Abstract
Background
In the era of more efficacious prevention of mother-to-child transmission (PMTCT) regimens, documenting the profile of drug resistance in HIV-infected infants and young children is critical to our efforts to improve care and treatment for children.
Methods
HIV drug resistance mutations in plasma virus were ascertained using population sequencing among 230 newly-diagnosed HIV-infected children under 2 years of age recruited in Johannesburg, South Africa, during 2011. By this time, more effective PMTCT regimens, including combination antiretroviral therapy (cART) for pregnant women, were being implemented.
Results
Two-thirds (67.4%) of HIV-infected children had been exposed to some form of maternal (89%) and/or infant (97%) PMTCT. Among PMTCT-exposed, 56.8% had non-nucleoside reverse transcriptase inhibitor (NNRTI), 14.8% nucleoside reverse transcriptase inhibitor (NRTI), and 1.3% protease inhibitor (PI) mutations. NNRTI mutations were strongly related to younger age. The remaining third (32.6%) had no reported or recorded PMTCT exposures but resistance to NNRTI was detected in 24.0%, NRTI in 10.7% and PI in 1.3%.
Conclusion
The new PMTCT strategies dramatically reduce the number of children who acquire infection but among those who do become infected, NNRTI resistance prevalence is high. In this South African setting with high PMTCT coverage, almost a quarter of children with no reported or recorded PMTCT also have drug resistance mutations. PMTCT history is an inadequate means of ruling out pre-treatment drug resistance. Our results support the use of PI-based first-line regimens in HIV-infected infants and young children regardless of PMTCT history.
Introduction
Non-nucleoside reverse transcriptase inhibitors (NNRTI) are still recommended as part of prevention of mother-to-child transmission (PMTCT) regimens, including option B/B+, despite the well-described selection of resistance mutations among a large proportion of PMTCT-exposed women and their infected infants [1, 2]. However, these data come predominantly from clinical trials and research cohorts and the frequency of prophylaxis-selected drug resistance in routine programs is less well-established [3].
Although PMTCT dramatically reduces the risk of pediatric HIV infection, it does not entirely prevent transmission [4]. Infants with no PMTCT exposure are at higher risk of infection than PMTCT-exposed infants, but the proportion exposed is a function of population coverage of PMTCT. In many settings, ritonavir-boosted lopinavir (LPV/r)-based regimens are only recommended for infants with reported PMTCT exposure on the assumption that NNRTI-associated mutations rarely occur outside of this group. However, the prevalence and patterns of drug resistance in HIV-infected infants with no reported history of PMTCT has not been described.
Our study was designed to describe drug resistance among newly-diagnosed, treatment-naïve, HIV-infected children under 2 years of age accessing routine services in Johannesburg, South Africa, a year after PMTCT guidelines were changed to support more effective interventions, including wider use of maternal combination antiretroviral therapy (cART) [5, 6].
Methods
Between January and December 2011, we aimed to recruit all newly-diagnosed, treatment-naive HIV-infected infants and young children under 2 years of age at three major hospitals, and two affiliated clinics, in Johannesburg, South Africa. Recruitment was conducted at routine PMTCT follow-up clinics and in-patient services where children were identified during hospitalization. Plasma from venous blood was stored for drug resistance testing. Detailed sociodemographic, clinical, treatment and PMTCT data were collected during a standardized interview. Maternal and pediatric medical records were sought to confirm drug exposures. PMTCT guidelines in place at the time recommended HIV testing at first antenatal visit with immediate CD4 testing to determine management. Women with CD4≤350 cells/μL were initiated on cART with nevirapine/tenofovir/lamivudine recommended as the preferred first-line regimen. Women with CD4>350 cells/μL initiated zidovudine from 14 weeks through labor, and single-dose nevirapine and single-dose emtricitabine/tenofovir were given post-delivery. All infants, regardless of maternal regimen or feeding practice, were given daily nevirapine for six weeks. Nevirapine was continued daily through breastfeeding for infants whose mothers were not on cART [5, 6]. Mothers signed informed consent and the study was approved by the Institutional Review Boards of the University of the Witwatersrand and Columbia University.
An in-house population sequencing method of HIV-1 polymerase optimized for subtype C infections and certified by the Virology Quality Assessment Program (VQA) was undertaken on children’s plasma samples [7]. HIV-1 RNA was isolated using a MagNa Pure LC Total Nucleic Acid Isolation kit on the MagNa Pure Automated System. A nested PCR was performed to generate a 1.7 kb amplicon spanning protease and reverse transcriptase genes [7, 8]. PCR products were sequenced using BigDye Terminators v3.1 on an ABI3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). Consensus sequences were aligned and manually edited using the Sequencher v5.0 (GeneCodes, Ann Arbor, MI). Resistance was defined as the presence of mutations associated with any level of impaired drug susceptibility, using the Stanford algorithm (http://hivdb.stanford.edu/).
