Abstract
Background
Exposure to ambient air pollution is linked to adverse pregnancy outcomes. Previous reports examining the relationship between ambient air pollution and Hypertensive Disorders of Pregnancy have been inconsistent.
Objectives
We evaluated the effects of ambient air pollution on the odds of Hypertensive Disorder of Pregnancy and whether these associations varied by body mass index (BMI).
Methods
We conducted a retrospective, case-control study among 298 predominantly Hispanic women (136 clinically-confirmed cases) who attended the Los Angeles County + University of Southern California Women’s and Children’s Hospital during 1996–2008. Trimester-specific carbon monoxide (CO), nitrogen dioxide (NO2), ozone (O3), and particulate matter with aerodynamic diameter <10µm and <2.5µm (PM10, PM2.5) exposure were estimated based on 24-hr exposure level at residential address. Logistic regression models were fitted to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for 2 standard deviation increase in exposure levels.
Results
Exposures to CO and PM2.5 in the first trimester were significantly associated with Hypertensive Disorders of Pregnancy, and these associations were modified by BMI. In non-obese women (BMI <30), first trimester exposures to PM2.5 and CO were significantly associated with increased odds of Hypertensive Disorder of Pregnancy (ORs per 2-standard deviation increase in PM2.5 (7µg/m3) and CO (1ppm) exposures were 9.10 [95% CI: 3.33–24.6] and 4.96 [95% CI: 1.85–13.31], respectively). Additionally, there was a significantly positive association between exposure to O3 in the second trimester and Hypertensive Disorder of Pregnancy (OR per 15ppb=2.05; 95% CI: 1.22–3.46).
Conclusion
Among non-obese women, first trimester exposure to PM2.5 and carbon monoxide are associated with increased odds of Hypertensive Disorder of Pregnancy.
Keywords: air pollution, Body Mass Index, carbon monoxide, particulate matter, preeclampsia
Introduction
Hypertensive disorders of pregnancy represent major obstetric complications that affect 5% to 7% of pregnancies and are a leading cause of maternal and neonatal morbidity and mortality (2000). In Hypertensive Disorder of Pregnancy cases, maternal inflammatory processes are exaggerated compared to the low level of inflammation in a normal pregnancy (Redman, 2005; Rusterholz, 2007). Therefore, factors that aggravate maternal systemic and/or placental inflammation have the potential to increase Hypertensive Disorder of Pregnancy risk.
Hypertensive Disorder of Pregnancy and cardiovascular disease appear to share common risk factors and a history of Hypertensive Disorder of Pregnancy increases the risk of subsequent hypertension, ischemic heart disease and stroke (Bellamy, 2007). Although obstetric (history of preeclampsia, family history of preeclampsia, primiparity, multiple pregnancies), clinical (chronic medical conditions such as hypertension and diabetes) and sociodemographic (race/ethnicity, obesity, maternal age) risk factors for Hypertensive Disorder of Pregnancy have been identified (North, 2011), the influence of environmental exposures has not been studied extensively. Because ambient and traffic-related air pollution affect blood pressure (Fuks, 2011), and vascular/systemic inflammation (Bauer, 2010), several studies have examined the possible association between air pollution and Hypertensive Disorder of Pregnancy (Wu, 2009; Rudra, 2011; van den Hooven, 2009) with mixed results. Differences in outcome definition and/or exposure assessment may have easily accounted for the inconsistent findings.
The first trimester likely represents a critical window of susceptibility for developing Hypertensive Disorder of Pregnancy, as it is during this period that trophoblast invasion into the maternal decidua occurs, establishing the fetal blood supply. The potential effects of air pollution in subsequent trimesters are unknown, but could be involved via pro-inflammatory processes. In addition to air pollution, obesity is associated with systemic inflammation (Ndumele, 2011), and air pollution may exaggerate systemic inflammation in obese individuals (Dubowsky, 2006). Whether pre-pregnancy body mass index (BMI) influences the relationship between air pollution and Hypertensive Disorder of Pregnancy risk is unknown, but given that body mass index is an established risk factor for preeclampsia, but not for Hemolysis Elevated Liver Enzyme Low Platelet (HELLP) count syndrome (Leeners, 2006), it could be an important modifier of disease risk. In this study, we explored the potential influence of BMI on the relationship between ambient pollutants and Hypertensive Disorder of Pregnancy beyond its role as an independent risk factor for Hypertensive Disorder of Pregnancy.
The aim of the current study was to investigate the role of trimester-specific ambient air pollution (particulate matter less than 2.5µm and 10 µm in diameter [PM2.5, PM10, respectively], nitrogen dioxide [NO2], carbon monoxide [CO], and ozone [O3]) on Hypertensive Disorder of Pregnancy risk. Specifically, we hypothesized that (1) the first trimester-specific ambient air pollution exposures are associated with Hypertensive Disorder of Pregnancy, and (2) maternal pre-pregnancy BMI modifies the associations between ambient air pollution and Hypertensive Disorder of Pregnancy occurrence. We tested these hypotheses in a study that was conducted in 298 predominantly Hispanic women.
Methods
As described previously (Wilson, 2009; Wilson, 2011), cases of clinically-defined preeclampsia (n = 136) and controls (n = 169) were recruited retrospectively from delivery logs at the Los Angeles County + University of Southern California Women’s and Children’s Hospital from 1999–2006 (103 subjects) and during their postpartum stay at the hospital from 2007–2008 (202 subjects). Medical charts were abstracted by one of the authors (M. W.) to verify case diagnosis, confirm the absence of significant hypertension among controls and to obtain information on comorbidities and other clinically relevant data for both cases and controls. The term “clinically-defined preeclampsia” refers to the diagnosis made by the doctor in charge of patient care (prior to chart review) and refers to patients with hypertension, plus one or more symptoms, including but not limited to proteinuria.
