Abstract
Background. Leishmaniasis is caused by parasites transmitted to the vertebrate host by infected sand flies. During transmission, the vertebrate host is also inoculated with sand fly saliva, which exerts powerful immunomodulatory effects on the host's immune response.
Methods. We conducted a prospective cohort analysis to characterize the human immune response to Lutzomyia intermedia saliva in 264 individuals, from an area for cutaneous leishmaniasis (CL) caused by Leishmania braziliensis.
Results. Antibodies were found in 150 individuals (56.8%); immunoglobulin G1 and G4 were the predominant subclasses. Recall responses to salivary gland sonicate showed elevated production of interleukin 10 (IL-10), interleukin 13, interferon γ, CXCL9, and CCL2 compared with controls. CD4+CD25+ T cells, including Foxp3+ cells, were the main source of IL-10. L. braziliensis replication was increased (P < .05) in macrophages cocultured with saliva-stimulated lymphocytes from exposed individuals and addition of anti–IL-10 reverted this effect. Positive correlation between antibody response to saliva and cellular response to Leishmania was not found. Importantly, individuals seropositive to saliva are 2.1 times more likely to develop CL (relative risk, 2.1; 95% confidence interval, 1.07–4.2; P < .05).
Conclusions. Exposure to L. intermedia sand flies skews the human immune response, facilitating L. braziliensis survival in vitro, and increases the risk of developing CL.
Keywords: sand fly saliva, L. braziliensis, cutaneous leishmaniasis, lutzomyia intermedia, ELISA, cytokines, chemokines, killing assay
Leishmaniases are neglected diseases caused by protozoa of genus Leishmania that affect millions of persons worldwide. Leishmania parasites are inoculated into the vertebrate host by infected sand flies and take up permanent residence within macrophages, where they replicate and cause disease. During parasite inoculation, the vertebrate host is simultaneously injected with sand fly saliva, which contains anticoagulants, vasodilators, and other molecules capable of modulating the host's immune response (reviewed in [1]).
In murine models, coinoculation of Leishmania plus sand fly saliva exacerbates Leishmania infection [2, 3]. On the other hand, immunization with sand fly saliva or with individual recombinant salivary proteins protects against subsequent challenge with inoculation of live parasites or with infected sand flies [4–6]. With regard to human leishmaniasis, an association between natural exposure to sand flies and disease outcome has been observed. For example, in an area endemic for visceral leishmaniasis (VL), individuals develop antibody responses to Lutzomyia longipalpis saliva and a positive Leishmania skin test result against Leishmania infantum-chagasi–soluble antigens [7]. A follow-up study demonstrated an increased incidence of positive Leishmania skin test results among individuals seropositive to L. longipalpis saliva [8]. Experimental exposure to L. longipalpis sand flies also induced the development of a T-helper (Th) 1–biased immune response to saliva, promoting L. infantum-chagasi killing in vitro [9]. In contrast, in an area endemic for cutaneous leishmaniasis (CL), individuals naturally exposed to Phlebotomus papatasi sand flies developed an interleukin 10 (IL-10)–mediated response, which the authors postulated could favor Leishmania major infection [10].
Infection with Leishmania braziliensis results in a variety of clinical phenotypes, including CL, mucosal leishmaniasis, and disseminated leishmaniasis [11, 12]. We previously found that experimental immunization with Lutzomyia intermedia saliva did not induce a classic delayed-type hypersensitivity (DTH) response [13], as described for the saliva of other sand fly species [5, 6]. Mice immunized with L. intermedia saliva displayed a mixed cytokine response and were not protected against challenge with live L. braziliensis [13]. In fact, immunization with L. intermedia saliva increased neutrophil migration and IL-10 expression on challenge with L. braziliensis [14]. In the endemic area, patients with CL displayed higher levels of antibodies to L. intermedia saliva than those with subclinical infection [13]. Collectively, these results suggest that prior exposure to L. intermedia saliva may negatively influence the outcome of L. braziliensis infection.
Based on the premise that natural exposure to L. intermedia sand flies modulates the immune response and impacts on the outcome of L. braziliensis infection, we followed a prospective cohort of individuals naturally exposed to L. intermedia sand flies, residing in an area endemic for CL caused by L. braziliensis. We evaluated their humoral immune response to L. intermedia saliva and characterized the cellular recall responses after peripheral blood stimulation. Coculture experiments using autologous lymphocytes and macrophages enabled us to investigate how the cytokine milieu affects the infection rate.
METHODS
Area of Study and Selection of Individuals
This study was conducted in Corte de Pedra, Bahia, Brazil, an area of L. braziliensis transmission where L. intermedia is present. A prospective cohort was established in January 2010 and was followed up to January 2013 [15]. Inclusion criteria for participating individuals consisted of a negative history of any type of Leishmania infection, established after a medical interview and examination for signs consistent with previous CL or mucosal leishmaniasis, such as scars on the skin or mucosal area. In addition, participating individuals were living in the same home as patients with CL, the latter diagnosed after parasite isolation or a positive polymerase chain reaction finding for L. braziliensis. This research was conducted with the approval of the ethical committee of the Hospital Professor Universitário Edgard Santos (Salvador, Bahia, Brazil; 240/2009) and Comissão Nacional de Ética em Pesquisa (Brazilian National Ethics Committee, Brazil), and informed consent was obtained from each participant.
