Abstract
Background. Little is known about how different antiretrovirals effect inflammation and monocyte activation in human immunodeficiency virus (HIV) infection.
Methods. We examined plasma specimens obtained during a randomized, double-blinded trial in antiretroviral therapy (ART)–naive HIV-infected adults which compared the efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (EVG/c/FTC/TDF) with that of efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV/FTC/TDF). From a random sample achieving an HIV type 1 RNA load of <50 copies/mL by week 48, changes over 24 and 48 weeks in levels of biomarkers of monocyte activation (soluble CD14 [sCD14] and soluble CD163 [sCD163]), systemic inflammation (soluble tumor necrosis factor α receptor I [sTNF-RI], interleukin 6 [IL-6], and high-sensitivity C-reactive protein [hsCRP]), and vascular inflammation (lipoprotein-associated phospholipase A2 [Lp-PLA2]) were compared. Multivariable linear regression was used.
Results. A total of 200 participants were included. Significant differences favoring EVG/c/FTC/TDF were noted for changes in sCD14, hsCRP, and Lp-PLA2 levels. Factors independently associated with a larger decrease in the sCD14 level included random assignment to receive EVG/c/FTC/TDF, higher baseline sCD14 level, and larger decreases in hsCRP and sCD163 levels; factors associated with a larger Lp-PLA2 decrease included higher baseline Lp-PLA2 and IL-6 levels, smaller increases in total cholesterol and triglycerides levels, a larger decrease in the sCD14 level, and a smaller decrease in the sCD163 level.
Conclusions. EVG/c/FTC/TDF led to greater decreases in sCD14, hsCRP, and Lp-PLA2 levels, compared with EFV/FTC/TDF. Randomization group independently predicted the change in sCD14 level, and changes in monocyte activation independently predicted the change in Lp-PLA2 level. There appears to be a more favorable effect of the integrase inhibitor EVG over efavirenz on immune activation, which may affect vascular inflammation.
Keywords: monocyte activation, vascular inflammation, systemic inflammation, antiretroviral-naive, HIV infection
(See the editorial commentary by Erlandson and Campbell on pages 339–42.)
Advances in antiretroviral therapy (ART) have had an impressive impact on morbidity and mortality due to human immunodeficiency virus (HIV) infection over the last 2 decades, such that life expectancy nears that of the general population in developed countries [1]. As AIDS-related mortality has fallen, the proportion of deaths due to cardiovascular disease (CVD) has increased [2]. Further, it has been shown that HIV-infected patients are at increased risk of myocardial infarction [3, 4] and stroke [5] and that CVD risk during HIV infection may accelerate faster with age than in the general population [6].
While traditional CVD-associated risk factors [7] and ART use [8] have been implicated in the risk of CVD among HIV-infected individuals, the role of persistent immune activation, specifically monocyte activation, has recently received much attention, as well [9–11]. Continued immune activation in HIV-infected persons during ART may be the result of enterocyte damage leading to microbial translocation [12, 13], coinfections [14–16], persistent low-level viral replication [17], or ART itself [18], and little is known about the differential effects of the many available antiretroviral medications on inflammation and immune activation in HIV infection. It is plausible that the integrase inhibitor class may decrease inflammation and immune activation more than other antiretroviral classes, because integrase inhibitors are more lipid friendly [19, 20] and may concentrate better in enterocytes [21]. Understanding the effect of specific antiretroviral medications on inflammation is important because there many medications available for the treatment of HIV infection and because inflammation has been linked to mortality in treated infection [22, 23].
The aim of this study was to compare changes from baseline to 24 and 48 weeks in levels of markers of monocyte activation and of systemic and vascular inflammation between ART-naive HIV-infected adults randomly assigned to receive an integrase inhibitor–based regimen, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (EVG/c/FTC/TDF) or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV/FTC/TDF), in the Gilead 102 study [24]. Our hypothesis was that the markers of monocyte activation and systemic and vascular inflammation would decrease to a greater degree in the EVG/c/FTC/TDF group.
METHODS
Gilead 102 is a 96-week, randomized, double-blinded, active-controlled clinical trial to evaluate the safety and efficacy of EVG/c/FTC/TDF versus that of EFV/FTC/TDF in HIV-infected ART-naive adults. The entry criteria for this study have been published previously [24]. In brief, eligibility criteria were age ≥18 years, HIV-1 RNA level of ≥5000 copies/mL, no prior ART use, genotypic susceptibility to emtricitabine, tenofovir, and efavirenz, estimated glomerular filtration rate (eGFR) of ≥70 mL/min by the Cockcroft-Gault equation, liver transaminase levels ≤5 times the upper limit of normal, absolute neutrophil count of ≥1000 neutrophils/mm3, platelet count of ≥50 000 platelets/mm3, hemoglobin level of ≥8.5 g/dL, life expectancy of ≥1 year from the time of enrollment, no AIDS-defining conditions diagnosed within 30 days, not currently receiving treatment for hepatitis C or other drugs known to interact with the study medications or systemic corticosteroids, no active infection or malignancy, and no current alcohol or substance use judged to potentially interfere with study compliance. Participants were randomly assigned at a ratio of 1:1 to receive EVG/c/FTC/TDF or EFV/FTC/TDF, as well as placebo matching the study drug administered to the other treatment group. The participants for this study were a random sample of Gilead 102 participants who achieved an HIV-1 RNA level of <50 copies/mL by week 48 and had a stored plasma specimen available from entry, week 24, and week 48 visits. All participants of the Gilead 102 study provided written informed consent, which included consent for use of stored blood specimens for clinical tests to be performed later. This study was approved by the University Hospitals Case Medical Center Institutional Review Board.