Results
Of 385 children initially approached attending the 5 centers, 292 were enrolled (15 refused, 37 were ineligible, 41 were discharged or died before enrollment). Drug resistance testing was undertaken in 255 children (37 had no blood collected). Three samples were excluded as they were duplicate enrollments, 3 samples did not amplify and 19 samples were excluded because the child had initiated cART prior to sample collection resulting in 230 children in the analysis.
Two-thirds (155/230, 67.4%) of children (median age 19 weeks, youngest 27 days) had been exposed to maternal and/or infant PMTCT. In 89.0% of PMTCT-exposed children this included a maternal component: cART [n=28, 18.1%], zidovudine during pregnancy and single-dose nevirapine at delivery [n=78, 50.3%], zidovudine only during pregnancy [n=23, 14.8%], or single-dose nevirapine [n=9, 5.8%]. In 97.4% of exposed children, this included infant prophylaxis (nevirapine [n=129], nevirapine and zidovudine [n=21] or zidovudine only [n=1]). The remaining third (75/230, 32.6%) had no self-reported or recorded PMTCT exposures (median age 42 weeks, youngest 32 days). Twelve women of 75 (16.0%) were not tested during pregnancy and 51 (68.0%) reported testing HIV negative during pregnancy; among these women 52 learned their own HIV status at the same time as child diagnosis.
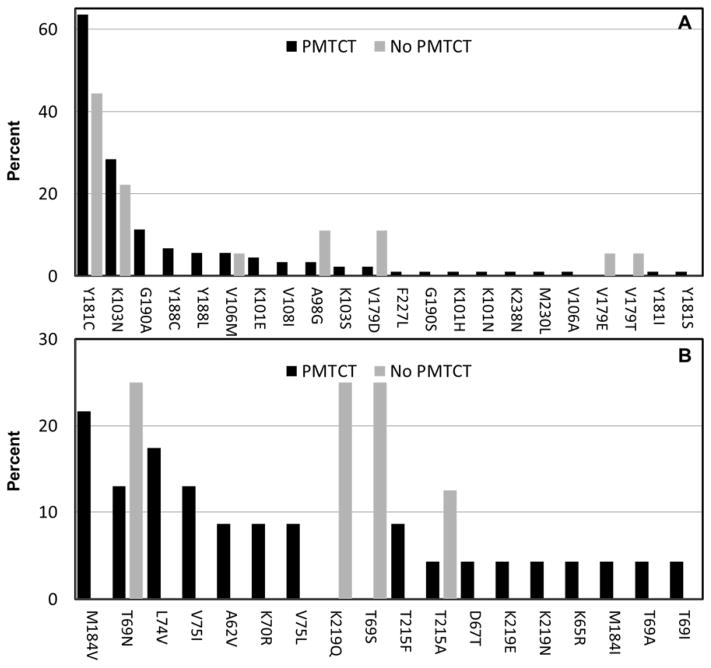
Among 155 PMTCT-exposed children, 56.8% (88) had any NNRTI mutations, 14.8% (23) had nucleoside reverse transcriptase inhibitor (NRTI) mutations (2.6% had more than one), and 1.3% (2) had protease inhibitor (PI) mutations. The most common NNRTI mutation was Y181C (56) constituting 63.6% of 88 children with any NNRTI mutations (Figure 1). Eighteen of 23 (78.3%) children with NRTI mutations also had NNRTI mutations. One child had multiple PI mutations (L10F, M46I, I54V, L76V, V82A) and one only M46L. Based on current drug-specificity in the classification of NNRTI mutations (http://hivdb.stanford.edu/) and among the PMTCT-exposed, 54.2% (54) had high level resistance to nevirapine, 21.9% (34) high level resistance to efavirenz and 2.3% (2) high level resistance to etravirine. The difference was largely driven by Y181C which is classified as conferring “intermediate level” resistance to efavirenz and etravirine.
Figure 1.
Profile of specific antiretroviral drug resistance mutations detected among newly-diagnosed HIV-infected children under 24 months of age in Johannesburg, South Africa, exposed to at least some prevention of mother-to-child transmission (PMTCT) interventions (black bars) or unexposed to PMTCT (gray bars). Panel A displays the percent with specific mutations among those with at least one non-nucleoside reverse transcriptase inhibitor (NNRTI) mutation (n=88 PMTCT; n=18 No PMTCT) and Panel B the percent with specific mutations among those with at least one nucleoside reverse transcriptase inhibitor (NRTI) mutation (n=23 PMTCT; n=8 No PMTCT).
