40 D antigen units of serotype 1, 8 D antigen units of serotype 2, and 32 D antigen units of serotype 3 poliovirus per 0.5 mL. Intramuscular injections were done into the deltoid muscle, and intradermal injections were done into the skin over the deltoid muscle. Intradermal injections were done using the NanoPass MicronJet600 device, a Food and Drug Administration (FDA)–approved microneedle-based device for intradermal injection. Occurrences of major leakage (defined as a visible drop >2 mm in diameter on the skin) and bleb formation were recorded after intradermal administration. Subjects also completed a questionnaire, and information, including laboratory data, medications, and comorbidities, was extracted from the medical records.
Subjects were given a diary to record adverse events during the first week and were called by the study coordinator within a week of enrollment, who asked about adverse reactions. The second study visit occurred 4–6 weeks after the enrollment visit and included collection of a second blood specimen and a follow-up questionnaire.
Sample Analysis
On the day of collection, blood samples were centrifuged, and the serum was stored at −80°C. After the study visits had been completed, aliquots of frozen serum samples were shipped on dry ice to Dr Konstantin Chumakov's laboratory at the FDA, where poliovirus neutralizing antibody assays were done in a blinded fashion according to the World Health Organization method [28]. The antibody titer was defined as the reciprocal of the highest dilution of serum that neutralized the virus, and all serum samples were diluted until the highest dilution was determined.
Immunity, or seroprotection, was defined as an antibody titer of ≥8. Vaccine response was defined as seroconversion in subjects not immune at baseline or as a ≥4-fold rise in titer in subjects immune at baseline.
Sample Size Calculations
Sample sizes were calculated to show equivalency in vaccine response between groups 1 and 3 (56 subjects were required in each group), a 15% increase in vaccine response in group 1 versus group 2 (42 subjects were required in each group), and a 25% increase in vaccine response in group 1 versus group 4 (21 subjects were required in each group), with an α level of 0.05, a β level of 0.20, and a 2-sided test for the equivalency study. Predicted vaccine response was based on the assumption that HIV infection would lead to a decrease of ≥5% in the vaccine response of approximately 85% reported in other studies (published by 2011, when our study was planned) investigating an IPV booster in HIV-uninfected subjects who received an OPV primary regimen [29, 30]. To compensate for an estimated 15% drop-out rate, enrollment in each group exceeded the required level by at least 18%.
Statistical Analysis
Descriptive and univariate analyses were performed using SAS, version 9.3 (SAS Institute, Cary, North Carolina). Baseline demographic characteristics were compared using t tests for continuous and χ2 tests for categorical variables. One-way analysis of variance tests with Bonferroni-corrected pairwise comparisons were used to assess associations between study groups and continuous outcomes (fold-rise in titer and geometric mean titers at baseline and after receipt of booster), and χ2 tests for categorical outcomes (immunity, vaccine response, and presence of side effects). Two-sided statistical tests were conducted at an α level of 0.05.
Post hoc, noninferiority for postbooster antibody titers was concluded if the lower limit of the 95% confidence interval of the difference between the log2 postbooster geometric mean titer (GMT) of the experimental group (minuend) and the control group (full-dose intramuscular IPV) did not exceed −1 for all 3 serotypes [11].
RESULTS
A total of 240 subjects consented to the study, of whom 9 did not meet screening criteria because of lack of laboratory data in the past 6 months or an HIV load of >400 copies/mL. We enrolled 231 subjects, of whom 97% completed both study visits (65, 63, 64, and 32 in groups 1, 2, 3, and 4, respectively). Demographic variables were not significantly different among the 4 groups (Table 1).
Table 1.
