To the Editor
The fluorescent dye sulforhodamine 101 (SR101) has been widely used for in vivo brain imaging because of its reported ability to exclusively label astrocytes, despite a note of caution in the original publication1. After administration, the dye diffuses through the astrocyte syncytium via gap junctions, brightly labeling astrocyte cell bodies, processes and perivascular endfeet. SR101 has been used extensively in combination with calcium-sensitive dyes to distinguish calcium signals derived from neurons and astrocytes2, 3.
However, gap-junction coupling occurs not only between astrocytes but also between astrocytes and oligodendrocytes4, 5. Given the presence of these gap junctions in the brain, the reason for the reported specificity of SR101 for astrocytes has remained a mystery.
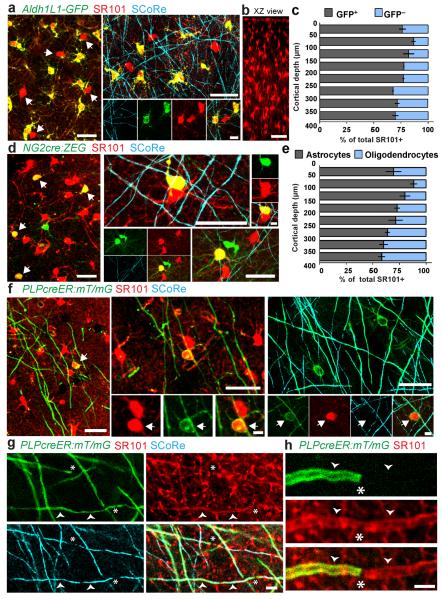
To directly test the specificity of SR101, we applied it topically to the cortex of Aldh1L1-GFP transgenic mice, which express GFP exclusively in astrocytes (Supplementary Methods). We found that a substantial proportion of SR101-labeled cells did not express GFP (Fig. 1a–c and Supplementary Video 1). We also applied SR101 to the cortex of NG2cre:ZEG transgenic mice, which express GFP in all cortical oligodendrocyte-lineage cells and vascular pericytes. In addition to astrocytes, a large proportion of GFP-expressing cells were labeled with SR101 (Fig. 1d and Supplementary Video 2). The morphology of these cells was distinct from that of NG2-expressing oligodendrocyte progenitors (polydendrocytes6) and pericytes but resembled that of mature oligodendrocytes (Fig. 1 and Supplementary Fig. 1). 99.0% ± 0.9% of SR101-labeled, GFP-expressing cells in Aldh1L1-GFP mice had at least one primary process terminating on a blood vessel (283/286 cells, 3 mice), whereas no SR101-labeled, GFP-negative cells exhibited this morphology (0/62 cells, 3 mice) (Supplementary Fig. 2), a result strongly suggesting that the latter were not astrocytes. To further confirm their identity, we used PLPcreER:mT/mG transgenic mice, which express membrane-bound GFP (mGFP) in a subset of oligodendrocytes after induction of Cre recombination. We found that 100% of mGFP labeled cells with myelinating oligodendrocyte morphology were labeled with SR101 (91/91 cells, 3 mice) (Fig. 1). Furthermore, in addition to the cell bodies, SR101 labeled the myelin sheaths (Fig. 1 and Supplementary Fig. 3). Finally, spectral confocal reflectance (SCoRe) microscopy7 in both NG2cre:ZEG and PLPcreER:mT/mG mice revealed that GFP- and mGFP-expressing, SR101-labeled cells had multiple myelinated processes. These data confirmed that the cells were indeed bona fide myelinating oligodendrocytes.
Figure 1.