The primary outcomes in this study were changes from baseline to week 48 in levels of soluble CD14 (sCD14) and soluble CD163 (sCD163), markers of monocyte activation; levels of soluble tumor necrosis factor α receptor I (sTNF-RI), interleukin 6(IL-6), and high sensitivity C-reactive protein (hsCRP), markers of systemic inflammation; and the level of lipoprotein-associated phospholipase A2 (Lp-PLA2), a marker of vascular inflammation. Secondary outcomes of interest were changes in levels of these markers from baseline to 24 weeks and relationships between changes in monocyte activation and inflammation markers.
Markers of Inflammation and Immune Activation
Participants had blood specimens collected after an 8-hour fast at entry, week 24, and week 48. Plasma specimens were stored at −80°C and never thawed until analysis. Stored plasma samples were analyzed using specific enzyme-linked immunosorbent assay kits as per the manufacturers' instructions (diaDexus [South San Francisco, California] for Lp-PLA2; R&D Systems [Minneapolis, Minnesota] for all others). Markers with compelling data linking them to CVD risk and mortality during HIV infection were selected [25–29].
Statistical Analysis
Data on demographic characteristics, clinical indices, and HIV-related factors are presented overall and by group at baseline. Median values and interquartile ranges (IQRs) are reported for continuous variables, and numbers and percentages are reported for categorical variables. Absolute and percentage changes from baseline to week 24 and from baseline to week 48 in levels of markers of monocyte activation and inflammation, as well as clinically relevant variables, were determined. All baseline variables and end points were compared between groups, using unpaired t tests or Wilcoxon rank sum tests, as warranted by the distribution of data, for continuous variables and using χ2 tests, Fisher exact tests, or Pearson exact χ2 tests, as appropriate, for categorical variables. Within-group changes were tested using paired t tests or Wilcoxon signed rank tests appropriate for the distribution of data.
For the regression analyses, all variables with nonnormally distributed data were log-transformed prior to model fitting. Univariable followed by multivariable linear regression was used to explore relationships of baseline factors with baseline log-transformed sCD14 and log-transformed Lp-PLA2. Variables tested in the univariable analyses included age, sex, race, hepatitis B and C status, weight, CD4+ T-cell count, HIV-1 RNA level, eGFR, hemoglobin level, glucose level, lipoprotein levels, and markers of monocyte activation and inflammation at baseline. All variables with a P value of < .25 in univariable analyses were considered for inclusion in the multivariable model, and backward elimination was used for model selection. Next, 2 separate analyses were performed in which change in sCD14 and Lp-PLA2 levels were the outcomes. First, analysis of covariance was used to adjust the mean percentage change from baseline to week 48 in log-transformed sCD14 and Lp-PLA2 levels for baseline levels of these 2 markers and for percentage changes from baseline to week 48 in clinically relevant variables, including weight, CD4+ T-cell count, hemoglobin level, eGFR, glucose level, and lipoprotein levels, with each variable on the same scale as the outcome variable. Last, univariable followed by multivariable linear regression was used to explore predictors of percentage change from baseline to week 48 in log-transformed sCD14 and Lp-PLA2 levels. Variables tested in the univariable analyses for models with percentage change from baseline to week 48 in log-transformed sCD14 or Lp-PLA2 levels as the outcome included all baseline variables listed above, as well as percentage change from baseline to week 48 in weight, CD4+ T-cell count, hemoglobin level, eGFR, glucose level, lipoprotein levels, and other markers of monocyte activation and inflammation, with each variable on the same scale as the outcome variable. All final models were checked to be sure the assumptions of linear regression were met.
All statistical tests were 2 sided, and differences with a P value of < .05 were considered statistically significant. Adjustments were not made in this significance level for multiple comparisons. Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
With 200 participants in this study and the assumption of a conservative common SD of 10%, our study had 80% power to detect a between-group difference as low as 4% in the percentage change in biomarker levels over 48 weeks, using an unpaired t test with a 2-sided 0.05 level of significance.
RESULTS
Baseline Characteristics
Two hundred participants were included in this study (100 in each group). Participants did not differ from the Gilead 102 population with regard to baseline characteristics (data not shown). At baseline, the 2 treatment groups were balanced with regard to all demographic and HIV-related factors, except there were more participants receiving antihypertensive medications in the EVG/c/FTC/TDF group (18% vs 8%; P = .04). When comparing individual classes of antihypertensive medications, only diuretic use was higher in the EVG/c/FTC/TDF group (13% vs 3%; P < .01); proportion of participants receiving medications from other classes were similar between groups (Table 1). Overall, the median age was 38 years (IQR, 30–44.5 years), and the majority of participants were men (89%) and white (65%); 30% were African American, and 5% were from other racial groups. The median baseline weight was 81 kg (IQR, 70.5–92.6 kg), and the median eGFR was 120.2 mL/min (IQR, 99.2–137.2 mL/min). Three and five participants had chronic hepatitis B virus and chronic hepatitis C virus infections, respectively. All participants were ART naive at study entry, by design, with a median baseline CD4+ T-cell count of 371.5 cell/mm3 (IQR, 267.5–484.5 cell/mm3) and a median HIV-1 RNA load of 64 900 copies/mL (IQR, 25 050–147 000 copies/mL). At baseline, 10% were receiving at least 1 cholesterol-lowering medication, including 4 participants receiving an HMG-CoA reductase inhibitor or statin.
Table 1.