Mild preeclampsia was defined as blood pressure ≥ 140 mmHg (systolic) or ≥ 90 mmHg (diastolic) on two or more occasions at least six hours apart plus proteinuria ≥300 mg/dL in a 24-hour urine collection or +1 on a dipstick in women who were normotensive in early pregnancy (less than 20 weeks gestation). Severe preeclampsia was defined as blood pressure ≥160 mmHg (systolic) or ≥110 mmHg (diastolic) on two or more occasions at least six hours apart plus proteinuria ≥500 mg/dL in a 24-hour urine collection or +3 on a dipstick. Gestational hypertension was defined as elevated blood pressure (mild or severe, as described above) without evidence of proteinuria. Eclampsia was defined as hypertension, with or without proteinuria, plus at least one observed seizure in a woman with no prior history of a seizure disorder, and Hemolysis Elevated Liver Enzyme Low Platelet count syndrome was defined as hemolysis (abnormal peripheral smear, bilirubin >1.2 mg/dl, or lactose dehydrogenase >600 IU/L), elevated liver enzymes (aspartate aminotransferase or alanine aminotransferase >70 IU/L) and low platelets (<100,000 mm3). Partial Hemolysis Elevated Liver Enzyme Low Platelet count syndrome was defined as two out of the previous three criteria (Egerman, 1999). Having any of these conditions resulted in a woman being categorized as having Hypertensive Disorder of Pregnancy. Women with lupus, chronic renal disease, multiple gestations, or sickle cell disease/trait were excluded.
Starting in 2004, recruitment letters were sent to all cases of Hypertensive Disorder of Pregnancy who were diagnosed at Los Angeles County + University of Southern California Women’s and Children’s Hospital and delivered between 1999 and 2006. Letters were also sent to 10 randomly selected controls for each case already in the study, matched on birth year and age ± 5 years. Due to an anticipated lower response rate among controls, 10 controls per case were initially contacted. Follow-up phone calls were made within 2–4 weeks if no response was received after the initial letter was sent. However, due to the high motility in this population, this method proved extremely inefficient.
From 2007–2008, cases and controls were identified while they were still on service in the Women’s and Children’s hospital. We approached every case of clinically diagnosed preeclampsia and randomly approached five controls for each case recruited (every woman not diagnosed with preeclampsia and without one of the exclusion criteria) for participation in the study. Participation rates during the in-house recruitment phase were 80% for cases and 77% for controls. Seven controls were excluded from the analysis due to missing data for the exposure assessment, and one case was excluded from the 3rd trimester analyses because she had delivered during the second trimester.
This study was approved by the University of Southern California Health Sciences Campus Institutional Review Board. All participants signed an informed consent for herself and her infant and, for women under the age of 18 at the time of recruitment (n=14), parental permission for participation was also obtained.
Air pollution exposure assignments were based on spatially mapped ambient air quality data obtained from the US Environmental Protection Agency’s Air Quality System (EPA, 2010) and additional exposure data available from the Southern California Children’s Health Study (Peters, 2004; Gauderman, 2007). Data for NO2, O3, CO, PM2.5 and PM10 ambient concentrations were acquired for California and Nevada for 1998–2008, the states and time-frame covering gestational locations and period for all our study subjects. These databases include gaseous NO2, O3, and CO concentrations that were monitored hourly at a network of 22 to 30 stations in southern California where all but 3 subjects lived. PM2.5 was measured by a variety of methods, including continuous Beta Attenuation Monitors, daily and once every third day integrated 24-hr filters (the Federal Reference Method – FRM-PM2.5), and two-week integrated filters (from the Children’s Health Study). PM10 was measured continuously by Tapered Element Oscillating Microbalance, Beta Attenuations Monitors, and by once every third or sixth day High Volume 24-hr integrated filters (Federal Reference Method – FRM-PM10). All PM measurements were standardized to represent Federal Reference Method measurements. PM measurements were available at 20 to 23 locations in southern California during this period. Only data that met a 75% completeness criterion were used to make exposure assignments, except at locations where only 1-in-3 day or 1-in-6 day daily data were collected (where 75% of expected data completeness was used). At four stations where Air Quality System and Children’s Health Study data overlapped, Children’s Health Study data were used when available because they received a higher level of quality assurance than the Air Quality System data.
Air quality data were first time averaged for the time periods relevant for each subject and then spatially mapped to the residence location. The relevant exposure time periods for the subjects were the trimesters of pregnancy. Trimester start dates were calculated as follows: 1st trimester start date: date of birth - gestational age at delivery, 2nd trimester start date: 1st trimester start date + 92 days, and 3rd trimester start date: 1st trimester start date + 185 days. Gestational age was dated using the date of last menstrual period and were confirmed by ultra-sound. The relevant locations were the geocoded residence locations in each time period. For subjects that moved, the trimester average exposures were calculated for the location with the longest duration in each trimester.
The time-averaged air pollution data were spatially mapped inverse distance-squared weighting of data from up to four closest stations located within 50 km (25 km for CO) of each participant residence (Kinney et al 1998). However, if one or more stations were located within 5 km of a residence then only data from the stations within 5 km were used for the interpolation. An additional requirement to assure consistency in this application is that the interpolations for all 3 periods were based on data from the same stations. The typical spatial patterns of O3, NO2, CO, and PM2.5 concentrations are illustrated in Figure 1 (annual average estimates for 2006). As indicated in the maps, many of the residences were in areas with good spatial monitoring coverage. As a result, 31%, 31%, 31%, 27%, and 22% of the trimester exposure assignments for O3, NO2, CO, PM2.5, and PM10, respectively, were based on data from stations located with 5 km of the residence. For 96% to 100% of the trimester average exposure assignments, the closest air quality station with valid data was located within 25 km of the residence.
Figure 1.
