Abstract
Background. Mechanisms mediating immunity to malaria remain unclear, but animal data and experimental human vaccination models suggest a critical role for CD4+ T cells. Advances in multiparametric flow cytometry have revealed that the functional quality of pathogen-specific CD4+ T cells determines immune protection in many infectious models. Little is known about the functional characteristics of Plasmodium-specific CD4+ T-cell responses in immune and nonimmune individuals.
Methods. We compared T-cell responses to Plasmodium falciparum among household-matched children and adults residing in settings of high or low malaria transmission in Uganda. Peripheral blood mononuclear cells were stimulated with P. falciparum antigen, and interferon γ (IFN-γ), interleukin 2, interleukin 10, and tumor necrosis factor α (TNF-α) production was analyzed via multiparametric flow cytometry.
Results. We found that the magnitude of the CD4+ T-cell responses was greater in areas of high transmission but similar between children and adults in each setting type. In the high-transmission setting, most P. falciparum–specific CD4+ T-cells in children produced interleukin 10, while responses in adults were dominated by IFN-γ and TNF-α. In contrast, in the low-transmission setting, responses in both children and adults were dominated by IFN-γ and TNF-α.
Conclusions. These findings highlight major differences in the CD4+ T-cell response of immune adults and nonimmune children that may be relevant for immune protection from malaria.
Keywords: CD4+ T cells, malaria, P. falciparum, cellular immunity, IL-10, IFN-γ
Naturally acquired immunity to malaria emerges slowly in malaria-endemic populations. In areas of high transmission, adults experience relatively few clinical episodes, compared with children, despite similar exposure to parasites, indicating that the adult immune system is able to control parasite burden and/or immunopathology associated with disease. Murine models have shown that cytokine-producing CD4+ T cells are critical for protection from malaria [1, 2]. In naturally exposed humans, interferon γ (IFN-γ) production by CD4+ T cells in response to merozoite [3, 4] and sporozoite [5–7] antigens has been associated with protection from disease. The importance of Plasmodium falciparum–specific CD4+ T-cell responses was further demonstrated by a study of naive adults vaccinated with an ultra-low dose of blood-stage parasites, which found that the CD4+ T-cell response was protective from malaria, even in the absence of antibody [8].
Advances in multiparametric flow cytometry have made it clear that assessment of pathogen-specific T cells by a single parameter is often inadequate for identifying correlates of protection. Antigen-specific T cells are functionally heterogeneous and can exhibit a complex variety of effector mechanisms. Mounting evidence from many infectious models suggests that the quality, rather than the quantity, of response is critical for immunity [9]. For instance, multifunctional CD4+ T cells coproducing IFN-γ, tumor necrosis factor α (TNF-α), and interleukin 2 (IL-2) correlate with vaccine-mediated protection from Leishmania major [10] and are implicated in protection induced by vaccinia virus immunization [11]. Multifunctional CD4+ T cells that coproduce TNF-α and/or IL-2 in conjunction with IFN-γ are thought to have increased function [12] and are associated with nonprogression of human immunodeficiency virus (HIV) infection [13]. In P. falciparum malaria, sterilizing immunity induced by experimental vaccination with whole sporozoites is associated with the induction of CD4+ T cells that coproduce IFN-γ, IL-2, and/or TNF-α [14–16]. CD4+ T-cell responses of a similar phenotype have been implicated in protection from malaria conferred by the vaccine RTS,S [17–19].
Among naturally exposed children, several effector phenotypes of P. falciparum–specific CD4+ T cells have been described, including distinct subsets producing inflammatory (TNF-α and IFN-γ) and regulatory (interleukin 10 [IL-10]) cytokines [20–22]. Recent studies suggest that the effector phenotype may be dependent on parasite exposure intensity, as recent symptomatic malaria is associated with increased frequencies of IFN-γ/IL-10–coproducing cells and a reduced frequency of TNF-α–producing cells [20, 21]. Murine models of malaria and other parasitic infections indicate that IFN-γ/IL-10–coproducing cells play an essential role in protecting the host from immunopathology, although this may come at the cost of reduced or delayed parasite clearance [23–25].
The identification of CD4+ T-cell responses in children susceptible to symptomatic malaria that differ from those in immune adults may provide important information regarding the mechanisms mediating protection. In this study, we performed a detailed assessment of P. falciparum–specific CD4+ T-cell responses among children and their adult caregivers in Uganda, in areas of high or low parasite transmission. We hypothesized that the frequency and functional characteristics of P. falciparum–specific CD4+ T-cell responses among clinically immune adults may differ from those of children who remain vulnerable to symptomatic malaria.
MATERIALS AND METHODS
Ethics Approval
Written informed consent was obtained from the adult caregiver of all study participants. The study protocol was approved by Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council of Science and Technology, and the University of California–San Francisco Committee on Human Research (institutional review board number 11-05995; reference number 067647).
