INTRODUCTION
Despite an overall trend towards less invasive oncologic care in the United States (US), rates of contralateral prophylactic mastectomy (CPM) in women diagnosed with unilateral breast cancer (UBC) have more than doubled over the past 15 years.1 The increased prevalence of CPM is thought to reflect pervasive overestimation of metachronous contralateral breast cancer (MCBC) risk by breast-cancer patients,2–4 increased dissemination of personalized genetic and immunohistochemical information to patients,5 improved post-mastectomy reconstruction options,6–8 and exposure to internet-based information that is often contradictory. It is unclear whether CPM is associated with improved survival or decreased recurrence in UBC patients, all of whom are at increased risk for MCBC,9,10 i.e., contralateral breast cancer (CBC) diagnosed subsequent to an index cancer. Definitions of MCBC vary throughout the literature. Depending on a given researcher’s decision as to what period of time is sufficiently long to distinguish a synchronous contralateral breast cancer (SCBC) from a metachronous one, MCBC has been defined as a new CBC diagnosed anywhere from one month to two years after an index tumor.11 But the magnitude of MCBC risk is not uniformly distributed among patients with UBC: among women without a BRCA mutation, less than 10% would be expected to eventually develop MCBC,2,12 but among women with a family history of breast cancer and/or an identified genetic mutation in BRCA1 or BRCA2, incidence of MCBC has been estimated to be anywhere from 12% to 47%.13–15 CPM has historically been prescribed for these higher risk patients as a means through which to decrease MCBC and, concomitantly, mortality associated with MCBC. But even among this subset of breast-cancer patients, the efficacy of CPM in improving long-term clinical outcomes is questionable.
Mirroring the difficulties of establishing a uniform definition of MCBC, survival – overall, breast-cancer-specific, and disease-free – in women with UBC has been defined in variable ways throughout the literature, and reports of the potential survival benefit CPM might confer on recipients have been similarly inconsistent. Among recent studies examining the relationship between CPM and overall survival (OS), neither Chung and colleagues’ 2012 study 6 nor the 2000 study by Peralta et al.16 demonstrated a CPM-associated benefit with regards to OS. Peralta and colleagues did, however, report prolonged disease-free survival (DFS), defined as time to any breast-cancer event (namely, a recurrent or second primary breast cancer including newly diagnosed CBCs) among CPM recipients. In contrast, Bedrosian et al.’s 2010 study based on Surveillance, Epidemiology, and End Results (SEER) data, Boughey et al.’s 2010 study from the Mayo Clinic, and Herrinton et al.’s 2005 Cancer Research Network study all reported a OS advantage potentially conferred by CPM; however, there are important subtleties in their findings.17–19 In the SEER data study by Bedrosian and colleagues, the observed CPM-associated survival benefit demonstrated in the full analysis was found in subgroup analysis to stem largely from the strong survival benefit (4.8%) conferred on young (i.e., under the age of 50) CPM recipients with early-stage (I and II), estrogen-receptor (ER)-negative disease who – by virtue of having more years to live and more aggressive tumor biology at baseline – had a higher absolute lifetime risk of MCBC compared to their older and ER-positive counterparts.17 In their cohort, Boughey et al. found CPM to be associated with improved OS but not with breast-cancer-specific survival (BCSS) and this discrepancy could be ascribed to CPM recipients’ being healthier at baseline, a conjecture supported by the fact that the 9% survival difference between recipients and non-recipients was greater than the absolute rate of CBCs in non-recipients (8.1%).18 Finally, in Herrinton et al.’s study, the 3.6% difference in breast-cancer-specific mortality (BCM) between CPM recipients and non-recipients (8.1% vs. 11.7%) is greater than the absolute reduction in CBC (0.5% vs. 2.7%), making it difficult to attribute the difference in disease-specific mortality to the effects of CPM and suggesting there may be some other contributing factor.19 Thus, it is unclear to what extent the observed survival benefit reported in these studies is secondary to decreased (though, notably, not eliminated) risk of MCBC following removal of contralateral breast tissue;9 selection bias, specifically confounding patient characteristics, such as younger age,9,17,20–22 that are both independently associated with better baseline health and a greater likelihood of undergoing CPM; or to receipt of treatments – such as tamoxifen and bilateral oophorectomy – that decrease the risk of BCM and/or all-cause mortality.23,24
Here, we present the results of a systematic review and meta-analysis of CPM in female patients with a personal history of UBC. Although a Cochrane review on prophylactic mastectomy (both CPM in UBC patients as well as bilateral prophylactic mastectomy for prevention of a first breast cancer) was published in 2004 and updated in 2010,25 our review is the first to include meta-analyses of clinical outcomes, focuses solely on CPM as a method of risk reduction in patients with breast-cancer diagnoses, and includes several large-scale studies published after the Cochrane review’s 2010 update. Our intention is to provide a quantitative summation of current evidence that can serve as a succinct guide for clinical practice and can inform the development of future research examining the efficacy of CPM in both average- and high-risk breast-cancer patients.