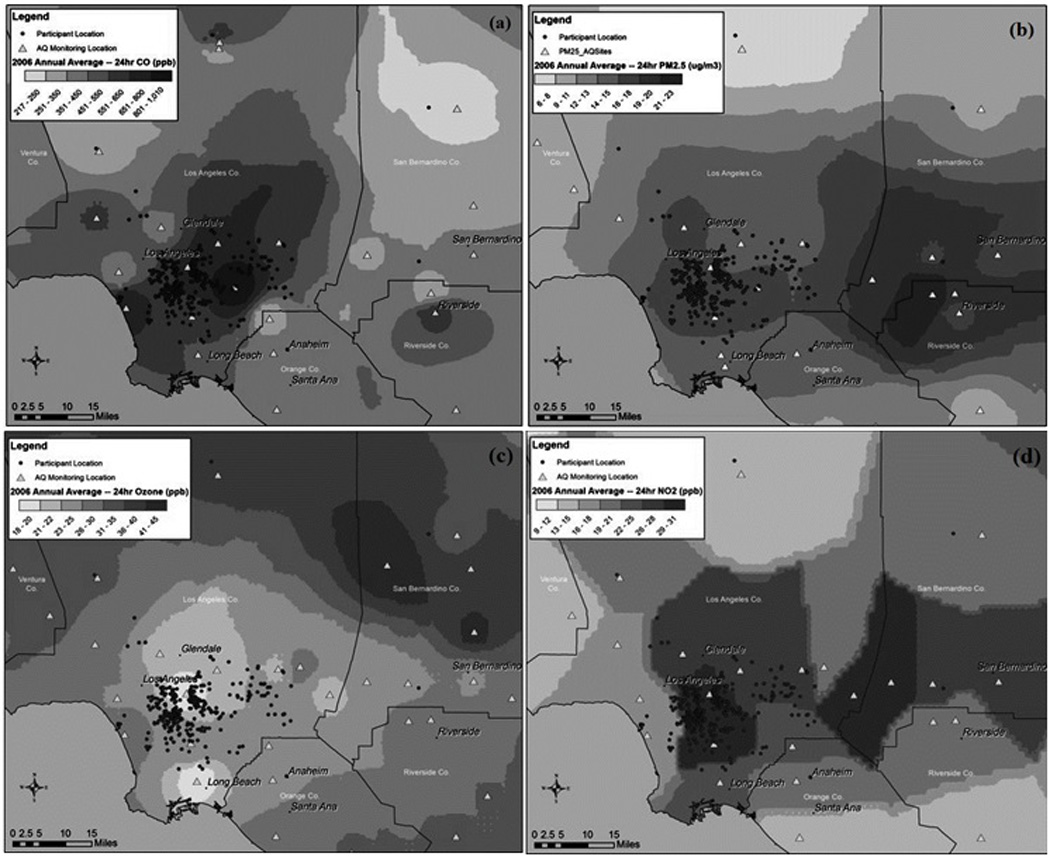
Residence locations, air quality monitoring stations, and estimated annual average concentrations in 2006 for (a) CO, (b) PM2.5, (c) Ozone, and (d) NO2 in the study area.
Correlation analyses were performed using Pearson’s correlation coefficients to assess the linear relationship between all air pollutants in this study. These analyses were performed separately for each pregnancy trimester. Unconditional Logistic Regression was used to examine the association between ambient air pollution and odds of Hypertensive Disorder of Pregnancy, adjusting for maternal age (continuous), parity (nulliparous vs. parous), maternal smoking status (recorded as yes/no) a nd exposure to secondhand tobacco smoke during pregnancy (yes/no). Covariates for the regression analysis were chosen on the basis of a priori knowledge. Both age and parity are known Hypertensive Disorder of Pregnancy risk factors; while maternal smoking is a known protective factor. We have also included an indicator variable for calendar year of pregnancy, as the air quality trends in the Los Angeles basin have been downward for CO, PM10, PM2.5 and NO2, and flat or slightly upward for O3. Specifically, children born after 2002 were exposed to less of the above mentioned pollutants compared to children born before 2002. Pre-pregnancy body mass index did not change the effect estimates by more than 10%, so we did not adjust for it in our models. To test for the modifying effect of BMI on the relationships between air pollution and Hypertensive Disorder of Pregnancy, we evaluated BMI as a dichotomous variable (Obese versus non-obese: BMI<30 and ≥30).
Odds ratios and 95% confidence intervals were computed for 2 standard-deviation increase in the pollutant level to obtain unit changes that are more comparable for interpretation purposes. The likelihood ratio test was used to test for interaction.
Results
Among 136 cases, 67 (49%) met the criteria for mild preeclampsia, 27 (20%) had severe preeclampsia, and 42 (31%) had gestational hypertension. Among those classified as having gestational hypertension, 30 (72%) had signs or symptoms of severe disease, including elevated liver enzymes, uric acid, or lactose dehydrogenase or decreased platelets (n=16); symptoms of preeclampsia such as headache, right upper quadrant pain, epigastric pain or visual disturbances (n=16); and/or a history of preeclampsia in a previous pregnancy (n=8). Among women with preeclampsia, five (4%) had superimposed preeclampsia, four (3%) had eclampsia, and six (5%) had Hemolysis Elevated Liver Enzyme Low Platelet count syndrome or partial Hemolysis Elevated Liver Enzyme Low Platelet count syndrome.
The patient population was 97% Hispanic, and cases and controls did not differ by race or maternal age (Table 1). Controls, on average delivered two weeks later than the cases, indicating that, as a group, they had ample opportunity to develop preeclampsia and be classified as cases. As expected, cases were more likely to be nulliparous than controls, have a higher BMI, and have infants with lower birth weights. Cases and controls did not differ on preexisting or comorbid conditions (Table 1). However, women with preeclampsia were more likely to have chronic hypertension (4% vs. 1%), have a history of previous Hypertensive Disorder of Pregnancy (11% vs. 4%), and have small for gestational age babies, defined as less than the 10th percentile of weight at each gestational age among babies in our data (12% vs. 5%). Moreover, as expected, the maximum systolic and diastolic blood pressures were significantly higher among cases than controls. Cases and controls did not differ in their smoking status; however, a higher proportion of cases were exposed to secondhand smoke than controls.