Study Sites, Participants, and Follow-up Procedures
Samples were obtained from children and adults in households enrolled in a longitudinal cohort study of malaria in 2 districts, peri-urban Walukuba subcounty, Jinja, and rural Nagongera subcounty, Tororo, in eastern Uganda [26]. Both sites experience year-round malaria transmission, with 2 annual peaks during October–January and April–July. Children ages 6 months to 10 years and 1 adult caregiver from 100 randomly selected households were enrolled between August 2011 and August 2012. At time of sample collection, all participants had been followed for the entire previous year. All adult caregivers were >20 years old. Exclusion criteria included any known chronic medical condition (including HIV infection) requiring specialized care. All medical care was provided free of charge at dedicated study clinics open 7 days/week. If participants presented with documented fever (tympanic temperature, ≥38°C) or a history of fever in the previous 24 hours, a thick blood smear was performed. If the smear was positive for any parasitemia, the patient received a diagnosis of malaria and was treated with artemether-lumefantrine. Quarterly routine evaluations, including thick blood smears, were performed for all study participants. Asymptomatic cases of Plasmodium infection identified during quarterly evaluations (defined as individuals with Plasmodium-positive blood smears and no fever) were not treated. All participants included in the current study were followed for at least 14 days following peripheral blood mononuclear cell (PBMC) collection for the development of symptomatic malaria. HIV serostatus was not assessed within the cohorts, but the Ugandan prevalence rates were estimated in 2011 as 7.1% in adults and 0.7% in children aged <5 years [27].
Measurement of CD4+ and CD8+ T-Cell Responses to Infected Red Blood Cells (RBCs)
PBMCs were isolated by density gradient centrifugation from whole-blood specimens collected into tubes containing acid citrate dextrose and cryopreserved in liquid nitrogen. T-cell responses were measured as previously described [20]. PBMCs were thawed using standard methods and rested overnight in 10% fetal bovine serum. A total of 106 PBMCs were stimulated with intact purified trophozoite/schizont-stage P. falciparum (clone 3D7)–infected RBCs (iRBCs) or uninfected RBCs at an effector to target ratio of 1:2. Brefeldin A and monensin (BD Pharmingen) were added at 6 hours (10 µg/mL). At 24 hours, cells were washed, and surface and intracellular staining was performed with the following antibodies: from BD Pharmingen, anti-CD3-PerCP (SK7), anti-CD8-APC-H7 (SK1), anti-IFN-γ-PE-Cy7 (B27), anti-IL-10-PE (JES3-19F1), and anti-TNF-α-FITC (6401.1111); from Biolegend, anti-CD4-BV650 (OKT4), anti-CD45RA-Brilliant violet 605 (HI100), anti-CD27-Brilliant violet 711 (O323), anti-CD14-Alexa700 (M5E2), anti-CCR7-FITC (G043H7), anti-CD3-Brilliant violet 650 (OKT3), anti-CD4-PerCP (RPA-T4), anti-CD19-Alexa700 (HIB19), anti-IL-2-Brilliant violet 421 (MQ1-17H12), anti-TNF-α-Alexa700 (MAb11), anti-CD14-Brilliant violet 511 (M5E2), and anti-CD19-Brilliant violet 511 (HIB19); from Miltenyi Biotec, anti-γδ2-APC (123R3); and from Invitrogen, Live/Dead aqua amine. Samples were acquired on a BD LSR2 flow cytometer with FACSDiva. A mean of 100 000 CD4+ T-cells (interquartile range [IQR], 85 000–130 000 cells) were collected, with a minimum of 10 000 cells collected. The proportion of CD45RA− cells was 40% (IQR, 31%–68%) and was higher in adults, compared with children (63% [IQR, 50%–71%] vs 35% [IQR, 28%–43%]).
Data Analysis
Flow cytometry data were analyzed using FlowJo software (Tree Star, San Carlos, California) and Pestle (version 1.7)/Spice (version 5.3; http://exon.niaid.nih.gov) [28]. Color compensation was performed using single-color cell controls or beads stained for each fluorochrome. Responding cells were gated as lymphocytes/singlets/CD14−CD19−Aqua−/CD3+ γδ Vδ2− cells and then as CD4+ or CD8+ and CD45RA− cells. Cytokine production was gated as CD4+CD45RA− or CD8+CD45RA− cells producing IFN-γ, IL-2, IL-10, or TNF-α, and Boolean gating was performed to categorize cells into 15 subsets. To calculate frequencies of P. falciparum–specific T cells, background responses to uninfected RBCs were subtracted from each subset. Responses were considered positive if the response was ≥0.01% of CD4+CD45RA− cells after background subtraction [28]. Based on this, total cytokine responses were considered positive if ≥0.15%, and total responses for individual cytokines were considered positive if ≥0.08%. To analyze the composition of the response, total individual cytokine responses were expressed as a fraction of the total response for each individual.