Sand Flies and Preparation of Salivary Gland Homogenate
Adult L. intermedia sand flies were captured in Corte de Pedra, Bahia. Sand flies were morphologically identified according to the identification key proposed by Young and Duncan. Salivary glands were dissected and stored in groups of 20 pairs in 20 µL of sodium chloride (150 mmol/L)–HEPES buffer (10 mmol/L; pH 7.4) at −70°C. Salivary gland homogenate (SGH) was prepared and tested for the presence of lipopolysaccharide, as described elsewhere [14].
Analysis of Anti–L. intermedia Saliva Antibodies by Enzyme-Linked Immunosorbent Assay
Humoral (immunoglobulin [Ig] G and IgG subclasses) response to L. intermedia SGH was determined as described elsewhere [9, 13]. The enzyme-linked immunosorbent assay (ELISA) cutoff value for each product was established as the mean optical density (OD) value plus 3 standard deviations, using serum samples from healthy volunteers (n = 80) from a nonendemic area. For the detection of IgE, serum samples were preincubated with RF-Absorbent reagent (Dade Behring) to eliminate IgG antibody competition, as described elsewhere [9].
Gel Electrophoresis and Western Blot Analysis
L. intermedia SGH (equivalent to 60 salivary bland pairs) was assayed on NuPAGE gel (8%–12%) (Invitrogen) for gel electrophoresis, according to manufacturer's instructions. Proteins were visualized by staining with SimplyBlue stain (Invitrogen) and transferred to nitrocellulose. Membranes were incubated with individual serum samples from exposed individuals diluted in phosphate-buffered saline/Tween/0.05% nonfat milk, followed by incubation with anti-human IgG alkaline phosphatase conjugate (Promega). Bands were visualized by adding alkaline phosphatase substrate (Promega).
Cell Culture and Cytokine Detection
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized venous blood layered over a Ficoll-Hypaque gradient (GE Healthcare). Cells were washed and resuspended in Roswell Park Memorial Institute 1640 medium supplemented with 10% human AB serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin (all from Invitrogen). Cells (3 × 106/mL) were plated in 24-well plates and stimulated with SGH (equivalent to 1.5 pair) for 72 hours at 37°C and 5% carbon dioxide (CO2). Cytokine levels in culture supernatants were determined by ELISA (R&D Systems).
Flow Cytometry
For flow cytometric analysis, 106 PBMCs were stimulated with SGH (equivalent to 1.5 pair), cultured for 24 hours, and stained for CD4, CD25, and Foxp3 using the One Step Staining Human Treg Flow Kit (Biolegend). For intracellular staining, brefeldin A (Sigma) (10 µg/mL) was added in the last 8 hours of culture. Cells were permeabilized using 0.2% saponin and stained with anti–IL-10 antibody (Ebioscience). Alexa Fluor 488, phycoerythrin, and peridinin-chlorophyll protein isotype controls were included in all experiments. Samples were acquired in a FACSCantoII flow cytometer (BD Pharmingen), and analysis was performed using FlowJo software (version 7.6.5; Tree Star).
In Vitro Killing Assay
PBMCs (3 × 106) were plated onto 4 well Lab-Tek chamber slides (Thermo Scientific) and incubated for 30 minutes at 37°C and 5% CO2. Nonadherent cells (autologous T cells) were removed and frozen; adherent cells were cultured for another 6 days. Macrophages were infected with stationary phase L. braziliensis (MHOM/BR/00/2000) [16] (5 parasites to 1 macrophage) for 2 hours at 37°C and 5% CO2. Noninternalized parasites were removed, and infected macrophages were further cultured alone, with autologous lymphocytes (2 lymphocytes to 1 macrophage), in the presence of autologous lymphocytes plus L. intermedia SGH (equivalent to 1.5 pair) or the autologous lymphocytes plus L. intermedia SGH (equivalent to 1.5 pair) plus anti–IL-10 (1 ng/mL) (Ebioscience). After 72 hours, slides were washed, stained with hematoxylin-eosin, and analyzed with light microscopy for determination of the percentage of infected cells and the number of amastigotes per 100 cells.
Statistical Analysis
Categorical data were compared using the Fisher exact test. Comparisons between 2 groups were performed by Mann–Whitney test and those among 3 or more groups by Kruskal–Wallis test followed by Dunn multiple comparison tests. The Wilcoxon paired test was used to assess differences between variables in the same subjects. The relative risk (RR) was calculated using the following formula: RR = I1/I0, where I1 is the incidence of CL in exposed individuals (seropositive to L. intermedia SGH), and I0 the incidence of CL in nonexposed individuals (seronegative to L. intermedia SGH). Analyses were conducted using GraphPad Prism version 5.00 for Windows (GraphPad Software), and differences were considered significant at P < .05.