Among 75 PMTCT-unexposed children, 24.0% (18) had NNRTI mutations, 10.7% (8) had single NRTI mutations and 1.3% (1) PI mutations (M46T). Similarly to PMTCT-exposed, the most common NNRTI mutation was Y181C (8) (Figure 1). The prevalence of mutations causing high level resistance to nevirapine was 17.3% (13), to efavirenz 6.7% (5) and none had high level resistance to etravirine.
There was a strong association between young age at testing and detection of NNRTI mutations in PMTCT-exposed children (p<0.0001) (Table 1). There was no association between age and NNRTI mutations in the unexposed group (p=0.66). A significantly higher prevalence of NNRTI mutations in the PMTCT-exposed vs. unexposed group persisted after adjusting for age (p=0.003).
Table 1.
Prevalence of mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors (NNRTI) among 155 newly-diagnosed HIV-infected children with exposure to at least some antiretroviral prophylaxis
| n/N (%) with NNRTI mutations | p-value | |
|---|---|---|
|
| ||
| Age when tested: | ||
| ≤8 weeks | 18/21 (85.7) | |
| 9–16 weeks | 39/53 (73.6) | |
| 17–26 weeks | 12/21 (57.1) | |
| 27–39 weeks | 9/25 (36.0) | |
| 40–52 weeks | 7/12 (58.3) | |
| 53–78 weeks | 3/12 (25.0) | |
| >78 weeks | 0/11 (0.0) | <0.001 |
|
| ||
| Sex: | ||
| Male | 35/70 (50.0) | |
| Female | 53/85 (62.4) | 0.12 |
|
| ||
| Plasma HIV RNA (copies/ml): | ||
| ≤100,000 | 11/16 (68.8) | |
| 100,001 – 1,000,000 | 26/52 (50.0) | |
| 1,000,000 – 10,000,000 | 35/61 (57.4) | |
| >10,000,000 | 9/13 (69.2) | 0.43 |
|
| ||
| CD4%: | ||
| <15 | 25/42 (59.5) | |
| 15–25 | 22/47 (46.8) | |
| ≥ 25 | 36/59 (61.0) | 0.30 |
|
| ||
| Any breastfeeding: | ||
| Yes | 27/53 (50.9) | |
| No | 60/100 (60.0) | 0.28 |
|
| ||
| Maternal antiretroviral regimen: | ||
| cART§ | 24/28 (85.7) | <0.001* |
| Zidovudine/nevirapine | 34/78 (43.6) | |
| Zidovudine alone | 17/23 (73.9) | |
| Nevirapine alone | 5/9 (55.5) | |
| None | 8/17 (47.1) | |
|
| ||
| Infant prophylaxis: | ||
| Nevirapine alone (1) | 83/129 (64.3) | |
| Zidovudine/nevirapine (2) | 4/21 (19.0) | <0.001** |
| Zidovudine alone | 0/1 | |
| None | 1/4 (25.0) | |
|
| ||
| Duration of infant nevirapine prophylaxis:*** | ||
| <4 weeks | 7/22 (31.8) | |
| 4–5 weeks | 13/24 (54.2) | |
| 6 weeks | 48/76 (63.2) | |
| >6 weeks | 11/15 (73.3) | 0.04 |
14 women received efavirenz-based, 11 women nevirapine-based and 3 women ritonavir-boosted lopinavir-based therapy.
compares cART to all other groups combined.
compares groups 1 and 2; association is attenuated (p=0.18) if adjusted for child age when tested.
excludes 13/150 children with nevirapine exposure who were missing duration data; association is attenuated (p=0.08) if adjusted for child age when tested.
Among PMTCT-exposed children, the prevalence of NNRTI mutations was not associated with child sex, viral load, CD4 count or breastfeeding in univariable analysis (Table 1).
NNRTI mutations were more common in children whose mothers had received cART but all had also received infant prophylaxis. Among the 28 women who had received cART during pregnancy, all but three received an NNRTI-based regimen (11 nevirapine, 14 efavirenz). There was no difference in the prevalence of mutations between these regimens. Lamivudine was used in all 25 women with stavudine (n=9), tenofovir (n=13) or zidovudine (n=3). Among 6/25 children with NRTI mutations, 4 mothers had used stavudine and 2 had used tenofovir. Three mothers had received PI-based regimens (LPV/r). One child had no resistance mutations, one had dual class resistance (Y181C,G190A,K219N) and one had multi-class resistance (Y188L and D67T,T69N,K70R,M184V,T215F,K219E and multiple PI mutations L10F,M46I,I54V,L76V,V82A).