Statistical Methods
All statistical analyses were performed using Prism 4.0 (GraphPad); Stata, version 12 (College Station, Texas); or Spice, version 5.3 (National Institute of Allergy and Infectious Diseases, Bethesda, Maryland). Responding cell frequencies were compared between children and adults and between low- and high-transmission sites, using the Wilcoxon rank sum test. Statistical comparisons of global cytokine profiles were performed by partial permutation tests, using Spice software, with a threshold of 0.01% [28].
RESULTS
Study Populations
Samples were obtained from 71 children and their 32 primary adult caregivers enrolled in a longitudinal cohort study at 2 study sites in Uganda, the peri-urban Walukuba subcounty, Jinja (low transmission; annual entomological inoculation rate, 2.8 infectious bites/person-year), and rural Nagongera subcounty, Tororo (high transmission; annual entomological inoculation rate, 310 infectious bites/person-year), between January and August 2013 [26]. Malaria incidence (based on all documented cases of malaria recorded at study clinics) was >20-fold lower in Walukuba than in Nagongera; among children from Walukuba, the malaria incidence in the year preceding collection of blood specimens was 0.1 episodes/person-year, while in Nagongera the incidence was 2.42 episodes/person-year (Table 1). The incidence was markedly lower among adult caregivers at both sites: no episodes of malaria in Walukuba adults were recorded, and 2 episodes of malaria in Nagongera adults were recorded (incidence, 0.09 episodes/person-year). Among Nagongera children, incidence declined with age (Spearman rho, −0.43; P = .0018), consistent with the acquisition of immunity. Nonetheless, 81% of Nagongera children aged >7 years experienced at least 1 episode of malaria in the prior year, indicating that even these older children were only partially immune. At the time of blood sample collection, no participants had symptomatic malaria. All participants from Walukuba were uninfected, whereas 17 of 51 children (33%) and 1 of 22 adults (4.6%) in Nagongera had asymptomatic P. falciparum infection detected by microscopy. Of these participants, 1 child (age, 2.5 years) developed symptomatic malaria within 14 days following blood sample collection. Exclusion of this participant did not change the results. Therefore, this participant was included in all analyses.
Table 1.
Characteristics of Children and Adults From Settings of Low (Walukuba) or High (Nagongera) Malaria Transmission
| Characteristic | Walukuba |
Nagongera |
||
|---|---|---|---|---|
| Children (n = 20) | Adults (n = 10) | Children (n = 51) | Adults (n = 22) | |
| Age, y, median (IQR) | 5.1 (3.7–6.5) | 28 (25–30) | 7.1 (4.6–8.7) | 54 (31–63) |
| Female sex, subjects, % | 55 | 100 | 47 | 77 |
| Malaria incidence in past year, cases/person-year, mean ± SD | 0.10 ± 0.31 | 0 | 2.42 ± 2.35 | 0.09 ± 0.29 |
| Asymptomatic with P. falciparum–positive blood smear, subjects, no. (%) | 0 | 0 | 17 (33) | 1 (4) |
Abbreviations: IQR, interquartile range; P. falciparum, Plasmodium falciparum.
Frequency of P. falciparum–Specific CD4+ T Cells Does not Differ Between Children and Adults but Is Greater in Settings of High Transmission
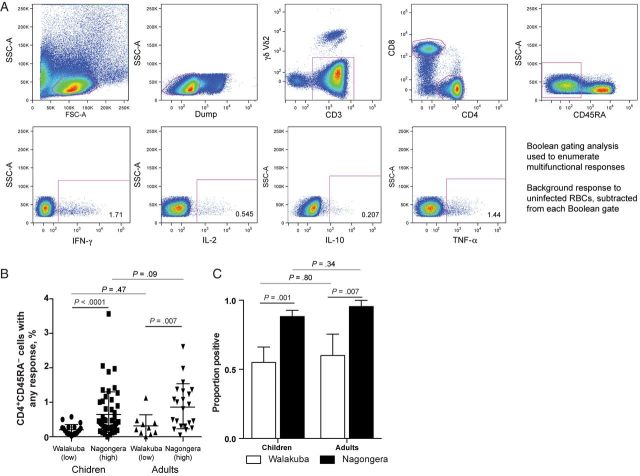
To investigate the frequency and function of T-cell responses, PBMCs were stimulated with P. falciparum iRBCs, and production of IFN-γ, IL-2, IL-10, and TNF-α by CD45RA− CD4+ and CD8+ T cells was analyzed (Figure 1A). Overall, higher frequencies of cytokine-producing CD4+CD45RA− cells were observed among both children and adults residing in Nagongera, compared with Walukuba. However, the magnitude of responses did not differ significantly between children and adults within each study site (Figure 1B). Similarly, the proportions of individuals with a detectable P. falciparum–specific CD4+ T-cell response were significantly higher among Nagongera children and adults (88% and 96%, respectively) than among Walukuba children and adults (55% and 60%, respectively), again with no significant difference between children and adults at each study site. Thus, both the frequency and prevalence of P. falciparum–specific CD4+ T-cell responses appear to be heavily influenced by parasite exposure. There was no difference in the overall frequency of cytokine-producing cells between uninfected children and asymptomatic infected children (as determined by microscopy) in Nagongera (P = .45). P. falciparum–specific CD8+ T-cell responses were infrequently observed in all participants; we observed 3 CD8+ T-cell IL-10 responses, 4 CD8+ T-cell IFN-γ responses, a single CD8+ T-cell TNF-α response, and no CD8+ T-cell IL-2 responses.