RESULTS
Humoral Immune Response in Individuals Exposed to L. Intermedia Sand Flies
The IgG response against L. intermedia saliva was evaluated by ELISA in 264 household contacts of patients with CL with a negative history of Leishmania infection. Negative serum samples were obtained from residents (n = 46) of a nonendemic area. Individuals exposed to L. intermedia displayed a higher (P < .001) anti-saliva IgG response than controls: 150/264 individuals (56.8%) tested positive for anti-saliva IgG (Figure 1A). The demographic and epidemiologic features of the population studied, according to the presence of anti-saliva IgG, are shown in Table 1. There was no significant difference between individuals who were seropositive and those who were seronegative to L. intermedia saliva regarding age, sex, and most epidemiologic features (Table 1). One exception was the documentation that seropositive individuals more frequently arrived home after 4 pm (P = .01; Fisher exact test).
Figure 1.
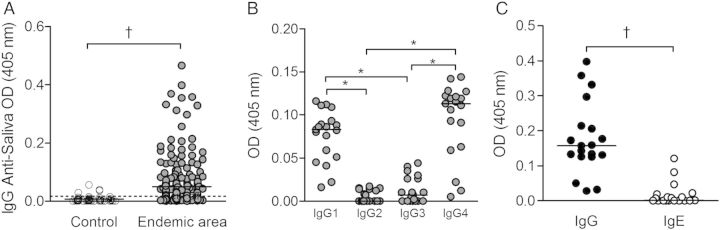
Humoral immune response to Lutzomyia intermedia saliva in a cutaneous leishmaniasis (CL)-endemic area. A, Total immunoglobulin (Ig) G response in residents of a nonendemic area (n = 46) (white circles) and a CL-endemic area (n = 264) (gray circles). B, IgG subclasses in a subset of residents (n = 19) from the endemic area represented in A who were seropositive to L. intermedia saliva. C, Comparison of IgG and IgE levels in the same subset. Circles represent individual values; horizontal lines, median optical density (OD) values; dotted line in A, cutoff level. *P < .05; †P < .001.
Table 1.
Demographic and Epidemiologic Features of Individuals From a CL-Endemic Area, by Seropositivity to Lutzomyia intermedia Saliva
| Characteristic | Seropositive (n = 150) | Seronegative (n = 114) | P Valuea |
|---|---|---|---|
| Median age, y (range) | 14 (2–53) | 15 (2–63) | .15 |
| Male sex, No. (%) | 74 (49.3) | 48 (42.1) | .20 |
| DTH to Leishmania, No. (%) | 15 (10) | 18 (15.7) | .17 |
| Occupation, No. (%) | |||
| Agriculture | 21 (14) | 23 (20.1) | .21 |
| Domestic | 35 (23.3) | 30 (26.3) | |
| Student/other | 94 (62.6) | 61 (53.5) | |
| Time living in endemic area, median (range), y | 14 (2–53) | 14.5 (2–63) | .18 |
| Time living in the same house, median (range), y | 9 (0–53) | 9 (0–56) | .63 |
| Home arrival after 4 pm, No. (%) | 38 (25.3) | 15 (13.1) | .01 |
Abbreviations: CL, cutaneous leishmaniasis; DTH, delayed-type hypersensitivity.
a Continuous variables were compared using the Mann–Whitney test, categorical variables using the Fisher exact test.
Next, we selected the 19 individuals with the highest IgG values against L. intermedia (group median OD, 0.1570; Figure 1A) saliva and evaluated the IgG isotypes within this group. IgG1 and IgG4 were the principal isotypes found (Figure 1B): IgG1 and IgG4 levels were higher (P < .05) than IgG2 and IgG3 levels, which were also detected in seropositive individuals (Figure 1B). The relative OD of anti-saliva IgG4 was higher than for IgG1, IgG2, and IgG3. We found pairwise correlations between IgG and IgG1 (r = 0.5; P = .009) and between IgG and IgG4 (r = 0.6; P = .004) (Supplementary Figure 1A and 1B, respectively). In the subset of 19 seropositive individuals, anti–L. intermedia saliva total IgG levels were higher (P < .05) compared with IgE (Figure 1C). Western blot analysis revealed that seropositive individuals preferentially recognized L. intermedia proteins of approximately 52, approximately 38, and approximately 30 kDa (Figure 2).
Figure 2.