Too few children were unexposed to infant nevirapine prophylaxis to examine this group separately but the prevalence of NNRTI mutations was lower if zidovudine was used in addition to nevirapine in univariable analysis (Table 1). However, this association was attenuated and was no longer significant if adjusted for infant age at testing. Similarly, the association between longer duration of infant nevirapine prophylaxis and NNRTI mutations (Table 1) was explained by younger age at testing.
Discussion
In this population of newly-diagnosed, treatment-naive HIV-infected infants and young children in five centers in Johannesburg, at least two-thirds had been exposed to some form of PMTCT intervention. NNRTI-associated mutations were detected by population sequencing in the majority (57%) of these PMTCT-exposed children. Had ultrasensitive methods such as allele-specific PCR been applied [9], this proportion would undoubtedly have been higher. The strongest predictor of NNRTI mutations in the PMTCT-exposed group was young age. This suggests that the decline in NNRTI mutations to below the detection threshold of the assays is a function of time since antiretroviral exposure, as we and others have previously shown [9–11].
Infants whose mothers received cART were more likely to have NNRTI-associated mutations. However, infant nevirapine prophylaxis was universal making it impossible to distinguish the independent effects of maternal regimen. Although this study was done before the shift to universal use of cART for PMTCT (Option B/B+), our results indicate that no decline in the frequency of resistance in infected children is to be expected based on this change. Although with increased maternal cART coverage, the number of newly-infected infants should decrease. PMTCT guidelines continue to recommend infant nevirapine prophylaxis even when maternal cART is given during pregnancy and breastfeeding [5, 6]. Whether there is additional benefit of infant prophylaxis in this circumstance is unknown. Infant prophylaxis may confer necessary transmission benefits when maternal antiretrovirals are started late in pregnancy or adherence is poor.
Infants who received nevirapine for a longer time were more likely to be diagnosed at a younger age, presumably because of better access to care. Thus the apparent relationship between longer duration of nevirapine prophylaxis and more NNRTI-associated mutations is most likely artifact. Given the high rates of NNRTI mutations with single-dose nevirapine [1, 2], there is likely a ceiling effect with additional doses having minimal relevance for additional selection of NNRTI resistance. Thus shifts away from Option A (i.e. long duration nevirapine prophylaxis) are unlikely to have an appreciable effect on the frequency of drug resistance in infected children.
As previously shown, Y181C predominates in nevirapine-exposed children in contrast to nevirapine-exposed adults where K103N predominates [1, 9]. While Y181C confers high level resistance to nevirapine, it confers “intermediate” level resistance to efavirenz and etravirine. Although use of efavirenz in children under three years of age is controversial and not recommended, its use in older children after initial suppression on LPV/r may not be as compromised by past PMTCT exposure as nevirapine-based treatment [12]. Etravirine is not yet available for young children.
Notably, NNRTI-associated mutations were observed in almost a quarter (24%) of newly-diagnosed, treatment-naive children with no reported or recorded PMTCT exposures. We hypothesize that poor maternal recall, lack of understanding, poor record- keeping or failure to recall exposures in prior pregnancies may explain some of these cases [13]. Mothers may have been infected with and transmitted resistant variants although rates are higher than expected based on the prevalence of resistance in drug-naïve adults from surveillance studies in this population [14]. There may also have been unacknowledged or inadvertent postnatal drug exposures. Measureable levels of antiretrovirals were observed in almost half of HIV-infected adults with undetectable viral load reporting no prior antiretroviral exposures in one large African trial [15]. Our results indicate that history of PMTCT exposure, even when rigorously ascertained, is a poor means of ruling out NNRTI mutations in the child. Similar data were reported in Swaziland and Zimbabwe[3] using WHO-recommended surveillance methods to assess HIV drug resistance in newly-diagnosed, treatment-naive children <18 months [16].
NRTI-associated mutations were observed in 15% of PMTCT-exposed children but <3% had more than one NRTI mutation. No unexposed children had more than one NRTI mutation. PI-associated mutations were rare. The mother of the one child with multiple PI-associated mutations had transitioned to second-line therapy during pregnancy. The two children with PI mutations but no history of PI exposure had M46L/T which may arise in the absence of drug pressure.