Figure 1.
Frequency of Plasmodium falciparum–specific CD4+ T-cell responses is dependent on exposure intensity. A, Gating strategy to identify CD4+CD45RA− cytokine cells producing interferon γ (IFN-γ), interleukin 2 (IL-2), interleukin 10 (IL-10), and tumor necrosis factor α (TNF-α) following stimulation with P. falciparum–infected red blood cells (RBCs). Background responses (to uninfected RBCs) were subtracted from each Boolean gate. B, Frequencies of CD4+ T cells producing any cytokine in response to P. falciparum–infected RBC stimulation are shown separately for children and adults from Walukuba (low-transmission setting) and Nagongera (high-transmission setting). C, The proportions of children and adults with a detectable CD4+ T-cell response to P. falciparum were higher in Nagongera, compared with Walukuba. Abbreviations: FSC, forward scatter; SSC, side scatter.
P. falciparum–Specific CD4+ T Cells in Children and Adults Differ Markedly in Their Functional Phenotype in a High-Transmission Setting
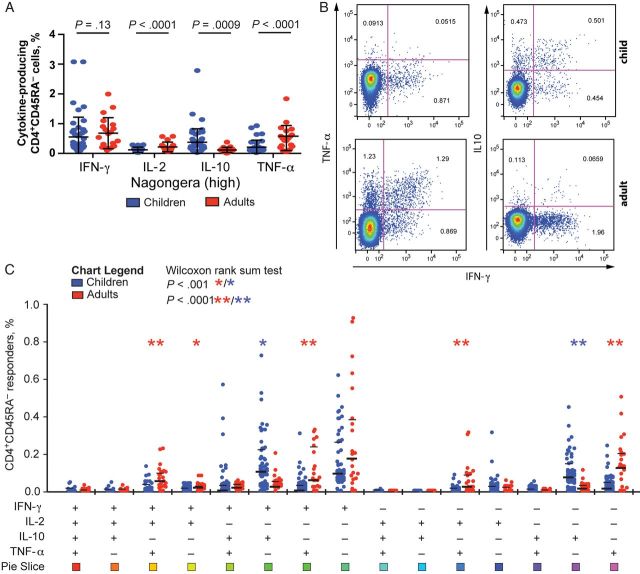
The functional quality of pathogen-specific CD4+ T cells, reflected in part by their effector cytokine production, is believed to be critical for protective immunity [9]. We compared the effector phenotype of P. falciparum–specific CD4+ T-cell responses between children and their adult caregivers who resided in the same household and who were thus similarly exposed to P. falciparum but with differing levels of immunity. In Nagongera, the overall frequencies of P. falciparum–specific IFN-γ producing CD4+ T cells were not statistically significantly different between children and adults. However, there were striking differences in other cytokines (Figure 2A–C). Among adults, higher frequencies of TNF-α–, TNF-α/IFN-γ–, TNF-α/IL-2–, IFN-γ/IL-2–, and TNF-α/IFN-γ/IL-2–producing CD4+ T cells were observed. In contrast, children had higher frequencies of IL-10– and IL-10/IFN-γ–producing cells. We also analyzed the composition of the response by calculating the proportional contribution of each cytokine to the total response. As with the frequencies of responding cells, in Nagongera the proportional contribution of IL-10–producing cells was dramatically higher in children (P < .0001), and the contributions of TNF-α– and IL-2–producing cells were greater in adults (P < .0001 and P = .0002, respectively; Figure 3A and 3B). Of interest, these age-related differences were not observed in Walukuba, where the proportion of cells producing IL-10 was somewhat higher in children (P = .053), but the proportions of cells producing IFN-γ, IL-2, and TNF-α were similar (Supplementary Figure 1).
Figure 2.