METHODS
Data collection
The aims of this project were to examine whether CPM for women with UBC is associated with significant improvements in OS (primary outcome) as well as the following secondary outcomes: BCM, incidence of CBC, and rates of distant/metastatic recurrence (DMR).
A medical librarian developed search strategies (Appendix 1) for Medline/PubMed, EMBASE, Scopus, ClinicalTrials.gov, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and the Health Economic Evaluations Database using a combination of standardized index terms and plain language to cover the concepts of CPM, UBC, randomized controlled trials (RCTs), and observational studies as comprehensively as possible. Searches were limited to English-language studies published through March 2012 using standard limitations provided by the databases. We also contacted the authors of 4 studies and asked whether they would share their study data with us in a format that would be more amenable to inclusion in our meta-analyses; the authors for two of these studies agreed to do so.17,26
Study selection
Our review was limited to published RCTs and observational studies that included and compared patients who had and had not received CPM. Case series and convenience samples were only included if they reported incidence of SCBC in CPM recipients. Conference abstracts were excluded. We defined CPM as any simple (total), subcutaneous, skin-sparing, modified radical, or radical mastectomy performed on a breast with no clinical or radiographic evidence of malignancy for the purpose of preventing CBC in a patient with a history of UBC. Included studies had to report survival, cause-specific mortality, CBC incidence, and/or recurrence for women who underwent CPM at any time after being diagnosed with primary UBC.
Data extraction
Data were independently abstracted and verified by two coders. Effect measures were deconstructed into component values (i.e., measures of incidence, length of follow-up) for both CPM and non-CPM cohorts. When this was not possible, study authors were contacted for primary data components of effect measures. In addition to outcome data, information on potentially confounding study-level characteristics (BRCA carrier status, family history) and cohort-level characteristics (age; length of follow-up; race; stage; lymph-node involvement; family history; receipt of chemotherapy, radiotherapy, and/or endocrine therapy; ER status; index tumor histology; multifocality/multicentricity; BRCA carrier status; and receipt of prophylactic oophorectomy) were extracted with the intention of conducting stratified subgroup analyses and meta-regression, respectively.
Statistical Analysis
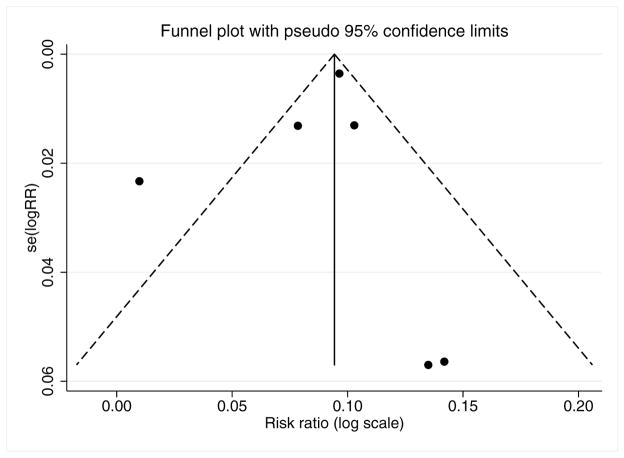
Included studies were independently assessed by two coders using a modified version of Downs and Black’s methodological quality checklist in which item 27 on sample-size calculation and power was converted into a yes/no question.27 In addition, publication bias was assessed by visual appraisal of a funnel plot, which plots the log of each study’s effect size against its standard error. If asymmetry suggesting publication bias was apparent, a Harbord test was performed, with two-tailed p<0.05 considered significant.
Relative risk (RR) was selected as the primary meta-analytic measure of association because not having individual patient follow-up time precluded calculation of hazard ratios. Risk difference (RD), i.e., absolute risk reduction, was also calculated. Statistical heterogeneity across trials was tested by Cochran’s Q statistic. An alpha value of 0.5 was taken to indicate between-trial heterogeneity, which is represented by I2 values. Fixed-effects models were used for meta-analyses in which there was no evidence of between-study heterogeneity, while random-effects models were used when there was significant heterogeneity. Meta-analyses were conducted to calculate pooled RRs and RDs for OS, BCM, incidence of MCBC, and incidence of DMR. Random-effects meta-analysis of proportions was used to calculate pooled incidence of SCBC.