Introduction of more efficacious PMTCT has reduced perinatal infections in this South African population to <3% [17]. Although there are declining numbers of new infections, our data confirm that the majority of newly-diagnosed HIV-infected infants and young children will carry NNRTI resistant virus. Resistance-associated mutations are also present in a considerable proportion of children with no reported or recorded antiretroviral drug exposures. Together, these observations support the current policy of utilizing LPV/r-based treatment as first-line in all infants and young children [18, 19].
Acknowledgments
Funding
The study was supported by the U.S. President’s Emergency Plan for AIDS Relief and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (supplement to HD 61255) and by the World Health Organization (through The Bill and Melinda Gates Foundation grant #38180). The clinical services that participants accessed were part of the South African government healthcare provision. The U.S. funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Bertagnolio is a staff of the World Health Organization and her views expressed in this publication do not necessarily represent the decisions or stated policies of WHO.
Footnotes
The authors have no conflicts of interest to disclose.
Author contributions:
Study design: LK, EA, AC
Clinical management and data collection: KT, SP, AC, VB
Drug resistance testing: GH, JL, LM
Data analysis: LK KT
Data interpretation: LK, EA, KT, AC, GH, LMSB, MP,
Writing: All authors.
References cited
- 1.Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis. 2013;207 (Suppl 2):S93–100. doi: 10.1093/infdis/jit110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ton Q, Frenkel L. HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission. Curr HIV Res. 2013;11:126–136. doi: 10.2174/1570162x11311020005. [DOI] [PubMed] [Google Scholar]
- 3.Penazzato M. WHO HIV drug resistance surveillance in children less than 18 months old newly diagnosed with HIV: results from Swaziland and Zimbabwe. 5th International Workshop on HIV Pediatrics; Kuala Lumpur, Malaysia. 28–29 June, 2013; (Abstract O_2012AB) [Google Scholar]
- 4.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Department of Health. Policy and Guidelines for the Implementation of the PMTCT Program. 2008. Pretoria: National Department of Health; 2008. [Google Scholar]
- 6.National Department of Health. Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) 2010. Pretoria: National Department of Health; 2010. [Google Scholar]
- 7.Pillay V, Ledwaba J, Hunt G, Rakgotho M, Singh B, Makubalo L, et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1-infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antivir Ther. 2008;13 (Suppl 2):101–107. [PubMed] [Google Scholar]
- 8.Zhou Z, Wagar N, DeVos JR, Rottinghaus E, Diallo K, Nguyen DB, et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One. 2011;6:e28184. doi: 10.1371/journal.pone.0028184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loubser S, Balfe P, Sherman G, Hammer S, Kuhn L, Morris L. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS. 2006;20:995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt GM, Coovadia A, Abrams EJ, Sherman G, Meyers T, Morris L, et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS. 2011;25:1461–1469. doi: 10.1097/QAD.0b013e3283492180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinson NA, Morris L, Gray G, Moodley D, Pillay V, Cohen S, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44:148–153. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn L, Coovadia A, Strehlau R, Martens L, Hu CC, Meyers T, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12:521–530. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinson NA, Morris L, Johnson J, Gray GE, Pillay V, Ledwaba J, et al. Women exposed to single-dose nevirapine in successive pregnancies: effectiveness and nonnucleoside reverse transcriptase inhibitor resistance. AIDS. 2009;23:809–816. doi: 10.1097/QAD.0b013e328323ad49. [DOI] [PubMed] [Google Scholar]
- 14.Hunt GM, Ledwaba J, Basson AE, Moyes J, Cohen C, Singh B, et al. Surveillance of transmitted HIV-1 drug resistance in Gauteng and KwaZulu-Natal Provinces, South Africa, 2005–2009. Clin Infect Dis. 2012;54 (Suppl 4):S334–338. doi: 10.1093/cid/cir1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle EM, Kashuba A, Baeten JM, Fife KH, Celem C, Mujugira A, et al. Unreported antiretroviral use by HIV-1 infected participants enrolling in a prospective research study. AIDS. doi: 10.1097/QAI.0b013e3182a2db02. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertagnolio S, Penazzato M, Jordan MR, Persaud D, Mofenson LM, Bennett DE. World Health Organization generic protocol to assess drug-resistant HIV among children <18 months of age and newly diagnosed with HIV in resource-limited countries. Clin Infect Dis. 2012;54 (Suppl 4):S254–260. doi: 10.1093/cid/cis003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goga AE, Dinh TH, Jackson DJ for the SAPMTCTE study group. Impact of the national prevention of mother-to-child transmission of HIV (PMTCT) program on perinatal mother-to-child transmission of HIV (MTCT) measured at six weeks postpartum, South Africa (SA). XIX International AIDS Conference; Washington. 2012. [Google Scholar]
- 18.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]