In a high-transmission setting, children have increased frequencies of regulatory CD4+ T-cell responses, and adults have increased frequencies of inflammatory CD4+ T-cell responses. A, The frequency of Plasmodium falciparum–specific CD4+CD45RA− cells producing interferon γ (IFN-γ), interleukin 2 (IL-2), interleukin 10 (IL-10), and tumor necrosis factor α (TNF-α) among children (blue) and adults (red) from Nagongera. B, Representative plots of CD4+CD45RA− cell responses from a child and an adult following stimulation with P. falciparum–infected red blood cells, showing typical coproduction of IL-10 and IFN-γ (for the child) and coproduction of TNF-α and IFN-γ (for the adult). C, Frequencies of P. falciparum–specific CD4−CD45RA− cells producing all possible cytokine combinations are shown separately for children and adults from Nagongera.
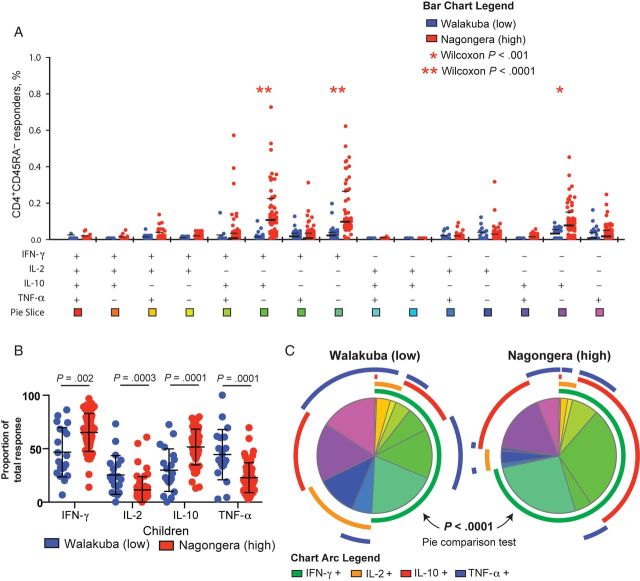
Figure 3.
In a high-transmission setting, the proportion of the Plasmodium falciparum–specific CD4+ T-cell response is dominated by regulatory responses in children and inflammatory responses in adults. A, Proportional contribution of interferon γ (IFN-γ)–, interleukin 2 (IL-2)–, interleukin 10 (IL-10)–, and tumor necrosis factor α (TNF-α)–producing cells to the total P. falciparum–specific CD4+ T-cell response differed between children and adults in Nagongera. B, The overall composition of responding cells differed significantly between Nagongera children and adults (P < .0001, by the partial permutations test).
P. falciparum–Specific CD4+ T Cells From Children in the High-Transmission Setting Produce More IL-10 Than Those From Children in the Low-Transmission Setting
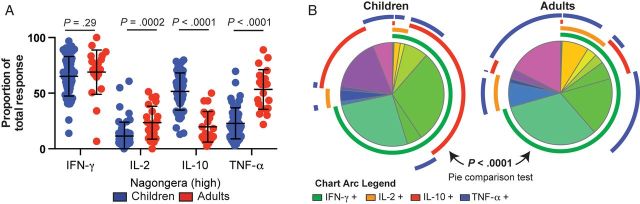
To assess the influence of parasite exposure on cytokine production by P. falciparum–specific CD4+ T cells, we compared responses of children living in a low-transmission setting with those of children living in a high-transmission setting. We found markedly higher frequencies of IFN-γ–, IL-10–, and IFN-γ/IL-10–producing CD4+ T cells among children from Nagongera (P < .0001 for all comparisons; Figure 4A). When analyzed as a fraction of the total response, the difference in cytokine production was more striking. Among Nagongera children, a greater proportion of P. falciparum–specific CD4+ T cells produced IFN-γ and IL-10 (P = .002 and P = .0001, respectively), whereas among Walukuba children a greater proportion of CD4+ T cells produced IL-2 and TNF-α (P = .0003 and P = .0001, respectively; Figure 4B and 4C). Among Nagongera children, we observed no differences in the composition of the cytokine response between asymptomatically infected and uninfected children, as defined by microscopy (Supplementary Figure 2). Together these data indicate that the IL-10–dominant phenotype of P. falciparum–specific CD4+ T cells observed among Nagongera children is related to residence in a high-transmission setting, because children from the low-transmission setting mount a CD4+ T-cell response that is more typical of that for adults, with a greater proportion of inflammatory cytokines. In contrast, there was little difference in the composition of responses observed among adults at these 2 sites. While the frequencies of CD4+ T cells producing IFN-γ, IL-2, IL-10, and TNF-α and the proportion of the response dominated by IFN-γ were all higher among adults from high-transmission as compared to low-transmission settings, the proportion of cells producing IL-2, IL-10, and TNF-α was similar (Supplementary Figure 3).
Figure 4.