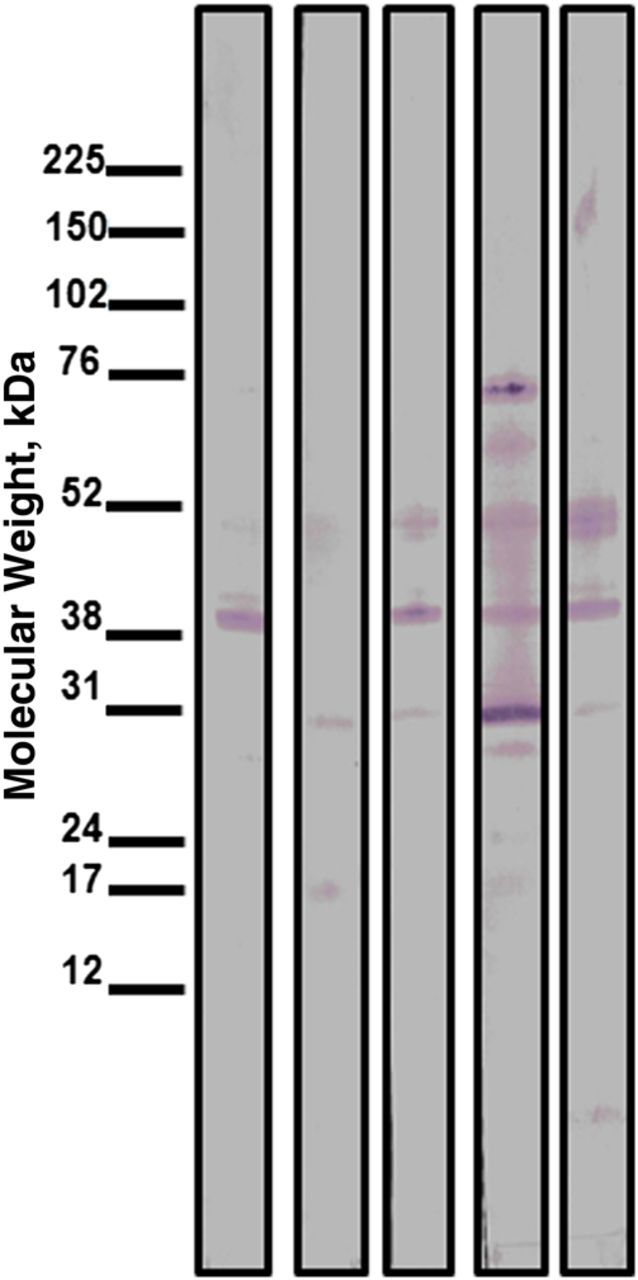
Detection of Lutzomyia intermedia salivary proteins by Western blot analysis. L. intermedia saliva was submitted to sodium dodecyl sulfate polyacrylamide gel electrophoresis and tested against serum samples from residents (n = 5) of a cutaneous leishmaniasis endemic area.
Recall Response in Individuals Exposed to L. intermedia Sand Flies
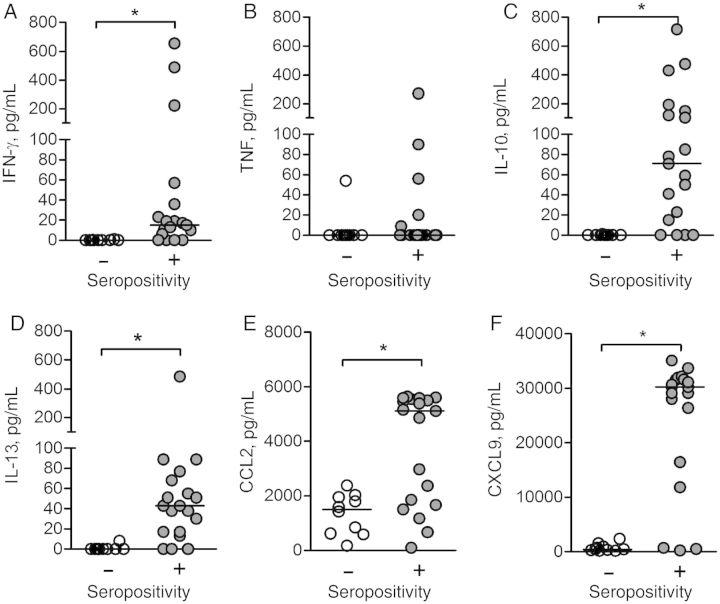
PBMCs from the same subset of 19 seropositive individuals were stimulated in vitro with L. intermedia saliva, and the presence of cytokines and chemokines was evaluated by ELISA. Controls consisted of 10 residents of the endemic area seronegative to L. intermedia saliva (Figure 1A). The levels of interferon (IFN) γ were higher (P < .05) in individuals who were seropositive to L. intermedia saliva than in those who were seronegative (Figure 3A). Tumor necrosis factor (TNF) levels were similar in both groups (Figure 3B); TNF was detectable in 5 of 19 tested culture supernatants (Figure 3B). On the other hand, IL-10 (Figure 3C) and interleukin 13 (IL-13) (Figure 3D) levels were higher (P < .05) in seropositive than in seronegative individuals. With regard to chemokines, CCL2 (Figure 3E) and CXCL9 (Figure 3F) levels were also higher in those who were seropositive. Therefore, individuals exposed to L. intermedia displayed a mixed response (Supplementary Figure 2A) with a predominance of IL-10, evidenced by elevated IL-10/IFN-γ and IL-10/IL-13 ratios (Supplementary Figure 2B).
Figure 3.
Cellular immune response to Lutzomyia intermedia saliva in a cutaneous leishmaniasis-endemic area. Peripheral blood mononuclear cells from a subset of residents who were seronegative (n = 10) (white circles) or seropositive (n = 19) (gray circles) to sand fly saliva were restimulated with L. intermedia saliva (equivalent to 1.5 pair) for 72 hours. Cytokine levels of interferon (IFN) γ (A), tumor necrosis factor (TNF) (B), interleukin 10 (IL-10) (C), interleukin 13 (IL-13) (D), CCL2 (E), and CXCL9 (F) were determined by enzyme-linked immunosorbent assay after 72 hours. Horizontal lines indicate median levels. *P < .05.