Table 1.
Selected Characteristics of the Study Population
| Variable | Controls (n=162) |
Cases(n=136) |
|---|---|---|
| Maternal age (mean ± standard deviation, years) | 27.0± 7.0 | 27.7± 7.4 |
| Gestational week | Range (28–42) | Range (25–41) |
| <37 (%) | 14(9) | 44(32) |
| 37–38 (%) | 48(29) | 43(30) |
| >38 (%) | 100(62) | 49(38) |
| Gestational week at first prenatal visit | Range (1–38) | Range (1–36) |
| <12 (%) | 121(77) | 102(78) |
| 12–20 (%) | 22(14) | 19(14.5) |
| >20(%) | 14(9) | 10(7.5) |
| Parity | ||
| Nulliparous (%) | 51(32) | 60(44) |
| Multiparous (%) | 111(68) | 74(56) |
| Race/Ethnicity | ||
| Hispanic White (%) | 157(97) | 131(96) |
| Hispanic Black (%) | 0 | 1(1) |
| Non-Hispanic Black (%) | 3(2) | 3(2) |
| Arab (%) | 1(0.5) | 1(1) |
| Filipino (%) | 1(0.5) | 0 |
| Birth weight (±Standard Deviation, grams) | 3290±536 | 2903± 884 |
| Maternal body mass index (mean ± standard deviation, kg/m2) | 25.7 ± 5.2 | 27.9 ±6.3 |
| Prenatal care (%) | 151 (93) | 119 (90) |
| Chronic Hypertension (%) | 2 (1) | 6 (4) |
| History of Hypertensive Disorder of Pregnancy (%) | 6 (4) | 15 (11) |
| Maximum systolic blood pressure (mmHg) | 117.9 ± 10.9 | 163 ± 15.9 |
| Maximum diastolic blood pressure (mmHg) | 68.8 ± 9.0 | 97.5 ± 9.9 |
| History of diabetes (%) | 6 (4) | 15 (11) |
| History of allergies (%) | 15 (9.0) | 17 (12.5) |
| History of asthma (%) | 8 (5) | 4 (3) |
| Mother’s smoking history (%) | 6 (4) | 9 (6) |
| Exposure to secondhand smoke (%) | 20 (14) | 22 (26) |
| Small for gestational age (%) | 8 (5) | 16 (12) |
4 cases and 9 controls were missing birth weight. Maternal body mass index was measured pre-pregnancy using height and weight. Body Mass Index was missing for 17 subjects (13 controls and 4 cases).
Chronic hypertension refers to those individuals who had high blood pressure prior to their pregnancies.
Similar patterns of correlations among the pollutants were seen across three trimesters (Table 2). Carbon monoxide, PM10, PM2.5, and NO2 were positively correlated with each other. Ozone was negatively correlated with CO, PM2.5, and NO2, and was uncorrelated with PM10.
Table 2.
Trimester-Specific Distributions of Ambient Air Pollutants and Correlations among Pollutants
| Exposure | mean ±SD | Correlations* |
|||
|---|---|---|---|---|---|
| NO2 | O3 | PM10 | PM2.5 | ||
| 1st Trimester | |||||
| CO (ppm) | 0.58± 0.47 | 0.86 | − 0.64 | 0.36 | 0.77 |
| NO2 (ppb) | 28.63± 7.1 | − 0.72 | 0.43 | 0.74 | |
| O3 (ppb) | 21.5± 7.4 | − 0.03 | − 0.45 | ||
| PM10 (µg/m3) | 34.5 ± 6.0 | 0.53 | |||
| PM2.5 (µg/m3) | 17 ± 3.5 | ||||
| 2nd Trimester | |||||
| CO (ppm) | 0.67± 0.50 | 0.84 | − 0.57 | 0.37 | 0.76 |
| NO2 (ppb) | 30 ±6.9 | − 0.74 | 0.44 | 0.80 | |
| O3 (ppb) | 18.2± 6.8 | 0.01 | − 0.53 | ||
| PM10 (µg/m3) | 34.9 ± 6.3 | 0.52 | |||
| PM2.5 (µg/m3) | 17.5 ± 3.5 | ||||
| 3rd Trimester | |||||
| CO (ppm) | 0.62 ±0.55 | 0.87 | − 0.66 | 0.54 | 0.79 |
| NO2 (ppb) | 30± 7.9 | − 0.78 | 0.61 | 0.81 | |
| O3 (ppb) | 18.2± 8.1 | − 0.18 | − 0.55 | ||
| PM10 (µg/m3) | 35.1 ± 7.2 | 0.68 | |||
| PM2.5 (µg/m3) | 18.1 ± 5.0 | ||||
Values obtained by Pearson’s correlation test.
Air pollution was associated with Hypertensive Disorder of Pregnancy in the 1st trimester (Table 3). Specifically, each 2-standard deviation increase in carbon monoxide (1ppm) increased the odds of developing Hypertensive Disorder of Pregnancy in the 1st trimester (OR = 2.83, 95% CI: 1.29, 6.20); however, CO exposure in the remaining two trimesters was not associated with Hypertensive Disorder of Pregnancy. Exposure to PM2.5 in the 1st trimester was also associated with Hypertensive Disorder of Pregnancy. Each 2-standard deviation increase in PM2.5 (7µg/m3) was associated with nearly a 4-fold increase in the odds of developing Hypertensive Disorder of Pregnancy (OR = 3.94, 95% CI: 1.82, 8.55). Similar to CO, exposure to PM2.5 in the remaining two trimesters were not associated with Hypertensive Disorder of Pregnancy. When 1st trimester PM2.5 and CO were jointly included in the model, the association between Hypertensive Disorder of Pregnancy and PM2.5 was attenuated but remained statistically significant (OR= 3.3; 95%CI: 1.30, 8.4), while CO exposure was no longer statistically significantly associated with Hypertensive Disorder of Pregnancy (OR = 1.4; 95% CI: 0.53, 3.6). Second trimester ozone was associated with a 2-fold increase in odds of Hypertensive Disorder of Pregnancy (OR per 2-standard deviation increase = 2.05, 95% CI: 1.22, 3.46). Exposures to PM10 and NO2 in any trimester were not significantly associated with Hypertensive Disorder of Pregnancy. We repeated the analyses excluding subjects with gestational hypertension (n=42) and found no change in the results (data not shown). Similarly, excluding women with chronic hypertension (2 controls and 6 cases) did not change our results (data not shown).