Composition of Plasmodium falciparum–specific CD4+ T-cell response differs between children in a low-transmission setting and those in a high-transmission setting. A, Frequencies of P. falciparum–specific CD4−CD45RA− cells producing all possible cytokine combinations are shown separately for children from Walukuba and those from Nagongera. B, Proportional contribution of interferon γ (IFN-γ)–, interleukin 2 (IL-2)–, interleukin 10 (IL-10)–, and tumor necrosis factor α (TNF-α)–producing cells to the total P. falciparum–specific CD4+ T-cell response differed between Nagongera children and Walukuba children. C, The overall composition of responding cells differed significantly between Nagongera children and Walukuba children (P < .0001, by the partial permutations test).
DISCUSSION
Here we show that the effector phenotype of the P. falciparum–specific CD4+ T-cell response is critically dependent on both age and P. falciparum exposure intensity. In high-transmission settings, CD4+ T cells from adults produced predominantly inflammatory cytokines (IFN-γ, TNF-α, and/or IL-2), while the response in children was of a more regulatory phenotype, dominated by IL-10– and IFN-γ/IL-10–producing CD4+ T cells. These findings suggest that induction of P. falciparum–specific CD4+ T cells producing the regulatory cytokine IL-10 is age dependent and likely requires recent or persistent exposure to a high P. falciparum antigen burden.
Production of IL-10 (with or without IFN-γ) by P. falciparum–specific CD4+ T cells in naturally exposed children has been described in several recent studies [20–22]. However, the frequency of IL-10–producing CD4+ T cells does not appear to be associated with prospective protection from symptomatic malaria [20, 22]. A similar population of IL-10-producing T-helper type 1 (Th1) cells has been shown to play an essential role in preventing immunopathology and tissue inflammation in murine models of Plasmodium and in other parasitic infections, although this appears to come at the cost of reduced or delayed parasite clearance [23, 24, 29–32]. Thus, parasite-specific IL-10–producing CD4+ T cells likely represent a peripheral tolerance mechanism that limits pathological inflammation but may interfere with the development of immunity. Interestingly, Th1 IL-10–producing cells suppress dendritic cell maturation [33] and production of interleukin 12 by antigen-presenting cells [23], preventing further Th1 differentiation. It is possible that, in P. falciparum–infected children, IL-10 provides local feedback to prevent the further development of CD4+ T cells with the inflammatory cytokine profile typical of adults.
In contrast to highly exposed children, highly exposed adults, and adults and children with low exposure exhibited a response that was dominated by coproduction of IFN-γ and TNF-α. In mouse models, IFN-γ and TNF-α production by CD4+ T cells is important for protection from Plasmodium [1, 2, 34]. Furthermore, CD4+ T-cell production of TNF-α, with or without IFN-γ and/or IL-2, induced by vaccination with RTS,S has been associated with protection in naturally exposed children and experimentally challenged adults [17–19] and in studies using whole-parasite vaccination strategies [8, 14, 15, 35]. Together, these data suggest that inflammatory CD4+ T-cell responses may contribute to protection from clinical disease. The dominance of this CD4+ T-cell phenotype among high exposed adults, who exhibit a high degree of clinical immunity to malaria, is consistent with a protective role. Longitudinal clinical follow-up studies will be required to determine the relationship between these Th1 cells and prospective protection from symptomatic malaria.
Our data suggest that the intensity of exposure to P. falciparum parasites, independent of age, influences the magnitude of the CD4+ T-cell response, while the functional phenotype of P. falciparum–specific CD4+ T cells is influenced by both age and exposure. In naturally exposed populations, protection from symptomatic malaria is strongly associated with age [36]. However, the degree to which this age-dependent acquisition of immunity is due to the accumulation of exposure to multiple parasite variants, as opposed to inherent differences in child and adult immune responses, remains unclear. Studies of Indonesian transmigrants suggest that the acquisition of immunity is age dependent regardless of exposure; in these studies, although adults were initially more susceptible to severe disease, they rapidly acquired protective immunity, while children remained susceptible to repeated episodes of symptomatic malaria [37, 38]. Age-dependent differences in the immune system itself [39] may result in different T-cell responses in children and adults with similar exposure levels. This possibility is supported by our data demonstrating that, although the composition of responses was remarkably different between children and adults from high-transmission settings, responses of adults from the low-transmission setting were similar to those of adults from high-transmission setting.