Baseline Demographic, Clinical, and Human Immunodeficiency Virus (HIV)-Related Indices, by Randomization Group
| Characteristic | EVG/c/FTC/TDF (n = 100) | EFV/FTC/TDF (n = 100) | P Values |
|---|---|---|---|
| Age, y | 37 (30.5–44.5) | 38 (30–44.5) | .98 |
| Male sex | 88 (88) | 90 (90) | .65 |
| Race | .69 | ||
| White | 62 (62) | 67 (67) | |
| African American | 33 (33) | 26 (26) | |
| Other | 5 (5) | 7 (7) | |
| Chronic hepatitis B | 0 (0) | 3 (3) | .25 |
| Chronic hepatitis C | 2 (2) | 3 (3) | >.99 |
| Weight, kg | 80.7 (69.6–89.8) | 81.2 (71.5–95.3) | .37 |
| eGFR, mL/min | 116.3 (97.8–139.4) | 121.5 (101.1–135.6) | .58 |
| Hemoglobin level, g/dL | 14.2 (12.9–15.1) | 14.3 (13.5–14.9) | .49 |
| Glucose level, mg/dL | 92 (86–98) | 91 (84–97) | .37 |
| Cholesterol level, mg/dL | |||
| Total | 161.5 (139–181) | 161.5 (141–185) | .72 |
| Low-density lipoprotein | 98 (79.5–118) | 94.5 (82–115) | .9 |
| High-density lipoprotein | 41 (35–48) | 40 (33–47) | .28 |
| Triglycerides level | 98 (79.5–118) | 100.5 (78–152) | .39 |
| Antihypertensive medication | |||
| Anya | 18 (18) | 8 (8) | .04 |
| Class | |||
| Renin-angiotensin system agent | 12 (12) | 8 (8) | .35 |
| β-blocker | 3 (3) | 0 (0) | .08 |
| Calcium channel blocker | 2 (2) | 2 (2) | >.99 |
| Diuretic | 13 (13) | 3 (3) | <.01 |
| Cholesterol-lowering medication | |||
| Anya | 11 (11) | 9 (9) | .64 |
| Class | |||
| Statin | 2 (2) | 2 (2) | >.99 |
| Fish oil | 8 (8) | 7 (7) | .79 |
| Fibrate | 2 (2) | 0 (0) | .16 |
| CD4+ T-cell count, cells/mm3 | 369 (279.5–473) | 380.5 (249–492) | .96 |
| HIV type 1 RNA level, copies/mL | 52 850 (19 550–144 000) | 79 300 (36 750–150 500) | .08 |
Data are median value (interquartile range) or no. (%) of patients.
Abbreviations: EFV/FTC/TDF, efavirenz/emtricitabine/tenofovir disoproxil fumarate; eGFR, estimated glomerular filtration rate; EVG/c/FTC/TDF, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate.
a Defined as use of ≥1 medication.
Changes in Markers After ART Initiation
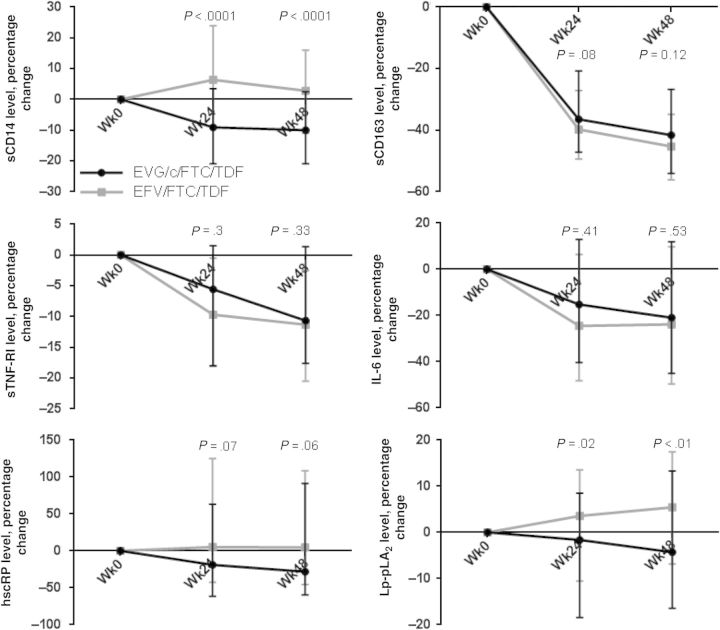
At baseline, levels of markers of monocyte activation and systemic and vascular inflammation were similar between groups. Baseline, week 24, week 48 values and absolute changes from baseline to week 24 and to week 48 in levels of all markers are shown in Table 2. Within the EVG/c/FTC/TDF group, levels of all markers decreased significantly relative to baseline by week 48, with the exception of Lp-PLA2, for which the decrease neared significance (P = .06). In the EFV/FTC/TDF group, the changes were mixed. Over 48 weeks, levels of sCD163, sTNF-RI, and IL-6 decreased significantly; however, the level of Lp-PLA2 increased, and the levels of sCD14 and hsCRP did not change significantly. Percentage changes in the markers are shown in Figure 1. Within-group percentage changes in levels of the markers were similar to the absolute changes except, within the EVG/c/FTC/TDF group, the change in hsCRP level was not statistically significant and, in the EFV/FTC/TDF group, increases in sCD14 and hsCRP levels were significant at both week 24 and week 48. Absolute and percentage changes from baseline to week 24 and from baseline to week 48 were significantly different for sCD14, hsCRP (absolute change only), and Lp-PLA2 levels, with changes favoring the EVG/c/FTC/TDF group. Changes were similar between groups for sCD163, sTNF-RI, and IL-6 levels. After adjustment for percentage changes from baseline to week 48 in weight, CD4+ T-cell count, hemoglobin level, eGFR, glucose level, and lipoprotein levels, percentage changes from baseline to week 48 in sCD14 and Lp-PLA2 levels remained significantly different between groups, with changes favoring EVG/c/FTC/TDF (data not shown).
