To the Editor
The use of antibodies against programmed cell death 1 (PD-1), which block inhibitory T-cell checkpoints, is a promising new therapy for advanced cancers.1 Recent trials have shown substantial clinical activity of anti–PD-1 antibodies in advanced cancers and led to the approvals of these agents, including pembrolizumab for melanoma and nivolumab for melanoma and squamous-cell lung cancer.2-4 Pneumonitis related to the use of antibodies against PD-1 is an immune-mediated toxic effect that resulted in three drug-related deaths in a phase 1 trial.1 Clinical identification and management of pneumonitis are contingent on radiographic assessment. We report three cases of pneumonitis associated with the use of anti–PD-1 antibodies in patients with melanoma.
A 70-year-old man (Patient 1) received nivolumab and ipilimumab sequentially. A 38-year-old woman (Patient 2) and a 58-year-old man (Patient 3) were treated with nivolumab alone; Patient 2 had previously received ipilimumab before starting the nivolumab trial. The onset of pneumonitis occurred 7.4 to 24.3 months after the initiation of therapy (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Computed tomographic (CT) imaging of pneumonitis at the time of diagnosis showed a spectrum of findings that are typically seen in interstitial pneumonias.5 These conditions were morphologically classified as acute interstitial pneumonia–acute respiratory distress syndrome (ARDS) in Patients 1 and 2 and as nonspecific interstitial pneumonia in Patient 3 (Table S2 in the Supplementary Appendix).
In Patients 1 and 2 with ARDS-pattern pneumonitis, diffuse ground-glass opacities, reticular opacities, consolidation, and traction bronchiectasis involved all lobes, with decreased lung volumes and effusions (Fig. 1A, 1B, and 1C). In Patient 1, the symptoms rapidly progressed during the 2 weeks before the diagnosis (Fig. 1A and 1B). The two patients were admitted to the intensive care unit and received intravenous antibiotic agents, glucocorticoids, and infliximab. Patient 1 required intubation, and his condition improved over the course of 10 weeks. Patient 2 died 4 weeks after the diagnosis of pneumonitis.
Figure 1. CT of the Chest Performed in Three Patients with Pneumonitis Associated with the Use of Anti– Programmed Cell Death 1 Antibodies.
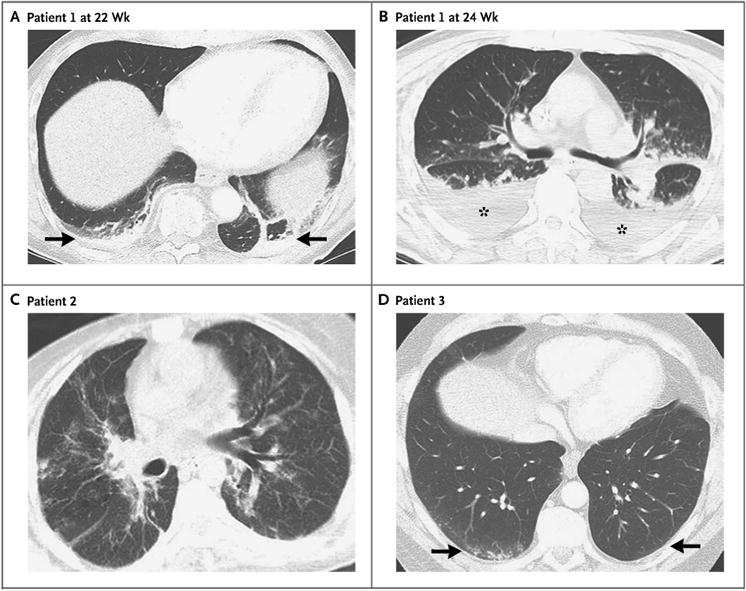
In Patient 1, a 70-year-old man with advanced melanoma who was treated in a trial of nivolumab given sequentially with ipilimumab, chest CT at 22 weeks of therapy revealed consolidation in the bilateral lower lobes with reticular and ground-glass opacities (Panel A, arrows). Two weeks later, the findings significantly progressed (Panel B, asterisks) and involved all lobes, with decreased lung volumes and pleural effusion. In Patient 2, a 38-year-old woman with advanced melanoma who was treated with nivolumab, chest CT at 15 weeks of therapy revealed diffuse ground-glass opacities, reticular opacities, consolidations, traction bronchiectasis, and areas of centrilobular nodularity (Panel C) involving all lobes and more than 50% of all lung zones, with decreased lung volumes. In Patient 3, a 58-year-old man with advanced melanoma who was treated with nivolumab, chest CT at 7 weeks of therapy revealed bilateral ground-glass opacities, reticular opacities, and small areas of consolidation in predominantly lower and peripheral distribution (Panel D, arrows), indicative of a pattern of nonspecific interstitial pneumonia.
Patient 3 had ground-glass opacities and reticular opacities in the peripheral and lower lungs, indicative of nonspecific interstitial pneumonia (Fig. 1D). He discontinued nivolumab for 8 weeks and received oral glucocorticoids as an outpatient, and the pneumonitis resolved after 2 weeks. The patient restarted nivolumab and has completed the 2-year treatment period with 12 cycles; he is currently participating in the follow-up period of the trial. He remains progression-free from melanoma, with no recurrent pneumonitis for 39 months.
The clinical oncology community has rapidly expanding access to a variety of immunotherapeutic agents for the treatment of several types of cancers. Thus, knowledge of the spectrum of manifestations of autoimmune pneumonitis may assist other clinicians in managing this rare but potentially serious toxic effect.
Supplementary Material
Acknowledgments
Supported by a grant from the National Cancer Institute (1K23CA157631, to Dr. Nishino).
Footnotes
References
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumabrefractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias: this joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