Demographic Characteristics of Study Subjects
| Characteristic | Group 1, 40% ID (n = 66) | Group 2, 20% ID (n = 66) | Group 3, 100% IM (n = 66) | Group 4, 40% IM (n = 33) | P Value |
|---|---|---|---|---|---|
| Age, y | |||||
| Mean ± SD | 45 ± 10 | 45 ± 11 | 46 ± 11 | 46 ± 11 | .96 |
| Range | 24–61 | 23–70 | 21–63 | 21–63 | |
| Female sex | 36 (24) | 36 (24) | 32 (21) | 21 (7) | .42 |
| Race | .68 | ||||
| White | 29 (19) | 26 (17) | 29 (19) | 33 (11) | |
| Black | 71 (47) | 73 (48) | 71 (47) | 64 (21) | |
| Other | 0 (0) | 2 (1) | 0 (0) | 3 (1) | |
| Born in the US | 98 (65) | 95 (63) | 91 (60) | 94 (31) | .38 |
| Lived or traveled internationally | 27 (18) | 29 (19) | 44 (29) | 36 (12) | .16 |
| Received all childhood vaccines | 97 (64) | 97 (64) | 91 (60) | 94 (31) | .43 |
| Year of HIV infection diagnosis, meana | 2001 | 2002 | 2001 | 2001 | .90 |
| Currently receiving ART | 97 (64) | 97 (64) | 100 (66) | 100 (33) | .90 |
| CD4+ T-cell count in cells/mm3, mean ± SD | |||||
| Most recent | 669 ± 361 | 630 ± 331 | 676 ± 354 | 569 ± 260 | .44 |
| Lowest in the medical record | 328 ± 269 | 287 ± 221 | 294 ± 294 | 322 ± 260 | .8 |
| Most recent HIV load (copies/mL), mean ± SD | 111 ± 95 | 82 ± 86 | 85 ± 78 | 99 ± 69 | .67 |
| Diagnosis of AIDS in the record | 58 (38) | 50 (33) | 59 (39) | 52 (17) | .69 |
| Ever homeless | 39 (26) | 33 (22) | 21 (14) | 21 (7) | .08 |
| Current smoker | 58 (38) | 47 (31) | 35 (23) | 39 (13) | .06 |
| Coinfected with hepatitis C virus | 18 (12) | 15 (10) | 9 (6) | 15 (5) | .48 |
| Coinfected with hepatitis B virus | 11 (7) | 6 (4) | 3 (2) | 0 (0) | .29 |
| History of hypertension | 42 (28) | 38 (25) | 42 (28) | 45 (15) | .89 |
| History of depression or bipolar disorder | 33 (22) | 33 (22) | 29 (19) | 27 (9) | .87 |
| IPV lot no. received | .92 | ||||
| H1452–1 | 9 (6) | 9 (6) | 14 (9) | 12 (4) | |
| H1604–1 | 29 (19) | 26 (17) | 21 (14) | 21 (7) | |
| H1605–2 | 62 (41) | 65 (43) | 65 (43) | 67 (22) |
Data are % (no.) of subjects, unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; ID, intradermal; IM, intramuscular; IPV, inactivated polio vaccine; SD, standard deviation.
a The SD for groups 1–3 was 8 years, and the SD for group 4 was 9 years.
Although 95% of subjects reported having received all their childhood vaccinations, most could not remember which specific polio vaccines they had received. However, 95% of subjects were born in the United States, and their vaccination history and wild poliovirus exposure can be estimated by their age (see “Materials and Methods” section for remarks on the history of polio vaccination in the United States). The 142 subjects (61%) who were 21–50 years old at enrollment were likely vaccinated with OPV and were likely not exposed to wild poliovirus. The 89 subjects (39%) who were 51–70 years old were likely vaccinated with IPV and may have been exposed to wild poliovirus (indeed, a 61-year-old subject reported history of paralytic poliomyelitis as a child). Of note, no subjects reported receiving polio vaccine as an adult for international travel.
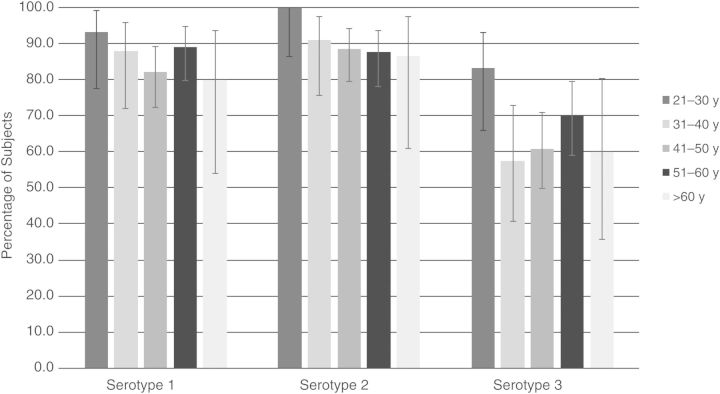
There were no significant differences in baseline polio immunity between study groups: 87%, 90%, and 66% of all subjects were immune to serotypes 1, 2, and 3, respectively. Baseline immunity rates against serotypes 1 and 2 were both significantly higher than that against serotype 3 (P < .0001 for both comparisons). Baseline immunity, stratified by age group, is shown in Figure 1.