Table 2.
Baseline, Week 24, Week 48, and Absolute Change in Biomarker Levels, by Randomization Group
| Biomarker, Time Point | EVG/c/FTC/TDF (n = 100) | EFV/FTC/TDF (n = 100) | P Valuesa |
|---|---|---|---|
| sCD14 level, ng/mL | |||
| Week 0 | 1529.5 (1329–1843.5) | 1593 (1328–1805.5) | .76 |
| Week 24 | 1418 (1235–1642) | 1649 (1454–1845) | <.0001 |
| Week 48 | 1368.5 (1255–1560) | 1654 (1436.5–1792) | <.0001 |
| Absolute change | |||
| Between weeks 0 and 24 | −99 (−349.5 to 45)b | 93 (−137.5 to 303.5)b | <.0001 |
| Between weeks 0 and 48 | −149 (−356 to 32.5)b | 46 (−176.5 to 227) | <.0001 |
| sCD163 level, ng/mL | |||
| Week 0 | 861.15 (679.98–1213.75) | 910.65 (728.9–1231.65) | .23 |
| Week 24 | 579.2 (389.75–777.4) | 549 (460–25–749.6) | .89 |
| Week 48 | 517.7 (378.3–690.35) | 525.2 (384.25–670.6) | .78 |
| Absolute change | |||
| Between weeks 0 and 24 | −310 (−485.25 to–125.75)b | −355.5 (−583.4–221.35)b | .09 |
| Between weeks 0 and 48 | −337.4 (−514.8–185.15)b | −407.1 (−628.4–252.5)b | .05 |
| sTNF-RI level, pg/mL | |||
| Week 0 | 1186 (948.5–1476.5) | 1172.5 (1017–1361) | .9 |
| Week 24 | 1068.5 (872–1301.5) | 1053.5 (917.5–1242.5) | .86 |
| Week 48 | 1089 (907–1235) | 1053 (881–1221.5) | .62 |
| Absolute change | |||
| Between weeks 0 and 24 | −54 (−227.5 to 17)b | −103 (−215.5 to 6)b | .28 |
| Between weeks 0 and 48 | −120.5 (−230 to 16.5)b | −123 (−247.5 to 30)b | .47 |
| IL-6 level, pg/mL | |||
| Week 0 | 1.695 (1.205–2.475) | 1.715 (1.27–2.765) | .6 |
| Week 24 | 1.38 (0.93–2.2) | 1.225 (0.905–2.42) | .77 |
| Week 48 | 1.365 (0.885–2.08) | 1.275 (0.895–2.185) | .91 |
| Absolute change | |||
| Between weeks 0 and 24 | −0.205 (−0.905 to 0.185)b | −0.34 (−1.05 to 0.085)b | .55 |
| Between weeks 0 and 48 | −0.27 (−0.935 to 0.135)b | −0.365 (−1.2 to 0.14)b | .53 |
| hsCRP level, ng/mL | |||
| Week 0 | 1479 (506.5–4977) | 1562.5 (751–3209.5) | .85 |
| Week 24 | 1214.5 (467.5–3499.5) | 1808.5 (781–4084) | .1 |
| Week 48 | 1538 (542.5–3702.8) | 1945.5 (745–4292.5) | .17 |
| Absolute change | |||
| Between weeks 0 and 24 | −108 (−1755 to 637.7) | 49.6 (−830 to 1793) | .04 |
| Between weeks 0 and 48 | −176.5 (−1872.5 to 860.5)b | 55 (−662.5 to 1759.5) | <.01 |
| Lp-PLA2 level, ng/mL | |||
| Week 0 | 171 (128.5–199) | 158 (134.5–188) | .24 |
| Week 24 | 157.5 (124.5–188) | 167.5 (136–191) | .32 |
| Week 48 | 156 (130–190) | 165.5 (147.5–193) | .1 |
| Absolute change | |||
| Between weeks 0 and 24 | −2 (−33 to 12)b | 4.5 (−19 to 21) | .02 |
| Between weeks 0 and 48 | −6 (−30.5 to 22.5) | 9 (−12 to 26.5)b | <.01 |
Data are median value (interquartile range).
Abbreviations: EFV/FTC/TDF, efavirenz/emtricitabine/tenofovir disoproxil fumarate; EVG/c/FTC/TDF, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; Lp-PLA2, lipoprotein-associated phospholipase A2; sCD14, soluble CD14; sCD163, soluble CD163; sTNF-RI, soluble tumor necrosis factor α receptor I.
a For between-group differences.
b P < .05 for within-group comparisons.
Figure 1.
Median percentage changes (interquartile range) in biomarker levels over 24 and 48 weeks, by randomization group. P values are for comparisons between groups in the percentage change from weeks 0 to 24 and weeks 0 to 48. Abbreviations: EFV/FTC/TDF, efavirenz/emtricitabine/tenofovir disoproxil fumarate; EVG/c/FTC/TDF, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; Lp-PLA2, lipoprotein-associated phospholipase A2; sCD14, soluble CD14; sCD163, soluble CD163; sTNF-RI, soluble tumor necrosis factor α receptor I.