Table 3.
Associations between Trimester-Specific Pollutant Exposures and Hypertensive Disorder of Pregnancy
| Pollutant | Trimester | Case/Control | Adjusted OR (95% CI)** |
|---|---|---|---|
| CO (ppm) | 1st | 136/162 | 2.83 (1.29, 6.20) |
| 2nd | 136/162 | 0.90 (0.45, 1.79) | |
| 3rd | 135/162 | 1.16 (0.61, 2.20) | |
| NO2 (ppb) | 1st | 136/162 | 1.42 (0.75, 2.67) |
| 2nd | 136/162 | 0.60 (0.33, 1.11) | |
| 3rd | 135/162 | 1.00 (0.56, 1.79) | |
| O3 (ppb) | 1st | 136/162 | 0.91 (0.54, 1.52) |
| 2nd | 136/162 | 2.05 (1.22, 3.46) | |
| 3rd | 135/162 | 1.19 (0.71, 1.98) | |
| PM2.5 (µg/m3) | 1st | 136/162 | 3.94 (1.82, 8.55) |
| 2nd | 136/162 | 1.86 (0.95, 3.63) | |
| 3rd | 135/162 | 1.44 (0.76, 2.70) | |
| PM10 (µg/m3) | 1st | 136/162 | 0.76 (0.43, 1.36) |
| 2nd | 136/162 | 0.76 (0.44, 1.32) | |
| 3rd | 135/162 | 1.41 (0.77, 2.57) | |
The odds ratios (ORs) and 95% confidence intervals (CIs) are reported per 2 standard deviations (2SD) increase in the pollutant concentration. The 2SDs for CO, NO2, O3, PM2.5, and PM10 were 1ppm, 14 ppb, 15 ppb, 7µg/m3 and 13µg/m3, respectively.
ORs adjusted for maternal age (continuous), parity, maternal smoking history, exposure to secondhand smoke during pregnancy, and year of conception (before or after 2002).
One case was excluded from the 3rd trimester analyses due to missing exposure.
Maternal BMI influenced the association between some of the pollutants and Hypertensive Disorder of Pregnancy occurrence (Table 4). Exposure to CO in the first trimester was significantly associated with increased odds of Hypertensive Disorder of Pregnancy among non-obese individuals (OR per 2-standard deviation = 4.68, 95%CI: 1.69, 12.99), while such exposure was not associated with Hypertensive Disorder of Pregnancy among obese individuals (P for interaction = 0.02). The first trimester PM2.5 exposure was significantly associated with Hypertensive Disorder of Pregnancy among non-obese women (OR = 8.63, 95%CI: 3.10, 24.14), but was not associated with Hypertensive Disorder of Pregnancy among obese women (OR = 0.72, 95%CI: 0.14, 3.56).
Table 4.
Associations between Trimester Specific Air Pollution Exposure and Hypertensive Disorder of Pregnancy, Stratified by BMI Categories
| Pollutant | Trimester | Non-Obese (BMI <30kg/m2) | Obese (BMI ≥30kg/m2) | Pinteraction | ||
|---|---|---|---|---|---|---|
| Case/control | OR (95% CI)* | Case/control | OR (95% CI)* | |||
| CO (ppm) | 1st | 94/119 | 4.68 (1.69, 12.99) | 38/30 | 0.81 (0.21, 3.10) | 0.02 |
| 2nd | 94/119 | 0.71 (0.27, 1.83) | 38/30 | 1.28 (0.34, 4.78) | 0.79 | |
| 3rd | 94/119 | 0.84 (0.37, 1.87) | 38/30 | 1.85 (0.45, 7.60) | 0.06 | |
| NO2 (ppb) | 1st | 94/119 | 2.05 (0.94, 4.48) | 38/30 | 0.51 (0.11, 2.40) | 0.04 |
| 2nd | 94/119 | 0.52 (0.24, 1.16) | 38/30 | 0.94 (0.27, 3.31) | 0.32 | |
| 3rd | 94/119 | 0.92 (0.46, 1.88) | 38/30 | 0.84 (0.24, 3.00) | 0.25 | |
| O3 (ppb) | 1st | 94/119 | 0.68 (0.36, 1.29) | 38/30 | 1.42 (0.44, 4.57) | 0.17 |
| 2nd | 94/119 | 2.16 (1.12, 4.17) | 38/30 | 1.36 (0.43, 4.26) | 0.34 | |
| 3rd | 94/119 | 1.44 (0.77, 2.70) | 38/30 | 1.03 (0.37, 2.91) | 0.04 | |
| PM2.5 (µg/m3) | 1st | 94/119 | 8.63 (3.10, 24.14) | 38/30 | 0.72 (0.14, 3.56) | 0.06 |
| 2nd | 94/119 | 1.74 (0.75, 4.01) | 38/30 | 2.41 (0.66, 8.76) | 0.51 | |
| 3rd | 94/119 | 1.28 (0.59, 2.78) | 38/30 | 1.39 (0.34, 5.60) | 0.23 | |
| PM10 (µg/m3) | 1st | 94/119 | 0.75 (0.36, 1.55) | 38/30 | 0.89 (0.26, 3.09) | 0.97 |
| 2nd | 94/119 | 0.63 (0.31, 1.28) | 38/30 | 0.75 (0.28, 2.00) | 0.64 | |
| 3rd | 94/119 | 1.99 (0.90, 4.41) | 38/30 | 0.68 (0.22, 2.04) | 0.09 | |
The odds ratios (ORs) and 95% confidence intervals (CIs) are reported per 2 standard deviations (2SD) increase in the pollutant concentration. The 2SDs for CO, NO2, O3, PM2.5, and PM10 were 1ppm, 14 ppb, 15 ppb, 7µg/m3 and 13µg/m3, respectively.