Alternatively, it is possible that the development of adaptive immunity to malaria in adults, mediated by antibody and/or cellular immune responses, prevents many infections and limits parasite antigen load, which in turn influences the evolution of the CD4+ T-cell response. In both viral and bacterial infections, the effector phenotype of pathogen-specific CD4+ T cells, particularly IL-10 production by Th1 cells, is influenced by antigen load and persistence [40, 41]. Experimental induction of IFN-γ/IL-10–producing CD4+ T cells specific for myelin basic protein requires repeated antigen dosing; the first dose results in IFN-γ and IL-2 effector responses, while repeated doses induce tolerance mediated via IFN-γ/IL-10–producing CD4+ T cells [42]. In murine models of Toxoplasma gondii, IFN-γ/IL-10 responses are short lived and dependent on continued antigen exposure [23].
Consistent with these models, our prior study of highly exposed children demonstrated that the frequency of IFN-γ/IL-10–producing CD4+ T cells declines markedly within months of a child's most recent symptomatic malaria episode [20]. Further, in another study in an area of seasonal malaria transmission, continued P. falciparum infection throughout the dry season was required for the maintenance of CD4+ T-cell IL-10 responses [21]. Together, these data suggest that these cells either have a relatively short half-life or are capable of functional plasticity and turn off IL-10 production over time. The latter possibility is supported by an elegant study of beekeepers exposed to repeated high doses of bee venom, which resulted in switching of cellular responses from an IFN-γ–dominant response to an IL-10-dominant response, even within clonal populations [43]. Maintenance of antigen-specific IL-10–producing cells required continuous antigen exposure, with a reversion of cells to IFN-γ dominance within 2–3 months following antigen withdrawal. In regions of exceptionally high transmission of parasites, such as Nagongera, where children regularly incur new high-burden blood-stage infections, exposure to parasite antigens may be nearly constant. In contrast, although adults presumably have similar environmental exposure, they do not experience comparable levels of antigenemia: their parasite burden is often only detectable via sensitive polymerase chain reaction (PCR) methods [44]. As a result, highly exposed children may develop IL-10 CD4+ T-cell responses, while in adults low-burden infection may maintain IFN-γ/TNF-α/IL-2 polyfunctional T-cell responses.
Our study has several limitations. Only T cells in peripheral blood specimens were studied; because the majority of memory T cells reside in tissues such as the spleen, our study may underestimate the total P. falciparum–specific T-cell response or provide biased assessments of function. Further, although we measured 4 different cytokine responses, other responses and/or cell types, such as regulatory T cells [45] or T follicular helper cells [46], may have critical importance in the development of immunity. Indeed, interleukin 4 responses to the P. falciparum antigen PfEMP1 have been identified in children and were associated with protection [22, 47]. With regard to clinical data, our study only measured current parasite infection via microscopy. It is possible that some microscopy-negative children may have had subpatent infection and that the identification of parasite-positive individuals via PCR may reveal differences not seen here. Our study also has potential to be biased by differences in other demographic and clinical characteristics, such as genetic composition, prevalence of coinfections, and/or socioeconomic factors, between adults and children and the populations at each study site. In particular, HIV serostatus was not measured in our study participants, but the seroprevalence is known to be significantly higher in adults than in children in Uganda [27].
In conclusion, among children and adults in malaria-endemic settings, CD4+ T-cell responses to P. falciparum are dependent on both transmission intensity and age. Adults in a high-transmission setting exhibited higher frequencies of CD4+ T cells producing combinations of IFN-γ, IL-2, and/or TNF-α, compared with children, whose responses were dominated by IL-10– and IL-10/IFN-γ–producing cells. In children, the increased frequency of IL-10–producing cells was dependent on transmission intensity. These differences may arise from intrinsic differences in the host immune responsiveness of children and adults, or they may be a result of changing parasite burden. The induction of regulatory CD4+ T-cell responses in heavily exposed children has important implications for vaccine development, as these responses may interfere with the induction of robust effecter mechanisms. These results contribute to our understanding of the acquisition of immunity in children and adults, and they provide important insights into cellular immune responses in naturally exposed populations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all parents and guardians, for kindly giving their consent; the study participants, for their cooperation; all members of the study teams, for their dedication and excellent work; and J. Legac and P. Rosenthal, for technical support and providing parasite cultures.