Stratified subgroup and bivariate meta-regression analyses were conducted for all potential confounders for which there were at least two (for stratification) or three (for meta-regression) studies with sufficient data for analysis. We report pooled RRs and RDs for OS, BCM, MCBC, and DMR and pooled proportions for SCBC with 95% confidence intervals (CI) at two-tailed p<0.05 significance. Statistical analyses were conducted in STATA 12 (Stata, College Station, Texas).
RESULTS
Study selection
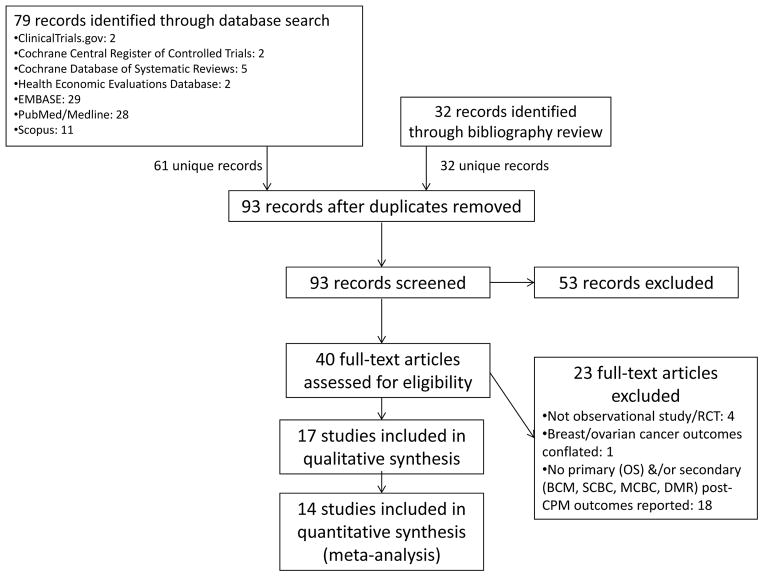
The initial database search yielded 79 reports, reduced to 61 after de-duplication (Figure 1). Abstracts for all 61 reports were screened, and 8 studies were initially selected as potentially appropriate for meta-analysis inclusion.6,16,17,19,21,24,28,29 An additional 14 studies were selected for bibliographic review,1,8,25,30–40 for a total of 22 articles that were initially read in full in addition to having their bibliographies reviewed. Bibliographic review yielded 32 additional references, whose abstracts were also screened. Thus, a total of 93 abstracts (61 from the database search and 32 from bibliographic review) were screened. Of these, 53 were excluded, leaving 40 reports for full-text review. Twenty-three reports were excluded for not meeting eligibility criteria following full-text review, leaving 17 studies for qualitative synthesis. For three of these studies, we were unable to obtain primary data amenable to meta-analysis,6,22,41 leaving 14 studies for quantitative synthesis (Table 1).13,16–19,21,24,26,42–47 On the modified Downs and Black study quality and bias assessment checklist (maximum score 28), the median score for included studies was 18 (range 14–22). Inter-rater reliability (i.e., Kappa score) was substantial at 0.8. Only one study had an a priori sampling strategy19 designed to optimize statistical power, and none were RCTs. A funnel plot representing potential publication bias among the studies included in the OS meta-analysis was fairly symmetric (Figure 2), and the Harbord test indicated no significant publication bias (p=0.627).
Figure 1.
Flow diagram of articles screened and selected for meta-analysis
Table 1.