Changes in Other Clinically Relevant Factors After ART Initiation
In this sample, absolute changes in CD4+ T-cell count from baseline to week 24 and from baseline to week 48 were similar between groups (185 cells/mm3 for EVG/c/FTC/TDF vs 155 cells/mm3 for EFV/FTC/TDF [P = .24] and 246 cells/mm3 for EVG/c/FTC/TDF vs 217 cells/mm3 for EFV/FTC/TDF [P = .23], respectively), as was the proportion of participants achieving an HIV-1 RNA level of <50 copies/mL by week 24 (95% and 96% for EVG/c/FTC/TDF and EFV/FTC/TDF, respectively; P = .72). By design, all participants had an undetectable HIV-1 RNA level by week 48. The decline in eGFR was greater in the EVG/c/FTC/TDF group, and this was apparent by week 24 (−11.3 and −0.2 mL/min for EVG/c/FTC/TDF and EFV/FTC/TDF, respectively; P < .0001). Absolute changes in lipoprotein levels were similar between groups, with the exception of the high-density lipoprotein (HDL) cholesterol level, which increased more in the EFV/FTC/TDF group by week 48 (5 vs 8 mg/dL; P = .045). The absolute change in weight was greater in the EVG/c/FTC/TDF group from baseline to week 24 (0.9 and 0 kg for EVG/c/FTC/TDF and EFV/FTC/TDF, respectively; P = .01) but was similar between groups by week 48 (1 and 0.7 kg for EVG/c/FTC/TDF and EFV/FTC/TDF, respectively; P = .13). Glucose levels increased to a greater degree in the EFV/FTC/TDF group from baseline to week 48 (2 vs 5.5 mg/dL; P = .02). Changes in hemoglobin levels were similar between groups.
Factors Associated With sCD14 and Lp-PLA2 Levels at Baseline
In an exploratory analysis, we determined factors independently associated with the log-transformed sCD14 level and the log-transformed Lp-PLA2 level at baseline (Table 3). Factors independently associated with higher sCD14 levels prior to ART initiation were lower weight, CD4+ T-cell count, hemoglobin level, and low-density lipoprotein (LDL) cholesterol level and higher HIV-1 RNA load, HDL cholesterol level, triglycerides level, sTNF-RI level, and IL-6 level. Factors independently associated with a higher Lp-PLA2 level prior to ART were male sex and higher total cholesterol and sTNF-RI levels.
Table 3.
Factors Independently Associated With Soluble CD14 (sCD14) and Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) Levels at Baseline
| Variable | Parameter Estimate ± SE | P Values |
|---|---|---|
| Log sCD14 levela | ||
| Weight, kg | −2.43 × 10−3 ± 8.19 × 10−4 | <.01 |
| CD4+ T-cell count, cells/mm3 | −1.77 × 10−4 ± 8.28 × 10−5 | .03 |
| HIV-1 RNA load, copies/mL | 2.19 × 10−7 ± 7.25 × 10−8 | <.01 |
| Hemoglobin level, g/dL | −2.56 × 10−2 ± 9.88 × 10−3 | .01 |
| HDL cholesterol level, mg/dL | 3.41 × 10−3 ± 1.6 × 10−3 | .03 |
| LDL cholesterol level, mg/dL | −1.13 × 10−3 ± 5.43 × 10−4 | .04 |
| Triglycerides level, mg/dL | 5.73 × 10−4 ± 2.14 × 10−4 | <.01 |
| Log sTNF-RI level, pg/mL | 0.348 ± 5.94 × 10−2 | <.0001 |
| Log IL-6 level, pg/mL | 7.67 × 10−2 ± 2.15 × 10−2 | <.001 |
| Log Lp-PLA2 levelb | ||
| Sex | 0.183 ± 6.4 × 10−2 | <.01 |
| Total cholesterol level, mg/dL | 1.99 × 10−3 ± 6.15 × 10−4 | <.01 |
| Log sTNF-RI level | 0.315 ± 7.3 × 10−2 | <.0001 |
Abbreviations: HDL, high-density lipoprotein; HIV-1, human immunodeficiency virus type 1; IL-6, interleukin 6; LDL, low-density lipoprotein; SE, standard error; sTNF-RI, soluble tumor necrosis factor α receptor I.
a Variables with a P value of < .25 in univariable analysis but not selected for inclusion in the final model included sex, race (white vs other), hepatitis C status, age, estimated glomerular filtration rate, log-transformed high-sensitivity C-reactive protein level, and log-transformed soluble CD163 level.
b Variables with a P value of < .25 in univariable analysis but not selected for in the final model included race (white vs other), hemoglobin level, triglycerides level, and log-transformed sCD14 level.
Factors Associated With Change in sCD14 and Lp-PLA2 Levels
We also determined factors independently associated with the percentage change from baseline to 48 weeks in log-transformed sCD14 and Lp-PLA2 levels (Table 4). For percentage change from baseline to week 48 week in the log-transformed sCD14 level, randomization group remained independently associated in the final model, such that being randomly assigned to the EVG/c/FTC/TDF group was associated with a larger decline in the sCD14 level over 48 weeks. Additional factors that were independently associated with a larger decrease in the sCD14 level included higher baseline sCD14 level, lower baseline CD4+ T-cell count, lower baseline HDL cholesterol level, larger increases in weight and eGFR, and larger decreases in sCD163 and hsCRP levels over 48 weeks. For percentage change from baseline to week 48 in the Lp-PLA2 level, factors independently associated with a larger decrease in the Lp-PLA2 level were higher baseline Lp-PLA2 and IL-6 levels and a lower baseline LDL cholesterol level, as well as smaller increases in total cholesterol and triglycerides levels, a larger decrease in the sCD14 level, and a smaller decrease in the sCD163 level. Randomization group was not independently associated with percentage change in the Lp-PLA2 level; however, after removing changes in sCD14 and sCD163 levels from the model, randomization group was associated with changes in the Lp-PLA2 level. We considered that changes in monocyte activation could be in the causal pathway between randomization group and changes in the Lp-PLA2 level, which is why changes in sCD14 and sCD163 levels were removed from this model.