Figure 1.
Baseline polio immunity, by age group in years. There were 30, 33, 79, 74, and 15 subjects aged 21–30, 31–40, 41–50, 51–60, and >60 years, respectively. The 95% confidence intervals of each proportion were calculated using the modified Wald method. A 2-tailed Fisher exact test revealed that the only significant differences in baseline immunity between age groups were for serotype 3 between the group aged 21–30 years and the groups aged 31–40 and 41–50 years (P = .03 and .04, respectively). Of note, the first 3 age groups (21–50 years) likely received oral polio vaccine as children and likely were not exposed to wild poliovirus, and the last 2 age groups (>51 years) likely received inactivated polio vaccine as children and may have been exposed to wild poliovirus.
There were no significant differences in rates of polio immunity 1 month after receipt of the IPV booster between study groups (Table 2). With the exception of a 61-year-old outlier in group 1 who had no measurable immunity to any serotype either before or after vaccination, every subject was immune to serotypes 1 and 2 after the IPV booster. All but 3 subjects were immune to serotype 3 after the IPV booster. Vaccine response for serotype 3 was significantly lower for group 2 (20% intradermal dose) versus group 3 (full intramuscular dose; P = .01; Table 2). Other differences were not significant.
Table 2.
Polio Immunity and Vaccine Response 1 Month After Inactivated Polio Vaccine (IPV) Booster Receipt by Study Group
| Group, IPV Formulation | Subjects, No. | Postbooster Immunity, by Serotype |
Vaccine Response, by Serotype |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| Group 1, 40% ID | 65 | 98 (64) | 98 (64) | 97 (63) | 91 (59) | 92 (60) | 91 (59) |
| Group 2, 20% ID | 63 | 100 (63) | 100 (63) | 98 (62) | 84 (53) | 84 (53) | 87 (55)a |
| Group 3, 100% IM | 64 | 100 (64) | 100 (64) | 100 (64) | 92 (59) | 94 (60) | 98 (63) |
| Group 4, 40% IM | 32 | 100 (32) | 100 (32) | 100 (32) | 94 (30) | 94 (30) | 97 (31) |
Data are % (no.) of subjects in each group for each serotype who were immune (defined as a polio neutralizing antibody titer of ≥8) after booster vaccination or who responded to the vaccine (defined as seroconversion in subjects who were not immune at baseline or at least a 4-fold rise in titer in subjects who were immune at baseline). Differences in postbooster immunity and vaccine response were not statistically significant between study groups, unless otherwise indicated.
Abbreviations: ID, intradermal; IM, intramuscular.
a P = .01, compared with group 3.
The baseline geometric mean titers (GMTs) and 1 month postbooster GMTs are shown in Table 3. There were no significant differences for baseline GMTs for any serotype. The postbooster GMTs were highest in group 1 (40% intradermal dose), but not significantly so. The fold-rises in titer were robust, with overall median fold-rises of 32, 42, and 161 for serotypes 1, 2, and 3, respectively. The fold-rise in titer for serotype 2 was significantly higher for group 1 (40% intradermal dose) versus group 2 (20% intradermal dose), but no other differences were significant.
Table 3.
Poliovirus Neutralizing Antibody Geometric Mean Titers (GMTs) at Baseline and After Receipt of Inactivated Polio Vaccine (IPV) Booster
| Group, IPV Formulation | Serotype 1, GMT (95% CI) |
Serotype 2, GMT (95% CI) |
Serotype 3, GMT (95% CI) |
|||
|---|---|---|---|---|---|---|
| Baseline | After Booster | Baseline | After Booster | Baseline | After Booster | |
| Group 1, 40% ID | 44 (31–64) | 1715 (1174–2504) | 33 (24–44) | 2188 (1507–3178) | 14 (10–20) | 2375 (1423–3963) |
| Group 2, 20% ID | 42 (29–59) | 976 (730–1304) | 53 (37–76) | 1438 (984–2101) | 20 (14–28) | 1698 (1114–2588) |
| Group 3, 100% IM | 42 (30–58) | 1249 (916–1705) | 36 (26–51) | 1489 (1041–2128) | 16 (11–21) | 1792 (1133–2835) |
| Group 4, 40% IM | 34 (20–56) | 1328 (795–2219) | 44 (29–66) | 1938 (1232–3047) | 11 (7–16) | 2075 (1225–3514) |
There were data on baseline GMT for 66, 66, 66, and 33 subjects and on postbooster GMT for 65, 63, 64, and 32 subjects for groups 1, 2, 3, and 4, respectively. There were no significant differences between study groups for either baseline or postbooster GMTs.