All models are adjusted for maternal age (continuous), parity, maternal smoking history, exposure to secondhand smoke during pregnancy, and year of conception (before or after 2002).
P values for interaction are obtained using the likelihood ratio test.
Subjects with missing BMI (N=17) were not included in this analysis (N=281).
Approximately 90% of this population had their first prenatal visit before 12 weeks gestation. Restricting the analyses to these women provided similar results (data not shown). All but one subject developed Hypertensive Disorder of Pregnancy during the last trimester. As such, while the air pollution exposures in the 1st and 2nd pregnancy trimesters represent pre-diagnosis exposures for the cases, the estimation of average exposures in the third trimester included exposures that occurred after diagnosis for a small minority of cases. Therefore, the third trimester-specific results should be interpreted with caution.
Discussion and Conclusion
In this study, we report that first-trimester exposures to PM2.5 and CO are associated with increased odds of developing Hypertensive Disorder of Pregnancy, particularly among non-obese women.
While the etiology of Hypertensive Disorder of Pregnancy is largely unknown, it is widely believed to begin in the first trimester with impaired placental development (Zhong, 2010; Redman, 2010). Early placentation occurs in a low oxygen (hypoxic) environment. During the second trimester, there is a switch from hypoxia to normoxia (Caniggia, 2000), which results in a dramatic shift in gene expression (Goldman-Wohl, 2002). Failure to undergo this essential switch results in inadequate trophoblast invasion into the spiral arteries, leading to shallow placentation, and in some cases, Hypertensive Disorder of Pregnancy (Goldman-Wohl, 2002; McMaster, 2004). Carbon monoxide can cross the placenta and has a greater affinity for fetal hemoglobin than oxygen (Sangalli, 2003). Thus, exposure to CO may result in prolonged hypoxia, contributing to the pathological conditions under which Hypertensive Disorder of Pregnancy may occur. Consistent with this hypothesis, only first trimester CO increased the odds of developing Hypertensive Disorder of Pregnancy in our population.
A link between CO and Hypertensive Disorder of Pregnancy has been previously suggested. Rudra and Williams reported a strong and positive association between CO and the odds of Hypertensive Disorder of Pregnancy (Rudra, 2011), although this association was no longer significant after adjustment for the year of conception. Vigeh et al, reported twice the rate of pregnancy hypertension in mothers exposed to higher CO concentrations than mothers exposed to lower CO concentrations (OR= 2.02, 95% CI= 1.35, 3.03) (Vigeh, 2011).
Exposure to CO has been linked to other adverse pregnancy outcomes including Intrauterine Growth Restriction. Like Hypertensive Disorder of Pregnancy, Intrauterine Growth Restriction is believed to be a disorder of early placental dysfunction in most, but not all cases (Furuya, 2008). Intrauterine Growth Restriction and Hypertensive Disorder of Pregnancy share common pathological pathways, which often result in their comorbid occurrence (Ness, 2006). Salam et al found that first trimester exposure to CO was associated with a 20% increased risk of Intrauterine Growth Restriction (Salam, 2005). Similarly Liu et al showed that each 1ppm increase in CO exposure during the 1st trimester was associated with a statistically significant increase in the risk of Intrauterine Growth Restriction (Liu, 2003). In the light of important shared etiologic factors between Hypertensive Disorder of Pregnancy and Intrauterine Growth Restriction, the finding of an association between Hypertensive Disorder of Pregnancy and CO exposure during the 1st trimester in women with Hypertensive Disorder of Pregnancy lends support to the putative importance of hypoxemia in the development of these conditions.
First trimester PM2.5 was strongly associated with Hypertensive Disorder of Pregnancy in non-obese subjects. Our results are consistent with an earlier study in which women exposed in the highest PM2.5 quartile during their pregnancy were at 42% increased risk of developing preeclampsia compared to those whose exposure was in the lowest quartile (Wu, 2009). Rudra et al reported a positive but non-significant association between PM2.5 exposure during the periconceptional (7-months surrounding the pregnancy) and the last 3-months of pregnancy and preeclampsia (Rudra, 2011). The reason for the difference in the windows of exposure (e.g., trimester-specific vs. periconceptional) between these studies is not clear.
The relationship between PM10 and Hypertensive Disorders of Pregnancy has been recently explored in a prospective cohort study in the Netherlands (van den Hooven, 2011). They reported a positive association between PM10 concentrations and the risk of Pregnancy Induced Hypertension (odds ratio 1.72 [95% CI 1.12 to 2.63] per 10 g/m3 increase), but not with preeclampsia (odds ratio 1.34 [95% CI 0.78 to 2.31] per 10 g/m3 increase) (van den Hooven, 2011). However, consistent with our own findings, the same authors reported no association between NO2 exposures and Pregnancy Induced Hypertension or preeclampsia (van den Hooven, 2011).
Interestingly, the observed associations between first trimester CO and PM2.5 on Hypertensive Disorders of Pregnancy occurrence were stronger and statistically significant only among non-obese women (BMI<30). One possible explanation is that the preexisting state of inflammation in obese women may have masked any additional influence of air pollution on Hypertensive Disorders of Pregnancy. In contrast, exposure to air pollution in non-obese subjects may have initiated inflammatory processes, which would trigger the subsequent pathophysiologic events leading to the development of Hypertensive Disorders of Pregnancy. Other alternatives include the possibility that biochemical pathways by which CO and PM2.5 are processed may vary between obese and non-obese women and the prospect that Hypertensive Disorders of Pregnancy heterogeneity – that the condition itself is different between obese and non-obese women – could explain observed differences.