Production of IL-10 by CD4+ T Cells From Individuals Exposed to L. intermedia Sand Flies
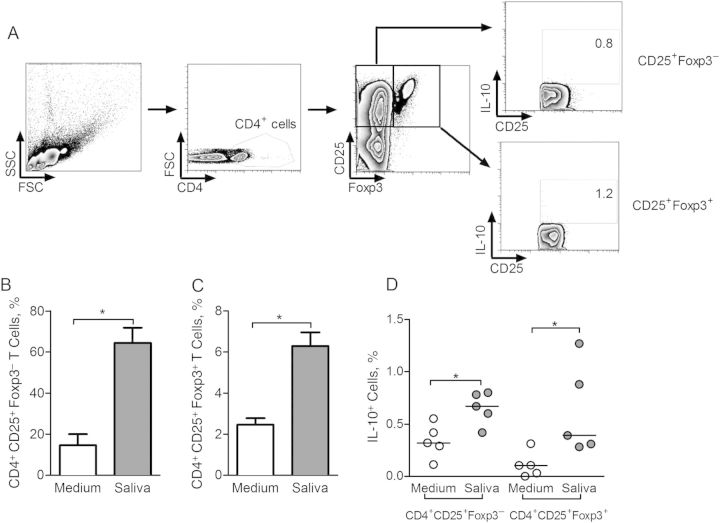
Next, PBMCs from 5 seropositive individuals were restimulated with L. intermedia saliva and the CD4+ and CD4− T-cell subsets were analyzed by flow cytometry for the production of IL-10 (Figure 4A and 4B). In the presence of L. intermedia saliva, the frequency of CD4+IL-10+ cells increased (P < .05) compared with control (unstimulated) cultures (Figure 4C). Within CD4− cells, the frequency of IL-10–secreting cells did not change despite stimulation with saliva (Figure 4C). Similar experiments were performed with cells obtained from control individuals residing in nonendemic areas, and the frequency of IL-10–secreting-cells did not change after stimulation with L. intermedia saliva (Supplementary Figure 3). Moreover, within the CD4+CD25+ compartment, we detected the presence of both Foxp3+ and Foxp3− cells secreting IL-10 (Figure 5A). After stimulation with L. intermedia saliva, the frequency of both CD4+CD25+Foxp3− (Figure 5B) and CD4+CD25+Foxp3+ subpopulations (Figure 5C) increased significantly, as did the frequency of IL-10–secreting cells within these 2 subpopulations (Figure 5D). Therefore, both Foxp3− and Foxp3+ cells contribute to IL-10 production after stimulation with L. intermedia saliva.
Figure 4.
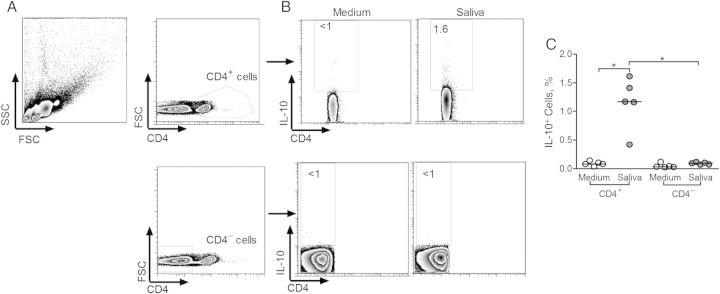
Frequency of interleukin 10 (IL-10)–producing cells in individuals exposed to Lutzomyia intermedia sand flies in a cutaneous leishmaniasis–endemic area. A, Gating strategy for flow cytometry analysis of CD4+ and CD4− cells after restimulation of peripheral blood mononuclear cells with L. intermedia saliva. In this sample gating, cells were first gated for size and granularity (FSC × SSC). The gated cells were further analyzed for expression of CD4. B, IL-10 expression was determined in both CD4+ and CD4− gated populations after culture in medium alone (control) or in the presence of L. intermedia saliva. C, Frequency of IL-10+ cells in CD4− and in CD4+ populations, in control (white circles) and saliva-stimulated (gray circles) cultures from 5 seropositive individuals. Circles represent individual values; horizontal lines, indicate median levels. *P < .05. Abbreviations: FSC, forward scattered light; SSC, side scattered light.
Figure 5.
Frequency of CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ T cells in individuals exposed to Lutzomyia intermedia sand flies in a cutaneous leishmaniasis-endemic area. A, Gating strategy for flow cytometry analysis of CD4+CD25+Foxp3+ cells after restimulation with L. intermedia saliva. In this sample gating, cells were first gated for size and granularity (FSC × SSC). CD25 and Foxp3 expression were determined in CD4+ gated cells and, within these, interleukin 10 (IL-10) expression was determined. B, Frequency of CD4+CD25+Foxp3− cells after culture in medium alone (white bar) or in the presence of L. intermedia saliva (gray bar). C, Frequency of CD4+CD25+Foxp3+ cells after culture in medium alone (white bar) or in the presence of L. intermedia saliva (gray bar). Data are shown as median with interquartile range. D, Frequency of IL-10+ cells in Foxp3− and in Foxp3+ populations, in control (white circles) and saliva-stimulated (gray circles) cultures from 5 seropositive individuals. Circles represent individual values; horizontal lines, median levels. *P < .05. Abbreviations: FSC, forward scattered light; SSC, side scattered light.