Abbreviations: CI, confidence interval; ID, intradermal; IM, intramuscular.
All experimental groups (groups 1, 2, and 4) were noninferior to the control group (group 3), based on postbooster antibody titers. For serotypes 1, 2, and 3, the 95% confidence intervals for the log2 postbooster GMTs of the experimental group, minus the control group, were −.24 to 1.16, −.18 to 1.29, and −.57 to 1.39, respectively, for group 1; −.96 to .25, −.79 to .69, and −.97 to .81, respectively, for group 2; and −.72 to .90, −.47 to 1.23, and −.86 to 1.28, respectively, for group 4.
Intradermal administration was preferred by most subjects who received IPV intradermally (54% preferred intradermal administration, 3% preferred intramuscular administration, and 42% did not care). Major leakage occurred with 12 of the 132 intradermal injections (9%), but all but one of these injections still had good bleb formation. Major leakage was not associated with lower antibody titers or the date of study enrollment. There were no significant differences between systemic side effects in the intradermal versus intramuscular groups, but the intradermal groups had higher rates of transient local effects, such as redness or itching at the injection site (Table 4). The only serious adverse event, which occurred 1 month after enrollment in a subject from group 1 and was considered unlikely to be related to the study, was hospitalization for chest pain and electrolyte abnormalities that were attributed to alcohol withdrawal.
Table 4.
Frequency of Adverse Events in the Week Following Vaccination, by Study Group
| Adverse Event | Group 1, 40% ID (n = 65) | Group 2, 20% ID (n = 63) | Group 3, 100% IM (n = 64) | Group 4, 40% IM (n = 32) |
|---|---|---|---|---|
| Anya | 51 (33) | 46 (29) | 28 (18) | 31 (10) |
| Fever | 0 (0) | 3 (2) | 0 (0) | 3 (1) |
| Rash | 2 (1) | 2 (1) | 6 (4) | 6 (2) |
| Redness at injection siteb | 29 (19) | 35 (22) | 6 (4) | 9 (3) |
| Swelling at injection site | 8 (5) | 11 (7) | 5 (3) | 6 (2) |
| Tenderness at injection site | 15 (10) | 13 (8) | 17 (11) | 16 (5) |
| Itching at injection sitec | 11 (7) | 6 (4) | 0 (0) | 0 (0) |
Data are (%) (no.) of subjects. Differences were not statistically significant, unless otherwise indicated.
Abbreviations: ID, intradermal; IM, intramuscular.
a P = .008 for group 1 vs group 3, and P = .04 for group 2 vs group 3.
b P = .0007 for group 1 vs group 3, P ≤ .0001 for group 2 vs group 3, P = .03 for group 1 vs group 4, and P = .008 for group 2 vs group 4.
c P = .007 for group 1 vs group 3, and P = .04 for group 2 vs group 3.
DISCUSSION
We report the results from the first human trial using a fractional intradermal dose of IPV that was >20% of the standard dose and the first trial of intradermal IPV in HIV-infected subjects. The 40%-dose intradermal IPV group achieved the highest postbooster antibody titers, compared with the other 3 groups (20%-dose intradermal IPV, full-dose intramuscular IPV, and 40%-dose intramuscular IPV), but the difference did not reach significance. Baseline and postbooster immunity were similar between the 4 study groups and for serotypes 1 and 2 for vaccine response, but the 20%-dose intradermal group had a significantly lower vaccine response to serotype 3, compared with the full-dose intramuscular group. Surprisingly, we found that adults with well-controlled HIV infection maintain high levels of polio immunity decades after polio vaccination and also have a robust memory response to booster IPV vaccination administered either intradermally or intramuscularly. Intradermal administration was well tolerated and preferred by a majority of subjects.