(a) In vivo two-photon (left) and combined confocal and spectral confocal reflectance (SCoRe) microscopy images (right) captured from the cortex of Aldh1L1-GFP mice labeled with SR101, showing SR101+GFP− cells (arrows). Scale bars, 25 μm (left and top) and 5 μm (bottom right). (b) xz projection showing in vivo SR101 labeling 400 μm deep into the cortex. Scale bar, 50 μm. (c) Proportion of SR101-labeled GFP+ and GFP− cells in postnatal day 90 (P90) Aldh1L1-GFP mice at various cortical depths. Error bars, s.d.; n = 3 mice. (d) In vivo two-photon (left) and combined fluorescence and SCoRe images (right) captured from the cortex of NG2cre:ZEG mice labeled with SR101, showing SR101+GFP+ oligodendrocytes (arrows). Scale bars, 25 μm (left, top, bottom right) and 5 μm (top right). (e) Proportion of SR101-labeled astrocytes (GFP−) and oligodendrocytes (GFP+) in P90 NG2cre:ZEG mice by cortical depth. Error bars, s.d.; n = 3 mice. (f) In vivo two-photon (left) and combined confocal fluorescence and SCoRe images (right) captured from the cortex of oligodendrocyte-specific PLPcreER:mT/mG mice labeled with SR101, showing SR101+mGFP+ myelinating oligodendrocytes (arrows). Scale bars, 25 μm (large images) and 5 μm (small images). (g) In vivo confocal and SCoRe image showing SR101 labeling in the myelin sheath (arrowheads). Scale bar, 5 μm. (h) High-magnification in vivo image showing the ends of two myelin internodes (arrowheads), both labeled with SR101, but only one labeled with mGFP owing to deliberate sparse reporter expression. Scale bar, 5 μm. Asterisks indicate nodes of Ranvier.
The density of cells dually labeled with GFP and SR101 in Aldh1L1-GFP and NG2cre:ZEG mice varied by cortical depth (Fig. 1c,e). Furthermore, we found an increase in the density of cells labeled with GFP and SR101 at postnatal days 30, 90 and 210 in NG2cre:ZEG mice, revealing age- and layer-dependent addition of oligodendrocytes (Supplementary Fig. 4). Finally, we established that SR101 labeling of oligodendrocytes occurred not only with cortical topical application but also after intravenous injection (Supplementary Fig. 5), confirming that oligodendrocyte labeling was not dependent on the route of administration.
To determine the mechanism by which oligodendrocytes were labeled by SR101, we examined the temporal dynamics of labeling by time-lapse imaging. Images acquired 40 min after application of SR101 revealed high background fluorescence in the tissue parenchyma and brightly labeled cell bodies (Supplementary Figs. 6,7,8). Reimaging of the same locations at 140 min after dye application revealed new SR101-labeled cells. The newly labeled cells were exclusively GFP- and mGFP-expressing oligodendrocytes in both NG2cre:ZEG and PLPcreER:mT/mG mice, respectively, and were GFP-negative cells in Aldh1L1-GFP mice (Supplementary Figs. 6,7,8 and Supplementary Videos 3 and 4). SR101 fluorescence intensity increased more strongly in the GFP-expressing oligodendrocytes from 40 to 140 min than in astrocytes initially labeled with SR101 at 40 min (Supplementary Fig. 6), suggesting that oligodendrocyte labeling occurs through transfer from astrocytes. Gap junctions were necessary for oligodendrocyte labeling, as the gap-junction blocker carbenoxolone inhibited SR101 labeling of both astrocytes and oligodendrocytes (Supplementary Fig. 9). Therefore, our time-lapse and pharmacological data, combined with previous reports demonstrating gap junctions between astrocytes and oligodendrocytes, suggested that oligodendrocyte labeling could be dependent on gap-junction coupling between these two cell populations.
Because of the difficulty in astrocyte identification and imaging due to heterogeneous labeling with antibody markers or transgenic reporters, and because of the usability of SR101 in wild-type mice and in other species, SR101 has been implemented extensively as an astrocyte-specific marker1. Using the same dye-labeling procedures as in numerous studies, we found that SR101 labels not only astrocytes but also mature myelinating oligodendrocytes. Given that astrocytes get labeled before oligodendrocytes, we propose that obtaining an initial image to map astrocyte locations (within ~45 min after dye application) in addition to performing detailed morphological analysis of single cells allows them to be distinguished from oligodendrocytes. Our data suggest that it could be interesting to reexamine a number of past studies that have used SR101.
Supplementary Material
References
- 1.Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F. Nat. Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, et al. Nat. Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 3.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massa PT, Mugnaini E. Neuroscience. 1982;7:523–538. doi: 10.1016/0306-4522(82)90285-8. [DOI] [PubMed] [Google Scholar]
- 5.Dermietzel R, Spray DC. Trends Neurosci. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- 6.Hill RA, Nishiyama A. Glia. 2014;62:1195–1210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schain AJ, Hill RA, Grutzendler J. Nat. Med. 2014;20:443–449. doi: 10.1038/nm.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.