Lack of Parasite Killing Upon Coculture of Autologous Lymphocytes and L. braziliensis–Infected Macrophages
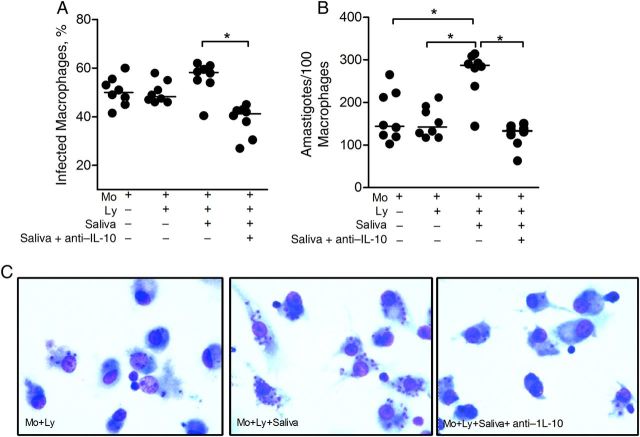
We next asked whether saliva-induced IL-10 secretion modulated L. braziliensis infection in vitro. Monocyte-derived macrophages obtained from exposed individuals were infected with L. braziliensis and cocultured with autologous lymphocytes, in the presence or absence of L. intermedia saliva. Addition of saliva to cocultures increased the percentage of infected cells (Figure 6A), although not significantly. However, the number of intracellular amastigotes was significantly increased in comparison with macrophages cultured alone or with autologous lymphocytes (Figure 6B). This effect was reversed in the presence of a neutralizing IL-10 antibody (Figure 6A and 6B). Representative photographs confirm the elevated number of intracellular amastigotes in the presence of saliva and the opposite effect in the presence of anti–IL-10 (Figure 6C). ELISA confirmed increased levels (P < .05) of IL-10 in cocultures stimulated with saliva (Supplementary Figure 4A), corroborating the higher parasite load (Figure 6B), whereas IFN-γ levels were not altered (Supplementary Figure 4B). Importantly, L. intermedia saliva per se does not alter L. braziliensis infection in human macrophages (Supplementary Figure 5).
Figure 6.
Infection rate of Leishmania braziliensis–infected macrophages after coculture with autologous lymphocytes and Lutzomyia intermedia saliva. Macrophages (Mo) from 8 individuals seropositive to L. intermedia saliva were infected with L. braziliensis promastigotes. Cells were cocultured with autologous lymphocytes alone (Ly), with Ly plus L. intermedia saliva, or with Ly plus saliva plus anti–interleukin 10 (IL-10). After 72 hours, glass coverslips were stained with hematoxylin-eosin and assessed with light microscopy for the percentage of infected macrophages (A) and for the number of amastigotes per 100 macrophages (B). Circles represent individual values; horizontal lines, median levels. *P < .05. C, Representative photomicrographs of cultures shown in A and B. Magnification ×40. Data are shown individually, horizontal lines indicate median levels.
Increased Risk of CL in Individuals Exposed to L. intermedia Sand Flies
During the 3-year follow-up, 38 of 264 residents of the endemic area (14.3%) developed CL. Importantly, 28 of 150 (18.6%) who were seropositive to saliva developed CL, compared with only 10 of 114 (8.7%) who were seronegative Based on these results, individuals naturally exposed to L. intermedia saliva are 2.1 times more likely to develop CL than nonexposed individuals (RR, 2.1; 95% confidence interval, 1.07–4.2; P < .05).
DISCUSSION
In the present work, we investigated how natural exposure to L. intermedia sand flies shapes the human immune response and whether such exposure affects the development of human CL. We conducted our study in a large cohort of 264 individuals residing an area endemic for CL caused by L. braziliensis, transmitted by L. intermedia sand flies. A seropositive response to saliva was detected in 56.8% of individuals, confirming our earlier findings regarding the immunogenicity of L. intermedia saliva [13] and also results obtained in areas of P. papatasi [17] and L. longipalpis [7]. We also found an association between seropositivity to L. intermedia saliva and home arrival after 4 pm, indicating that the antibody response correlates with the increased presence of sand flies at dusk [18].
We found that IgG1 and IgG4 subclasses predominated, similar to results reported after experimental exposure to L. longipalpis [9] and, in the case of IgG4, similar to results after P. papatasi exposure [19]. We found positive correlations between total IgG/IgG1 and total IgG/IgG4, suggesting that both IgG1 and IgG4 are markers of exposure in serologic assays. In contrast to mosquito saliva [20], exposure to L. intermedia did not induce a strong IgE response, indicating lack of allergic reaction, nor was there a correlation between IgE and IgG4 levels. Antibodies to L. intermedia were directed mainly against approximately 52, approximately 38 and approximately 30 kDa proteins, suggesting that yellow-related protein, apyrase (putative), and proteins from the 33-kDa Phlebotomine family are the immunodominant antigens in L. intermedia saliva. Indeed yellow proteins and apyrase were also immunodominant in in VL-endemic areas [21, 22].