Among prior published randomized, controlled trials comparing seroconversion rates after 20%-dose intradermal IPV with those after full-dose intramuscular IPV in children, 3 showed significantly inferior seroconversion rates in the intradermal group [12–14], and 2 showed similar seroconversion rates [7, 15]. However, all prior trials showed significantly lower antibody titers after 20%-dose intradermal IPV, compared with full-dose intramuscular IPV [7, 11–15]. Our results are consistent with these studies but are the first to demonstrate that a booster of 40%-dose intradermal IPV results in antibody titers that are not only noninferior to full-dose intramuscular IPV but are actually higher, although not significantly so. The clinical significance of lower antibody titers that are above the threshold for seroprotection remains unclear. However, studies have suggested that high antibody titers (≥128) after IPV immunization are needed for reduction of fecal transmission [31], and 2 recent clinical trials showed that lower antibody titers at the time of an OPV challenge are associated with significantly higher OPV shedding [4, 5]. Because IPV as a primary regimen is known to induce less intestinal immunity than OPV [32], and because reducing fecal transmission is essential to stopping community circulation of poliovirus, choosing an IPV vaccination strategy that results in high antibody titers would be beneficial.
The high levels of baseline polio immunity in our HIV-infected subjects, even up to 5 decades after they should have last received a polio vaccination, is encouraging for the global polio eradication effort. We have previously shown that HIV-infected infants have a significantly lower immunologic response to OPV than uninfected infants [20]. However, the results from this current study suggest that, for the 33 million HIV-infected adults globally [33], most of whom were infected with HIV years after polio vaccination, such as the subjects in this study, polio immunity levels may remain high. Of note, these high levels of baseline polio immunity were evident even though most of our study subjects had a history of AIDS and even though OPV has not been used in the United States since 2000, so there would have been no recent boosting in our subjects due to community spread of OPV. Although the polio eradication effort has primarily focused on polio immunity in children, the large 2010 outbreak of wild poliovirus infection in the Republic of the Congo primarily affected adults (74% of cases) [34]. In this outbreak, older age was associated with a 7-fold higher risk of death [35]. A study using mathematical modeling suggested that the contribution of older children and adults to the spread of wild poliovirus in this outbreak was also significant [36]. Consequently, it is reassuring that polio immunity can remain high for decades despite HIV infection as an adult. Our data are consistent with 4 smaller studies from the 1990s that evaluated whether HIV-infected adults maintain polio seroprotection [24–26, 37]. These studies found seroprotection rates of 73%–80%, 73%–95%, and 54%–87% against poliovirus serotypes 1, 2, and 3, respectively. Lower immunity to serotype 3, compared with serotypes 1 and 2, following polio vaccination has been well documented and is a reason behind the OPV formulation change in the early 1990s [27] and the IPV formulation change in 1987 [38].
Surprisingly, the 40%-dose intramuscular IPV group had a similar vaccine response and antibody titers to both the full-dose intramuscular IPV group and the 40%-dose intradermal IPV group. Our preclinical study in rats showed that dose response was more consistent with intradermal IPV, compared with intramuscular IPV, in that antibody titers increased with increasing intradermal doses up to the maximum intradermal dose tested (40%), whereas antibody titers with increasing intramuscular doses were erratic [39]. It is possible that, given the high levels of preexisting immunity in our cohort, the maximal intramuscular response plateaued at a lower booster dose. However, our results suggest that in populations with high baseline levels of immunity, a full booster dose of IPV may not be needed even with intramuscular administration.
Intradermal administration of fractional IPV doses of up to 40% seems to be safe and well tolerated. Although the overall rate of adverse events was significantly higher in the intradermal groups, this was due to transient local adverse events such as redness and itching at the injection site. Rates of systemic adverse events such as fever and rash were low overall and did not differ significantly between groups. The majority of subjects who received intradermal vaccination said that they preferred intradermal to intramuscular administration. This is consistent with past intradermal IPV studies in infants that used needle-free intradermal delivery devices [7, 12]. In these studies, transient local adverse events were also significantly higher in the intradermal group, but the parents strongly preferred intradermal over intramuscular administration because it was less likely to make their infants cry.
We conducted this study in HIV-infected adults because they have suboptimal responses to many vaccines even with well-controlled HIV infection [16, 17, 19], a finding considered to be related to chronic immune activation [40]. Consequently, we felt that this population could function as a surrogate for populations in the developing world with suboptimal vaccine responses. However, the fold-rise in titers and postbooster GMTs in our study population were quite high and were actually comparable to those from a Dutch study investigating booster intramuscular and intradermal IPV doses in healthy adults who received IPV as children [11]. This similar booster response may have been because most of our subjects likely received OPV, not IPV, as children. However, it still suggests that while well-controlled HIV infection may impair the primary response to a vaccine, it might not impair the boosting response to a vaccine first received as a child prior to HIV infection.