We found that O3 was significantly associated with Hypertensive Disorders of Pregnancy in the second trimester. While there are currently no studies investigating the role of O3 in predisposing to Hypertensive Disorders of Pregnancy, Liu et al showed a significant association between O3 and Intrauterine Growth Restriction during the 2nd trimester of pregnancy (OR = 1.08, 95%CI= 1.01, 1–15) (Liu, 2003). This finding was subsequently confirmed by Salam et al (Salam, 2005). The exact mechanism by which ozone acts to increase risk is unknown; however, it is likely that increased levels of O3 leads to increased lipid peroxidation, resulting in the release of pro-inflammatory cytokines into the circulation. Lipid peroxides are involved in oxidative stress, which is believed to be one of the main etiologic factors in formation of Hypertensive Disorders of Pregnancy (reviewed in Wilson, 2003). Circulating cytokines in maternal circulation could impair placental circulation (Larini, 2005) due to endothelial cell dysfunction, which in turn could lead to increased risk for Hypertensive Disorders of Pregnancy.
We acknowledge the following study limitations. Exposure misclassification, especially with regard to CO, is a frequent concern of any study investigating this pollutant. CO levels exhibit monthly variation with higher amounts in spring and summer compared to fall and winter. However; the impact of this limitation on our findings is minimal since we have roughly equal distribution of births during winter and fall compared to summer and spring months. Moreover, in the presence of any possible exposure misclassification, it would be non-differential with respect to case/control status, which tends to result in a bias toward the null.
Pollutant exposure is also subject to misclassification by work environment. While we collected information on occupation, we did not have detailed data on occupational history, location and time-activity at workplace. However, because over 90% of women in the study population reported “housewife” as their occupation during their pregnancy (the exposure period of interest), we do not expect that information on occupational exposure would significantly alter the classification status of women in the study.
Exposure misclassification during the third trimester remains a concern, since average exposure assignment to women diagnosed with Hypertensive Disorders of Pregnancy during the 3rd trimester covered both pre- and post-diagnosis levels. In this study the majority of cases were diagnosed with Hypertensive Disorders of Pregnancy after 35 weeks. Therefore, the third trimester analyses may be somewhat biased due to exposure misclassification as a result of mixing of pre- and post-diagnosis exposure levels among cases. Additionally, we could not examine the 3rd trimester exposure based on diagnostic (birth) dates due to complications in exposure assignment to controls. For instance, one month before birth would usually represent exposure levels during gestational ages 37–40 weeks in controls, while one month before birth among cases (due to the earlier delivery date) would usually represent exposure levels during gestational ages 31–34 weeks. The resultant comparison would be between different at-risk periods for cases and controls. Thus, we recommend that the third trimester results be interpreted with caution.
We used two different recruitment methods to ascertain our subjects. However, we can find no evidence that method of recruitment resulted in any differences in the study populations selected. To evaluate the possibility that subjects recruited retrospectively (1999–2006) differed from those recruited while on service (2007–2008), we evaluated the distribution of all covariate data with respect to these two methods of recruitment and found no significant differences between the groups. Furthermore, restricting the analyses to subjects who were recruited in 2007–2008 produced results in the similar direction but not significant (data not shown).
We were unable to calculate an exact response rate due to the high proportion of disconnected phone numbers, undeliverable mail and homeless women for the retrospective enrollment phase of the study (1999–2006). However, based on the number of women diagnosed with Hypertensive Disorders of Pregnancy at the Los Angeles County + University of Southern California Women’s and Children’s Hospital during the time period in question and the number of women recruited during that period, our recruitment rates during retrospective recruitment were approximately 39% and 8% among cases and controls, respectively. These rates represent an underestimate of our true recruitment rate.
Last, as this study was conducted among a population that was 97% Hispanic, generalizability to other ethnic groups may be limited. We acknowledge that the relatively small sample size available for this study may have limited our power to comprehensively examine the association between ambient air pollution and Hypertensive Disorders of Pregnancy across different pregnancy trimesters, especially given the heterogeneity of the outcome. These results will need to be confirmed in a larger, ideally prospective study.
This study has several advantages compared to similar studies. We were able to test the associations of five criteria pollutants in a trimester-specific manner. Los Angeles County has varying levels of air pollution, and the study subjects came from different parts of the County. This provided the opportunity to examine associations between a wide range of air pollution on Hypertensive Disorders of Pregnancy occurrence. Moreover, we were able to examine the impact of O3 in this study, which has not been reported previously. The study subjects were primarily Hispanics, who represent the fastest growing population in America and represent an under-studied segment of the population. Finally, the extensive chart review for all of the subjects provided detailed information on case diagnosis as well as on co-morbid conditions on both cases and controls.
In conclusion, PM2.5 and CO exhibited significant associations with Hypertensive Disorders of Pregnancy during the 1st pregnancy trimester, and the associations were stronger in non-obese women. Our results may have important public health implications in promoting maternal and child health and advocating for public policy changes regarding pollutant levels. Since Hypertensive Disorders of Pregnancy is itself a risk factor for future maternal cardiovascular disease (Freibert, 2011) as well as a number of diseases among children born to mothers with preeclampsia (Wu, 2011), further reductions in ambient air pollution is likely to provide a wide array of health benefits.
Acknowledgments
We would also like to thank all of the women who participated in this study, without whom this research would not have been possible.
Funding
This work was supported by the Southern California Environmental Health Sciences Center (NIEHS grant#5P30ES007048, and T32 ES013678) and R21 HD046624-02.
Footnotes
Details of ethics approval
This study was approved by the University of Southern California Health Sciences Institutional Review Board (protocol ID no HS-03C050).
Disclosure of interests
None of the authors have any conflicts of interest to report.