Table 4.
Factors Independently Associated With Percentage Change in Soluble CD14 (sCD14) and Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) Levels Over 48 Weeks
| Variable | Parameter Estimate ± SE | P Values |
|---|---|---|
| Percentage change in log sCD14 level between wks 0 and 48a | ||
| Randomization group | −2.02 × 10−2 ± 3.05 × 10−3 | <.0001 |
| Week 0 data on | ||
| Log sCD14 level | −5.44 × 10−2 ± 6.14 × 10−3 | <.0001 |
| Log CD4+ T-cell count | 6.3 × 10−3 ± 2.45 × 10−3 | .01 |
| Log HDL cholesterol level | 1.04 × 10−2 ± 5.26 × 10−3 | .05 |
| Percentage change between wks 0 and 48 in | ||
| Log weight | −0.313 ± 0.104 | <.01 |
| Log eGFR | −0.109 ± 5.03 × 10−2 | .03 |
| Log sCD163 level | 0.1 ± 2.99 × 10−2 | <.01 |
| Log hsCRP level | 3.11 × 10−2 ± 7.45 × 10−3 | <.0001 |
| Percentage change in Lp-PLA2 level between wks 0 and 48b | ||
| Randomization group | −2.64 × 10−2 ± 2.56 × 10−2 | .3 |
| Week 0 data on | ||
| Lp-PLA2 level | −1.87 × 10−3 ± 2.24 × 10−4 | <.0001 |
| LDL cholesterol level | 1.23 × 10−3 ± 4.85 × 10−4 | .01 |
| IL-6 level | −2.56 × 10−3 ± 1.16 × 10−3 | .03 |
| Percentage change between wks 0 and 48 in | ||
| Total cholesterol level | 0.169 ± 6.54 × 10−2 | .01 |
| Triglycerides level | 5.33 × 10−2 ± 1.85 × 10−2 | <.01 |
| sCD14 level | 0.229 ± 6.19 × 10−2 | <.001 |
| sCD163 level | −0.122 ± 5.4 × 10−2 | .02 |
Abbreviations: eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HIV-1, human immunodeficiency virus type 1; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; sCD163, soluble CD163; SE, standard error; sTNF-RI, soluble tumor necrosis factor α receptor I.
a Variables with a P value of < .25 in univariable analysis but not selected for in the final model included week 0 log-transformed weight, log-transformed HIV-1 RNA load, log-transformed eGFR, log-transformed hemoglobin level, log-transformed triglycerides level, log-transformed sCD163 level, log-transformed sTNF-RI level, log-transformed IL-6 level, and log-transformed hsCRP level and percentage change between weeks 0 and 48 in log-transformed CD4+ T-cell count, log-transformed hemoglobin level, log-transformed HDL cholesterol level, log-transformed LDL cholesterol level, log-transformed triglycerides level, log-transformed sCD163 level, log-transformed sTNF-RI level, and log-transformed Lp-PLA2 level.
b Variables with a P value of < .25 in univariable analysis but not selected for in the final model included week 0 randomization group, sex, weight, CD4+ T-cell count, HIV-1 RNA level, eGFR, HDL cholesterol level, triglycerides level, sCD14 level, and sTNF-RI level and percentage change between weeks 0 and 48 in weight, CD4+ T-cell count, HDL cholesterol level, and sTNF-RI level.
DISCUSSION
To date, there are limited data describing the differential effects of currently recommended first-line antiretroviral regimens on immune activation and inflammation during HIV infection. As long as viral suppression remains the goal of HIV treatment, choosing ART with the least long-term toxicity and highest benefit is of great priority in the management of this chronic illness. To our knowledge, this is the first study to compare the effects of ART initiation with an integrase inhibitor (elvitegravir)– and nonnucleoside reverse transcriptase inhibitor (NNRTI; efavirenz)– based regimen on markers of monocyte activation and systemic and vascular inflammation. We show here that, over 48 weeks, greater improvements in sCD14, hsCRP, and Lp-PLA2 levels are achieved with EVG/c/FTC/TDF, compared with EFV/FTC/TDF.
In HIV infection, published studies have consistently demonstrated that ART initiation leads to decreases in levels of systemic markers of inflammation, with the exception of hsCRP [30–35], and decreased levels of immune activation [36, 37]. However, there are few studies that compare the effects of specific antiretrovirals and only one that included the integrase inhibitor class [34, 35, 38]. The results of these studies are consistent with findings for the EFV/FTC/TDF group in our study, in which levels of sTNF-RI and IL-6 decreased and the level of hsCRP remained similar. In a randomized, open-label trial of efavirenz or lopinavir/ritonavir (LPV/r) in combination with zidovudine/lamivudine initially, sTNF-RI and sTNF-RII levels decreased significantly over 24 weeks; however, the sTNF-RI level tended to increase to baseline by week 96, whereas decreases in the sTNF-RII level were maintained. There were no between-group differences in the changes observed [34]. In A5224s, a substudy of A5202, in which ART-naive, HIV-infected participants were randomly assigned to receive abacavir/lamivudine (ABC/3TC) or tenofovir/emtricitabine (TDF/FTC) with efavirenz or atazanavir/ritonavir (ATV/r) in a factorial design, changes in markers of inflammation over 24 and 96 weeks were evaluated. Levels of most markers, including sTNF-RI and sTNF-RII, TNF-α, IL-6, and adhesion molecules (sVCAM-1 and sICAM-1) decreased significantly by week 96, without significant differences between arms. However, the hsCRP level decreased less in ABC/3TC recipients, compared with TDF/FTC recipients, and at 96 weeks the hsCRP level was significantly higher than at baseline for the ABC/3TC plus efavirenz group [35]. Finally, to date, ACTG 5260s is the only ART initiation study that compared markers of immune activation and inflammation in the setting of an integrase inhibitor (raltegravir). This study randomly assigned ART-naive participants to receive TDF/FTC along with open-labeled raltegravir, ATV/r, or darunavir/ritonavir (DRV/r). In this study, the hsCRP level decreased with raltegravir and ATV/r by 96 weeks, the IL-6 level decreased with only raltegravir, the D-dimer level decreased with ATV/r and DRV/r, and levels of T-cell activation markers and sCD163 decreased in all groups [38]. Neither sTNF-RI nor Lp-PLA2 levels were measured in this study.