Our study has limitations. The booster responses were much higher than anticipated, so our predetermined sample sizes may have been too low to detect differences. The polio vaccination history was not known for individual subjects but could only be assumed on the basis of US vaccination policies when the subjects were children. Finally, HIV-infected adults in the United States are only a surrogate for the groups in whom intradermal IPV may be most relevant.
Despite these limitations, we demonstrate that a 40% fractional dose of IPV administered intradermally results in at least noninferior antibody titers, compared with the full dose administered intramuscularly, and that it results in higher antibody titers than a 20% intradermal dose. Intradermal IPV administration at a fractional dose of >20% should be considered as a means to make IPV more affordable for developing countries, balancing sufficient immunity with cost reduction.
Notes
Acknowledgments. We thank the providers, staff, and especially patients at the EVMS C3ID clinic for their generous support and cooperation.
S. B. T. had full access to all of the data and is responsible for the content of the article.
Disclaimer. The funders had no role in the study design, data collection, data analysis, manuscript preparation, or the decision to submit the manuscript for publication.
Financial support. This work was supported by the Doris Duke Charitable Foundation (Clinical Scientist Development Award 2012061 [principal investigator, S. B. T.]). S. B. T. also received salary support while working on this project from the National Institutes of Health (Career Development Award 5K23AI093678 [principal investigator, S. B. T.]).
Potential conflicts of interest. E. K. and Y. L. are employed by NanoPass Technologies. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Moturi EK, Porter KA, Wassilak SG, et al. Progress toward polio eradication--Worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep 2014; 63:468–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Diop OM, Burns CC, Wassilak SG, Kew OM. Update on vaccine-derived polioviruses - worldwide, July 2012-December 2013. MMWR Morb Mortal Wkly Rep 2014; 63:242–8. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Polio eradication and endgame strategic plan 2013–2018: executive summary. Geneva: WHO, 2013. [Google Scholar]
- 4.John J, Giri S, Karthikeyan AS, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. Lancet 2014; 384:1505–12. [DOI] [PubMed] [Google Scholar]
- 5.Jafari H, Deshpande JM, Sutter RW, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science 2014; 345:922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutter R, Suleiman A, Malankar P, et al. Trial of a supplemental dose of four poliovirus vaccines. N Engl J Med 2000; 343:767–73. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed A, AlAwaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med 2010; 362:2351–9. [DOI] [PubMed] [Google Scholar]
- 8.Nelson KS, Janssen JM, Troy SB, Maldonado Y. Intradermal fractional dose inactivated polio vaccine: A review of the literature. Vaccine 2012; 30:121–5. [DOI] [PubMed] [Google Scholar]
- 9.Hung IF, Levin Y, To KK, et al. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 2012; 30:6427–35. [DOI] [PubMed] [Google Scholar]
- 10.Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008; 198:650–8. [DOI] [PubMed] [Google Scholar]
- 11.Soonawala D, Verdijk P, Wijmenga-Monsuur AJ, et al. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013; 31:3688–94. [DOI] [PubMed] [Google Scholar]
- 12.Resik S, Tejeda A, Lago P, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis 2010; 201:1344–52. [DOI] [PubMed] [Google Scholar]
- 13.Resik S, Tejeda A, Sutter RW, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013; 368:416–24. [DOI] [PubMed] [Google Scholar]
- 14.Estívariz CF, Jafari H, Sutter RW, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis 2012; 12:128–35. [DOI] [PubMed] [Google Scholar]
- 15.Cadorna-Carlos J, Vidor E, Bonnet MC. Randomized controlled study of fractional doses of inactivated poliovirus vaccine administered intradermally with a needle in the Philippines. Int J Infect Dis 2012; 16:e110–6. [DOI] [PubMed] [Google Scholar]