Our results show that stimulation of PBMCs from seropositive individuals induced IFN-γ, IL-10, and IL-13 production. This mixed response recapitulates our previous results after immunization of BALB/c mice with L. intermedia saliva [13] and, importantly, shows a predominance of IL-10 over IFN-γ (Supplementary Figure 2). Individuals exposed to P. papatasi also developed an IL-10–dominant response [10], possibly resulting from the presence of adenosine and adenosine monophosphate in sand fly saliva [23, 24]. Adenosine increases IL-10 production [25] and decreases interleukin 12 [26]. However, individuals exposed to Phlebotomus duboscqi were categorized as Th1, Th2, or mixed responders [27] and those exposed to L. longipalpis produced IFN-γ, IL-10, and TNF-α [9]. In a study of P. papatasi–exposed individuals [10], the authors also found that saliva-stimulated cells did not produce IFN-γ but, rather, induced IL-10 secretion, primarily from CD8+ T cells. In contrast, we found that the source of IL-10 was both CD4+CD25+Foxp3+ and Foxp3− cells. Foxp3 is a transcription factor expressed in naturally occurring regulatory T cells (Tregs), which suppress effector T cells and are important for maintenance of peripheral tolerance [28]. Tregs mediate suppression by distinct mechanisms, including IL-10 production [29]. In CL caused by L. braziliensis, the presence of Tregs (CD4+CD25+Foxp3+) suggested suppression of the effector response, facilitating parasite survival [30, 31].
In the current study, coculture of lymphocytes from L. intermedia–exposed individuals with autologous L. braziliensis–infected macrophages significantly increased infection rates and amastigote numbers, findings that were associated with elevated IL-10 and, importantly, reversed on neutralization of IL-10. Therefore, we suggest that exposure to L. intermedia sand flies stimulates a regulatory or suppressive response, favoring L. braziliensis replication. These results differ from those reported for L. longipalpis [9] and P. papatasi sand flies [4, 5]. In fact, they argue against the hypothesis that the initial response to whole L. intermedia saliva can alter the immune response to Leishmania, in the context of protection against CL caused by L. braziliensis (reviewed in [1]).
In addition to natural (CD4+CD25+Foxp3+) Tregs, it is also possible that activated CD4+ T cells or even Treg type 1 (Tr1) cells (CD4+CD25−Foxp3−) [32] cells contribute to IL-10 production. Likewise, Tr1 cells suppress effector T cells through IL-10 production [33] or through contact-dependent mechanisms. Interestingly, IL-10 secreted by Tr1 cells regulates IgG4 secretion [34], and we found that IgG4 levels were increased in individuals exposed to L. intermedia. Because IgG4 cannot properly bind complement [35], preventing antibody-mediated complement activation and pathogen uptake and destruction, this could be another mechanism by which L. intermedia salivary molecules modulate the host's immune response.
Individuals who were seropositive to L. intermedia saliva displayed increased CCL2 and CXCL9 production. These data corroborate our recent findings regarding elevated CXCL9 messenger RNA in response to exposure to L. intermedia saliva [36]. Lesions of patients with CL also display high expression of CXCL9 [37]. Finally, both L. intermedia [14] and L. longipalpis [38] saliva induce CCL2 production in murine cells. Given that CCL2 induces macrophage recruitment, the definitive host cells for Leishmania parasites, this could also affect Leishmania infection.
In studies conducted in VL-endemic areas, a significant correlation was found between presence of antibodies to L. longipalpis saliva and a positive cellular response (DTH) to Leishmania [39]. Later, a prospective cohort study showed that the incidence of anti-Leishmania DTH was higher in subjects who also presented antibodies to L. longipalpis saliva [8]. These findings indicated that mounting an immune response to L. longipalpis saliva paralleled the development of a cell-mediated immune response to Leishmania. Moreover, immunization with L. longipalpis salivary proteins confers protection against experimental VL [6, 40]. On the contrary, in the current study we showed that exposure to L. intermedia saliva increases the risk of developing CL in an endemic area for L. braziliensis.
This finding recapitulates our previous results in the experimental model of infection [13] but, more importantly, points to a yet unappreciated role regarding natural exposure to L. intermedia sand flies. The presence of antibodies to P. papatasi saliva was also associated with increased risk of CL in a Tunisian endemic area [19]. Given that a minority of sand flies are infected with parasites, it is likely that residents will be constantly exposed to noninfected bites. In this scenario, the IL-10–biased immune response developed by individuals exposed to L. intermedia sand flies adds yet another layer of complexity to CL caused by L. braziliensis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Phillip Scott for critical review.