References
- Bauer M, Moebus S, Mohlenkamp S, Dragano N, Nonnemacher M, Fuchsluger M, Kessler C, Jakobs M, Memmesheimer R, et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56:1803–1808. doi: 10.1016/j.jacc.2010.04.065. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;(Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman RS, Sibai BM. HELLP syndrome. Clin Obstet Gynecol. 1999;42:381–389. doi: 10.1097/00003081-199906000-00022. [DOI] [PubMed] [Google Scholar]
- EPA. [accessed 1 February 2010];U.S. Environmental Protection Agency’s Air Quality System. 2010 http://www.epa.gov/ttn/airs/airsaqs/;
- Freibert SM, Mannino DM, Bush H, Crofford LJ. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Womens Health (Larchmt) 2011;20:287–293. doi: 10.1089/jwh.2010.2097. [DOI] [PubMed] [Google Scholar]
- Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, Dragano S, Mohlenkamp H, Jakobs C, Kessler R, Erbel B, Hoffmann Long-Term Urban Particulate Air Pollution, Traffic Noise and Arterial Blood Pressure. Environ Health Perspect. 2011;119:1706–1711. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Ishida J, Aoki I, Fukamizu A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc Health Risk Manag. 2008;4:1301–1313. doi: 10.2147/vhrm.s4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, Lurmann F, Avol E, Kunzli N, Jerrett M, Peter J. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol. 2002;187:233–238. doi: 10.1016/s0303-7207(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Kinney PL, Aggarwal M, Nikiforov SV, Nadas A. Methods development for epidemiologic investigations of the health effects of prolonged ozone exposure. Part III. An approach to retrospective estimation of lifetime ozone exposure using a questionnaire and ambient monitoring data (U.S. sites) Res Rep Health Eff Inst. 1998;81:79–108. [PubMed] [Google Scholar]
- Larini A, Bocci V. Effects of ozone on isolated peripheral blood mononuclear cells. Toxicol In Vitro. 2005;19:55–61. doi: 10.1016/j.tiv.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Leeners B, Rath W, Kuse S, Irawan C, Imthurn B, Neumaier-Wagner P. BMI: new aspects of a classical risk factor for hypertensive disorders in pregnancy. Clin Sci (Lond) 2006;111:81–86. doi: 10.1042/CS20060015. [DOI] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;111:1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24:540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- Ndumele CE, Nasir K, Conceicao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- North RA, McCowan LM, Dekker GA, Poston L, Chan EH, Stewart AW, Black MA, Taylor RS, Walker JJ, Baker PN, Kenny LC. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342:d1875. doi: 10.1136/bmj.d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Avol E, Berhane K, Gauderman J, Gilliland F, Jerrett M, Kunzli N, London S, McConnell R, Navidi W, Rappaport E, Thomas D, Lurmann FW, Roberts PT, Alcorn SH, Funk T, Gong H, Linn WS, Cass G, Margolis H. Epidemiologic investigation to identify chronic effects of ambient pollutants in southern California. Final report prepared for the California Air Resources Board and the California Environmental Protection Agency by the University of Southern California, Department of Preventative Medicine, Los Angeles, CA, Sonoma Technology, Inc., Petaluma, CA, Los Amigos Research and Education Institute, Downey, CA, and Technical and Business Systems, Santa Rosa, CA, Contract No. 2004 May;:94–331. Available on the Internet at < http://www.arb.ca.gov/research/apr/past/94-331a.pdf>.
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- Rudra CB, Williams MA, Sheppard L, Koenig JQ, Schiff MA. Ambient carbon monoxide and fine particulate matter in relation to preeclampsia and preterm delivery in western Washington State. Environ Health Perspect. 2011;119:886–892. doi: 10.1289/ehp.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- Salam MT, Millstein J, Li YF, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children’s Health Study. Environ Health Perspect. 2005;113:1638–1644. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangalli MR, McLean AJ, Peek MJ, Rivory LP, Le Couteur DG. Carbon monoxide disposition and permeability-surface area product in the foetal circulation of the perfused term human placenta. Placenta. 2003;24:8–11. doi: 10.1053/plac.2002.0877. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, Jaddoe VW, de Kluizenaar Y, Hofman A, Mackenbach JP, Steegers EA, Miedema HM, Pierik FH. Residential traffic exposure and pregnancy-related outcomes: a prospective birth cohort study. Environ Health. 2009;8:59. doi: 10.1186/1476-069X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, de Kluizenaar Y, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, Mackenbach JP, Steegers EA, Miedema HM, Jaddoe VW. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: the generation R study. Hypertension. 2011;57:406–412. doi: 10.1161/HYPERTENSIONAHA.110.164087. [DOI] [PubMed] [Google Scholar]
- Vigeh M, Yunesian M, Shariat M, Niroomanesh S, Ramezanzadeh F. Environmental carbon monoxide related to pregnancy hypertension. Women Health. 2011;51:724–738. doi: 10.1080/03630242.2011.633599. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Goodwin TM, Pan VL, Ingles SA. Molecular epidemiology of preeclampsia. Obstet Gynecol Surv. 2003;58:39–66. doi: 10.1097/00006254-200301000-00022. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Desmond DH, Goodwin TM, Miller DA, Ingles SA. Maternal and fetal variants in the TGF-beta3 gene and risk of pregnancy-induced hypertension in a predominantly Latino population. Am J Obstet Gynecol. 2009;201(295):e1–e5. doi: 10.1016/j.ajog.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Brueggmann D, Desmond DH, Mandeville JE, Goodwin TM. Ingles the GCM1 gene is associated with pregnancy induced hypertension in a predominantly hispanic population. Int J Mol Epidemiol Genet. 2:196–206. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;117:1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Diseases in children born to mothers with preeclampsia: a population-based sibling cohort study. Am J Obstet Gynecol. 2011;204(157):e1–e5. doi: 10.1016/j.ajog.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and Intrauterine Growth Restriction. Prenat Diagn. 2010;30:293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]