Findings from our study are consistent with those from studies involving HIV-infected participants with virological suppression who were randomly assigned to continue their current regimen or switch to an integrase inhibitor-based regimen (raltegravir). In the SPIRAL study (n = 233), switch to raltegravir from a protease inhibitor (PI) led to improvements in hsCRP, IL-6, TNF-α, and D-dimer levels that could only partially be attributed to improvements in lipoprotein levels in the raltegravir arm [39]. Similarly, in the ANRS 138 trial (n = 164), immediate or delayed switch to raltegravir from an enfuvirtide-based regimen led to improvements in all inflammatory markers tested, including IL-6, hsCRP, and D-dimer levels [40]. Last, in a small study (n = 37), in which woman with virological suppression during their current PI- or NNRTI-based ART regimen were randomly assigned to undergo immediate or delayed switch to raltegravir, the sCD14 level (but not IL-6, hsCRP, or sCD163 levels) decreased significantly in both the immediate and delayed switch groups and was different between the raltegravir and PI/NNRTI groups at week 24 [41].
sCD14, a marker of monocyte activation and response to lipopolysaccharide, has been linked to subclinical atherosclerosis [11] and mortality [28] during HIV infection. In an observational study, the sCD14 level did not change over a 2-year study period in treatment-naive participants initiating ABC/3TC or TDF/FTC treatment along with EFV, LPV/r, or ATV/r [33]. The lack of decrease in the sCD14 level in the EFV/FTC/TDF group in our study is consistent with findings in this latter study. Interestingly, in our study, the sCD14 level decreased significantly by 24 weeks after initiation of the EVG-based regimen, a finding that may translate into a significant clinical benefit. Indeed, the association seen in our study between changes in sCD14 levels and Lp-PLA2 levels could have significant mechanistic implications because monocytes and their products could be an important driver of vascular inflammation and atherosclerosis, a hypothesis supported by prior studies linking monocyte activation markers to noncalcified coronary plaques [25]; coronary calcifications [26]; aortic inflammation, as measured by positron emission tomography [9]; and acute coronary syndrome [42].
Lp-PLA2, an enzyme produced by monocytes/macrophages, among other cells, that hydrolyzes oxidized phospholipids on LDL-cholesterol, thereby producing highly inflammatory mediators, has become an important marker of vascular inflammation and CVD risk, independent of other factors [43, 44]. An increased Lp-PLA2 level predicts both primary and recurrent CVD events in the general population [29, 45] and has been used to further stratify patients with an intermediate 10-year risk for CVD [46]. In our study, the Lp-PLA2 level decreased significantly in the EVG/c/FTC/TDF group, whereas an increase in the Lp-PLA2 level was seen in the EFV/FTC/TDF group. Interestingly, while changes in Lp-PLA2 levels were significantly different between the 2 groups, randomization group was only predictive of change in the Lp-PLA2 level over 48 weeks when 2 monocyte activation markers, sCD14 and sCD163, were removed from the model. This suggests that the effect of EVG/c/FTC/TDF on Lp-PLA2 may be mediated through a decrease in monocyte activation.
Mechanistically, it is possible that elvitegravir resulted in more-favorable changes in the sCD14 level owing to possibly higher concentration of drug in enterocytes [21], which could result in full suppression of viral replication in gut-associated lymphoid tissues and better control over bacterial translocation. Alternatively, since oxidized lipids are known to drive immune activation in the pathogenic development of coronary plaque [47–49] and because integrase inhibitors overall have more-favorable effect on lipids [19, 20], it is possible that integrase inhibitors decrease levels of oxidized lipids more than other classes of drugs, and this should be investigated. Last, it has been shown that integrase inhibitors cause a more rapid decline in the HIV-1 RNA level, compared with NNRTIs [24]. Although there was no difference in the proportion of participants achieving an HIV-1 RNA level of <50 copies/mL by 24 weeks in this study, it is possible that this contributed to the differences seen.
Strengths of this study include randomized treatment allocation and large sample size, compared with other published studies evaluating biomarkers longitudinally. Limitations include the relatively short duration over which changes in levels of the markers were evaluated. Although inflammation and immune activation likely improve most dramatically in the first year after ART initiation, a longer duration of follow-up would provide additional information, given that marker levels after suppressive ART are still higher than expected for HIV-uninfected individuals [50]. Last, whether unboosted integrase inhibitors will lead to the same result is unknown. Therefore, these results should not be generalized to patients receiving unboosted integrase inhibitor–based regimens.
In conclusion, initiation of EVG/c/FTC/TDF therapy led to greater decreases in sCD14, hsCRP, and Lp-PLA2 levels than EFV/FTC/TDF. Randomization group independently predicted changes in the sCD14 level, and changes in monocyte activation independently predicted changes in the Lp-PLA2 level. There appears to be a favorable effect of the integrase inhibitor elvitegravir, compared with efavirenz, on HIV-related immune activation, which may, in turn, affect vascular inflammation.