Financial support. This work was supported by the National Institutes of Health (grant AI 30639 to E. M. C.), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant 564689/2008-4 to C. I. d. O.), and Fundação de Amparo à Pesquisa da Bahia (fellowships to A. M. C. and J. R. C.). A. B., E. M. C., and C. I. d. O. are senior researchers at CNPq.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gomes R, Oliveira F. The immune response to sand fly salivary proteins and its influence on Leishmania immunity. Front Immunol 2012; 3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 1988; 239:1306–8. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med 1998; 188:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science 2000; 290:1351–4. [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela JG, Belkaid Y, Garfield MK, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med 2001; 194:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes R, Teixeira C, Teixeira MJ, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci U S A 2008; 105:7845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes RB, Brodskyn C, de Oliveira CI, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis 2002; 186:1530–4. [DOI] [PubMed] [Google Scholar]
- 8.Aquino DM, Caldas AJ, Miranda JC, Silva AA, Barral-Netto M, Barral A. Epidemiological study of the association between anti-Lutzomyia longipalpis saliva antibodies and development of delayed-type hypersensitivity to Leishmania antigen. Am J Trop Med Hyg 2010; 83:825–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinhas V, Andrade BB, Paes F, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol 2007; 37:3111–21. [DOI] [PubMed] [Google Scholar]
- 10.Abdeladhim M, Ben Ahmed M, Marzouki S, et al. Human cellular immune response to the saliva of Phlebotomus papatasi is mediated by IL-10-producing CD8+ T cells and Th1-polarized CD4+ lymphocytes. PLoS Negl Trop Dis 2011; 5:e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llanos Cuentas EA, Cuba CC, Barreto AC, Marsden PD. Clinical characteristics of human Leishmania braziliensis braziliensis infections. Trans R Soc Trop Med Hyg 1984; 78:845–6. [DOI] [PubMed] [Google Scholar]
- 12.Turetz ML, Machado PR, Ko AI, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis 2002; 186:1829–34. [DOI] [PubMed] [Google Scholar]
- 13.de Moura TR, Oliveira F, Novais FO, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis 2007; 1:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Moura TR, Oliveira F, Rodrigues GC, et al. Immunity to Lutzomyia intermedia saliva modulates the inflammatory environment induced by Leishmania braziliensis. PLoS Negl Trop Dis 2010; 4:e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnorr D, Muniz AC, Passos S, et al. IFN- γ production to leishmania antigen supplements the leishmania skin test in identifying exposure to L. braziliensis infection. PLoS Negl Trop Dis 2012; 6:e1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Moura TR, Novais FO, Oliveira F, et al. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect Immun 2005; 73:5827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology 2005; 130:493–9. [DOI] [PubMed] [Google Scholar]
- 18.Souza NA, Andrade-Coelho CA, Peixoto AA, Rangel EF. Nocturnal activity rhythms of Lutzomyia intermedia and Lutzomyia whitmani (Diptera: Psychodidae) in a transmission area of American cutaneous leishmaniasis in Rio de Janeiro State, Brazil. J Med Entomol 2005; 42:986–92. [DOI] [PubMed] [Google Scholar]
- 19.Marzouki S, Ben Ahmed M, Boussoffara T, et al. Characterization of the antibody response to the saliva of Phlebotomus papatasi in people living in endemic areas of cutaneous leishmaniasis. Am J Trop Med Hyg 2011; 84:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Z, Beckett AN, Engler RJ, Hoffman DR, Ott NL, Simons FE. Immune responses to mosquito saliva in 14 individuals with acute systemic allergic reactions to mosquito bites. J Allergy Clin Immunol 2004; 114:1189–94. [DOI] [PubMed] [Google Scholar]
- 21.Souza AP, Andrade BB, Aquino D, et al. Using recombinant proteins from Lutzomyia longipalpis saliva to estimate human vector exposure in visceral leishmaniasis endemic areas. PLoS Negl Trop Dis 2010; 4:e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira C, Gomes R, Collin N, et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis 2010; 4:e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro JM, Katz O, Pannell LK, Waitumbi J, Warburg A. Salivary glands of the sand fly Phlebotomus papatasi contain pharmacologically active amounts of adenosine and 5′-AMP. J Exp Biol 1999; 202:1551–9. [DOI] [PubMed] [Google Scholar]
- 24.Katz O, Waitumbi JN, Zer R, Warburg A. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. Am J Trop Med Hyg 2000; 62:145–50. [DOI] [PubMed] [Google Scholar]
- 25.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-α, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 1996; 157:4634–40. [PubMed] [Google Scholar]
- 26.Hasko G, Kuhel DG, Chen JF, et al. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 2000; 14:2065–74. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira F, Traore B, Gomes R, et al. Delayed-type hypersensitivity to sand fly saliva in humans from a leishmaniasis-endemic area of Mali is Th1-mediated and persists to midlife. J Invest Dermatol 2013; 133:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 29.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campanelli AP, Roselino AM, Cavassani KA, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis 2006; 193:1313–22. [DOI] [PubMed] [Google Scholar]
- 31.Costa DL, Guimaraes LH, Cardoso TM, et al. Characterization of regulatory T cell (Treg) function in patients infected with Leishmania braziliensis. Hum Immunol 2013; 74:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol 2006; 18:120–7. [DOI] [PubMed] [Google Scholar]
- 33.Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol 2009; 183:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol 2005; 174:4718–26. [DOI] [PubMed] [Google Scholar]
- 35.van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol 1986; 64:415–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Weinkopff T, de Oliveira CI, de Carvalho AM, et al. Repeated exposure to Lutzomyia intermedia sand fly saliva induces local expression of interferon-inducible genes both at the site of injection in mice and in human blood. PLoS Negl Trop Dis 2014; 8:e2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritter U, Korner H. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol 2002; 24:295–301. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira CR, Teixeira MJ, Gomes RB, et al. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. J Immunol 2005; 175:8346–53. [DOI] [PubMed] [Google Scholar]
- 39.Barral A, Honda E, Caldas A, et al. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg 2000; 62:740–5. [DOI] [PubMed] [Google Scholar]
- 40.Collin N, Gomes R, Teixeira C, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog 2009; 5:e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.