Financial support. The work was supported by the University of California–San Francisco Centers for AIDS Research (supplement P30AI027763 to M. E. F.) and Resource Allocation Global Health Policy Award Program (to M. J. B.); the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants U19AI089674 to G. D., K24AI113002 and RO1AI093615 to M. E. F., and K23AI100949 to P. J.); the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene (to P. J.); the National Health and Medical Research Council, Australia (early-career fellowship to M. J. B. and Infrastructure for Research Institutes Support Scheme funds to the Burnet Institute); and the Victoria, Australia, state government (operational infrastructure support to the Burnet Institute).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Meding SJ, Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol 1991; 21:1433–8. [DOI] [PubMed] [Google Scholar]
- 2.Stephens R, Albano FR, Quin S, et al. Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood 2005; 106:1676–84. [DOI] [PubMed] [Google Scholar]
- 3.Moormann AM, Sumba PO, Chelimo K, et al. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis 2013; 208:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 1999; 179:980–8. [DOI] [PubMed] [Google Scholar]
- 5.John CC, Moormann AM, Sumba PO, Ofulla AV, Pregibon DC, Kazura JW. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against Malaria. Infect Immun 2004; 72:5135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todryk SM, Bejon P, Mwangi T, et al. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS One 2008; 3:e2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reece WHH, Pinder M, Gothard PK, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med 2004; 10:406–10. [DOI] [PubMed] [Google Scholar]
- 8.Pombo DJ, Lawrence G, Hirunpetcharat C, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 2002; 360:610–7. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8:247–58. [DOI] [PubMed] [Google Scholar]
- 10.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843–50. [DOI] [PubMed] [Google Scholar]
- 11.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med 2007; 204:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007; 81:8468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannanganat S, Kapogiannis BG, Ibegbu C, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol 2007; 81:12071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roestenberg M, Teirlinck AC, McCall MBB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 2011; 377:1770–6. [DOI] [PubMed] [Google Scholar]
- 15.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009; 361:468–77. [DOI] [PubMed] [Google Scholar]
- 16.Sedegah M, Kim Y, Ganeshan H, et al. Identification of minimal human MHC-restricted CD8+ T-cell epitopes within the Plasmodium falciparum circumsporozoite protein (CSP). Malar J 2013; 12:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olotu A, Moris P, Mwacharo J, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P. falciparum clinical malaria. PLoS One 2011; 6:e25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumsden JM, Schwenk RJ, Rein LE, et al. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-α producing effector and central memory CD4 T cells. PLoS One 2011; 6:e20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200:337–46. [DOI] [PubMed] [Google Scholar]
- 20.Jagannathan P, Eccles-James I, Bowen K, et al. IFNγ/IL-10 co-producing cells dominate the CD4 response to Malaria in highly exposed children. PLoS Pathog 2014; 10:e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portugal S, Moebius J, Skinner J, et al. Exposure-Dependent control of malaria-induced inflammation in children. PLoS Pathog 2014; 10:e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitau EN, Tuju J, Karanja H, et al. CD4+ T cell responses to the Plasmodium falciparum erythrocyte membrane protein 1 in children with mild Malaria. J Immunol 2014; 192:1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankovic D, Kullberg MC, Feng CG, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 2007; 204:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med 2007; 204:285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freitas do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol 2012; 42:549–55. [DOI] [PubMed] [Google Scholar]
- 26.Kamya M, Arinaitwe E, Wanzira H, et al. Malaria transmission, infection and disease at three sites with varied transmission intensity in Uganda: implications for Malaria control. Am J Trop Med Hyg 2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uganda Ministry of Health, Center for Disease Control and Prevention. Uganda AIDS Indicator Survey (AIS), 2011. http://dhsprogram.com/pubs/pdf/AIS10/AIS10.pdf. Accessed 10 January 2015.
- 28.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011; 79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freitas do Rosario AP, Lamb T, Spence P, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 2012; 188:1178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couper KN, Blount DG, Wilson MS, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 2008; 4:e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002; 420:502–7. [DOI] [PubMed] [Google Scholar]
- 32.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 1996; 157:798–805. [PubMed] [Google Scholar]
- 33.Gabrysová L, Nicolson KS, Streeter HB, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med 2009; 206:1755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog 2010; 6:e1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seder RA, Chang L-J, Enama ME, et al. Protection against Malaria by intravenous immunization with a nonreplicating sporozoite Vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 36.Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9:725–32. [DOI] [PubMed] [Google Scholar]
- 37.Baird JK, Jones TR, Danudirgo EW, et al. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg 1991; 45:65–76. [DOI] [PubMed] [Google Scholar]
- 38.Baird JK. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol 1998; 92:367–90. [DOI] [PubMed] [Google Scholar]
- 39.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004; 4:553–64. [DOI] [PubMed] [Google Scholar]
- 40.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol 2005; 174:1037–45. [DOI] [PubMed] [Google Scholar]
- 41.Millington KA, Innes JA, Hackforth S, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol 2007; 178:5217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabrysová L, Wraith DC. Antigenic strength controls the generation of antigen-specific IL-10-secreting T regulatory cells. Eur J Immunol 2010; 40:1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meiler F, Zumkehr J, Klunker S, Rückert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med 2008; 205:2887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 2012; 3:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholzen A, Minigo G, Plebanski M. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol 2010; 26:16–25. [DOI] [PubMed] [Google Scholar]
- 46.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gitau EN, Tuju J, Stevenson L, et al. T-cell responses to the DBLα-tag, a short semi-conserved region of the Plasmodium falciparum membrane erythrocyte protein 1. PLoS One 2012; 7:e30095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.