Notes
Financialsupport. This work was supported by Gilead (investigator-initiated research grant to G. A. M.), the National Heart, Lung, and Blood Institute (grant K23HL116209 to C. O. H.), and the Center for AIDS Research at Case Western Reserve University (grant AI 36219).
Potential conflicts of interest. V. S.-G., K. M., and J. S. are each employed by and hold stock in Gilead Sciences. G. A. M. has received research grants from BMS, Gilead Sciences, and GSK; has served as a consultant to BMS, GSK, and Merck; and has served as a speaker for BMS and Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS 2014; 28:1181–91. [DOI] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS 2014; 28:1911–9. [DOI] [PubMed] [Google Scholar]
- 6.Petoumenos K, Reiss P, Ryom L, et al. Increased risk of cardiovascular disease (CVD) with age in HIV-positive men: a comparison of the D:A:D CVD risk equation and general population CVD risk equations. HIV medicine 2014; 15:595–603. [DOI] [PubMed] [Google Scholar]
- 7.Currier JS, Kendall MA, Zackin R, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS 2005; 19:927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaked I, Hanna DB, Gleissner C, et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol 2014; 34:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 13.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane M, Avihingsanon A, Rajasuriar R, et al. Lipopolysaccharide, immune activation, and liver abnormalities in HIV/hepatitis B Virus (HBV)-coinfected individuals receiving HBV-active combination antiretroviral therapy. J Infect Dis 2014; 210:745–51. [DOI] [PubMed] [Google Scholar]
- 15.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurochko AD, Huang ES. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol 1999; 162:4806–16. [PubMed] [Google Scholar]
- 17.Chun TW, Nickle DC, Justement JS, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest 2005; 115:3250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 2014; 111:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez E, Larrousse M, Llibre JM, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS 2010; 24:1697–707. [DOI] [PubMed] [Google Scholar]
- 20.Ofotokun I, Ribaudo H, Na L, et al. Darunavir or atazanavir vs raltegravir lipid changes are unlinked to ritonavir exposure: ACTG 5257 [abstract 746]. In: Program and abstracts of the 21st Conference on Retroviruses and Opportunistic Infections (Boston). San Francisco: International Antiviral Society-USA 2014; 485–6. [Google Scholar]
- 21.Patterson KB, Prince HA, Stevens T, et al. Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS 2013; 27:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 25.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AC, Rizk N, O'Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009; 49:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 2010; 375:1536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry K, Kitch D, Dube M, et al. C-Reactive protein levels over time and cardiovascular risk in HIV-infected individuals suppressed on an indinavir-based regimen: AIDS Clinical Trials Group 5056s. AIDS 2004; 18:2434–7. [PubMed] [Google Scholar]
- 31.Shikuma CM, Ribaudo HJ, Zheng Y, et al. Change in high-sensitivity c-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses 2011; 27:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hattab S, Guihot A, Guiguet M, et al. Comparative impact of antiretroviral drugs on markers of inflammation and immune activation during the first two years of effective therapy for HIV-1 infection: an observational study. BMC Infect Dis 2014; 14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. [DOI] [PubMed] [Google Scholar]
- 35.McComsey GA, Kitch D, Daar ES, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS 2012; 26:1371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taiwo B, Matining RM, Zheng L, et al. Associations of T cell activation and inflammatory biomarkers with virological response to darunavir/ritonavir plus raltegravir therapy. J Antimicrob Chemother 2013; 68:1857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funderburg NT, Andrade A, Chan ES, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One 2013; 8:e83514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelesidis T, Trann TTT, McComsey GA, et al. Comparison of effects of ATV, RAL or DRV with FTC/tenofovir on biomarkers of systemic inflammation, macrophage and T-cell activation: ACTG A5260s. International AIDS Conference Melbourne, Australia, 2014. [Google Scholar]
- 39.Martinez E, D'Albuquerque PM, Llibre JM, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS 2012; 26:2315–26. [DOI] [PubMed] [Google Scholar]
- 40.Silva EF, Charreau I, Gourmel B, et al. Decreases in inflammatory and coagulation biomarkers levels in HIV-infected patients switching from enfuvirtide to raltegravir: ANRS 138 substudy. J Infect Dis 2013; 208:892–7. [DOI] [PubMed] [Google Scholar]
- 41.Lake J, McComsey G, Hulgan T, et al. Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the Women, Integrase and Fat Accumulation Trial. HIV medicine 2014; 15:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakkinen T, Luoma JS, Hiltunen MO, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol 1999; 19:2909–17. [DOI] [PubMed] [Google Scholar]
- 44.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 2005; 25:923–31. [DOI] [PubMed] [Google Scholar]
- 45.Sabatine MS, Morrow DA, O'Donoghue M, et al. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2007; 27:2463–9. [DOI] [PubMed] [Google Scholar]
- 46.Davidson MH, Corson MA, Alberts MJ, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol 2008; 101:51F–7. [DOI] [PubMed] [Google Scholar]
- 47.Horkko S, Binder CJ, Shaw PX, et al. Immunological responses to oxidized LDL. Free Radic Biol Med 2000; 28:1771–9. [DOI] [PubMed] [Google Scholar]
- 48.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001; 21:1876–90. [DOI] [PubMed] [Google Scholar]
- 49.Samson S, Mundkur L, Kakkar VV. Immune response to lipoproteins in atherosclerosis. Cholesterol 2012; 2012:571846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Pablo-Bernal RS, Ruiz-Mateos E, Rosado I, et al. TNF-alpha levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother 2014; 69:3041–6. [DOI] [PubMed] [Google Scholar]