- 16.Crum-Cianflone NF, Eberly LE, Duplessis C, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis 2011; 52:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010; 202:1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurence JC. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am J Med 2005; 118(suppl 10A):75S–83. [DOI] [PubMed] [Google Scholar]
- 19.Yao ZQ, Moorman JP. Immune exhaustion and immune senescence: two distinct pathways for HBV vaccine failure during HCV and/or HIV infection. Arch Immunol Ther Exp (Warsz) 2013; 61:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troy SB, Musingwini G, Halpern MS, et al. Vaccine poliovirus shedding and immune response to oral polio vaccine in HIV-infected and -uninfected zimbabwean infants. J Infect Dis 2013; 208:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbi M, Bardare M, Luraschi C, Zehender G, Clerici Schoeller M, Ferraris G. Antibody response to inactivated polio vaccine (E-IPV) in children born to HIV positive mothers. Eur J Epidemiol 1992; 8:211–6. [DOI] [PubMed] [Google Scholar]
- 22.Bardare M, Barbi M, Plebani A, Ferraris G, Zanetti A. Humoral response to inactivated poliovaccine in anti-HIV positive infants. Adv Exp Med Biol 1989; 257:221–4. [DOI] [PubMed] [Google Scholar]
- 23.Blanche S, Le Deist F, Fischer A, et al. Longitudinal study of 18 children with perinatal LAV/HTLV III infection: attempt at prognostic evaluation. J Pediatr 1986; 109:965–70. [DOI] [PubMed] [Google Scholar]
- 24.Kroon F, van Dissel J, Labadie J, van Loon A, van Furth R. Antibody response to diphtheria, tetanus, and poliomyelitis vaccines in relation to the number of CD4+ T lymphocytes in adults infected with human immunodeficiency virus. Clin Infect Dis 1995; 21:1197–203. [DOI] [PubMed] [Google Scholar]
- 25.Vardinon N, Handsher R, Burke M, Zacut V, Yust I. Poliovirus vaccination responses in HIV-infected patients: correlation with T4 cell counts. J Infect Dis 1990; 162:238–41. [DOI] [PubMed] [Google Scholar]
- 26.Varon D, Handsher R, Dardik R, et al. Response of hemophilic patients to poliovirus vaccination: correlation with HIV serology and with immunological parameters. J Med Virol 1993; 40:91–5. [DOI] [PubMed] [Google Scholar]
- 27.Sutter R, Kew O, Cochi S, Aylward RB. Poliovirus vaccine-live. In: Paul O, Orenstein W, Plotkin S, eds. Vaccines. 6th ed China: Elsevier/Saunders, 2013:598–645. [Google Scholar]
- 28.World Health Organization (WHO). Manual for the virological investigation of polio. WHO/EPI/GEN/97.01 Geneva: WHO, 1997. [Google Scholar]
- 29.Samuel B, Cherian T, Sridharan G, Mukundan P, John T. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet 1991; 338:343–4. [DOI] [PubMed] [Google Scholar]
- 30.Moriniere B, van Loon F, Rhodes P, et al. Immunogenicity of a supplemental dose of oral versus inactivated poliovirus vaccine. Lancet 1993; 341:1545–50. [DOI] [PubMed] [Google Scholar]
- 31.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol 1999; 150:1001–21. [DOI] [PubMed] [Google Scholar]
- 32.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog 2012; 8:e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS, 2013.
- 34.Patel MK, Konde MK, Didi-Ngossaki BH, et al. An outbreak of wild poliovirus in the Republic of Congo, 2010–2011. Clin Infect Dis 2012; 55:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregory CJ, Ndiaye S, Patel M, et al. Investigation of elevated case-fatality rate in poliomyelitis outbreak in Pointe Noire, Republic of Congo, 2010. Clin Infect Dis 2012; 55:1299–306. [DOI] [PubMed] [Google Scholar]
- 36.Blake IM, Martin R, Goel A, et al. The role of older children and adults in wild poliovirus transmission. Proc Natl Acad Sci U S A 2014; 111:10604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pregliasco F, Minolfi V, Boschin A, Andreassi A, Profeta ML. A seroepidemiologic survey of immunity against poliomyelitis in a group of HIV positive and HIV negative drug addicts. Eur J Epidemiol 1995; 11:693–5. [DOI] [PubMed] [Google Scholar]
- 38.Bonnet MC, Dutta A. World wide experience with inactivated poliovirus vaccine. Vaccine 2008; 26:4978–83. [DOI] [PubMed] [Google Scholar]
- 39.Kouiavskaia D, Mirochnitchenko O, Dragunsky E, et al. Intradermal inactivated poliovirus vaccine: a preclinical dose-finding study. J Infect Dis 2015; 211:1447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]