Studies included in Contralateral Prophylactic Mastectomy (CPM) Meta-Analysis
| Study | Year | Country | Study Design | Data Source | CPM N= (F/U) | No CPM N= (F/U) | Year Range | Meta-Analyses |
|---|---|---|---|---|---|---|---|---|
| Babiera et al.42 | 1997 | United States | Retrospective cohort | M.D. Anderson Cancer Center, Houston, TX | 18 (52 mos) a | 115 (70 mos) a | 1978–1993 d | MCBC, DMR |
| Bedrosian et al.17 | 2010 | United States | Retrospective cohort | SEER | 8748 (47 mos) a | 95,283 (47 mos) a | 1998–2003 d | OS, BCM |
| Boughey et al.43 | 2006 | United States | Retrospective cohort | M.D. Anderson Cancer Center, Houston, TX | 382 (NR) | - | 2001–2005 e | SCBC |
| Boughey et al.18 | 2010 | United States | Case-control | Mayo Clinic, Rochester, MN | 385 (18 yrs) a | 385 (16.4 yrs) a | 1971–1993 e | OS, BCM, MCBC, DMR |
| Goldflam et al.44 | 2004 | United States | Case series | M.D. Anderson Cancer Center, Houston, TX | 239 (7.8 yrs) b | - | 1987–1997 e | SCBC |
| Herrinton et al.19 | 2005 | United States | Retrospective cohort | Cancer Research Network | 908f; 1072g (5.7 yrs) a | 46,368f; 317g (4.8 yrs) a | 1979–1999 d | OS, BCM, SCBC, MCBC |
| Kiely et al.21 | 2010 | Australia/New Zealand | Prospective cohort | kConFab | 154 (8 yrs) b | 864 (11.7 yrs) b | Up to 2008 e | OS, SCBC, MCBC |
| King et al. – JCO26 | 2011 | United States | Retrospective cohort | Memorial Sloan-Kettering Cancer Center, New York, NY | 407 (4.4 yrs) a,c | 2572 (6.8 yrs) a,c | 1997–2005 e | OS, MCBC, DMR |
| King et al. – A Surg45 | 2011 | United States | Case series | 407 (4.4 yrs) a | - | SCBC | ||
| McDonnell et al.13 | 2001 | United States | Case series | Mayo Clinic, Rochester, MN | 745 (10 yrs) a | - | 1960–1993 d | SCBC |
| Metcalfe et al.23 | 2004 | Canada/United States | Retrospective cohort | The Hereditary Breast Cancer Clinical Study Group | 146 (9.2 yrs) b | 336 (9.2 yrs) b | 1975–2000 d | MCBC |
| Montgomery et al.47 | 1999 | United States | Convenience sample | National Prophylactic Mastectomy Registry | 296 (4.9 yrs) a | - | 1954–1998 e | SCBC |
| Peralta et al.16 | 2000 | United States | Retrospective cohort | City of Hope National Medical Center, Duarte, CA | 64 (6.8 yrs) b | 182 (6.8 yrs) b | 1973–1998 e | SCBC, MCBC, DMR |
| Van Sprundel et al.24 | 2005 | The Netherlands | Retrospective cohort | Leiden University Medical Center; The Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam | 79 (7.4 yrs) b | 69 (10.5 yrs) b | Up to 1993 d | OS, BCM, SCBC, MCBC, DMR |
Median;
Mean;
For overall survival meta-analysis, minimum follow-up time data ≥ 2 years, median=6.1 years; CPM n=392, no CPM n=2521;
dates of diagnosis;
dates of procedure;
OS, BCM;
SCBC, MCBC
A Surg, Annals of Surgery; BCM, breast-cancer-specific mortality; CA, California; DMR, distant/metastatic recurrence; F/U, mean/median follow-up time; ILR, ipsilateral locoregional recurrence; JCO, Journal of Clinical Oncology; kConFab, Kathleen Cunningham Foundation consortium for Research into Familial Breast Cancer; MCBC, metachronous contralateral prophylactic mastectomy; MD, Maryland; MN, Minnesota; mos, months; NR, not reported; NY, New York; OS, overall survival; SCBC, synchronous contralateral breast cancer; SEER, Surveillance, Epidemiology, and End Results database; TX, Texas; yrs, years
Figure 2.
Funnel plot assessing publication bias in overall survival meta-analysis
Results of meta-analyses (Table 2)
Table 2.
Summary of Meta-Analysis Results
| Analysis | Outcome | Measure | Point Estimate | 95% CI | p value | No. of Studies | CPM (n=) | No CPM (n=) |
|---|---|---|---|---|---|---|---|---|
| All studies17–19,21,24,26 | OS | RR | 1.09 | 1.06, 1.11 | <0.001 | 6 | 10,666 | 145,490 |
| RD | 7.4% | 5.6%, 9.3% | <0.001 | |||||
|
| ||||||||
| FGR – Elevated18,21,24 | RR | 1.09 | 0.97, 1.24 | 0.157 | 3 | 618 | 1318 | |
| RD | 6.6% | −1.2%, 14.3% | 0.096 | |||||
|
| ||||||||
| FGR – Varying17,19,26 | RR | 1.10 | 1.09, 1.11 | <0.001 | 3 | 10,048 | 144,172 | |
| RD | 8.4% | 7.8%, 8.9% | <0.001 | |||||
|
| ||||||||
| All studies17–19,24 | BCM | RR | 0.69 | 0.56, 0.85 | 0.001 | 4 | 10,120 | 142,105 |
| RD | −3.5% | −4.0%, −3.0% | <0.001 | |||||
|
| ||||||||
| FGR – Elevated18,24 | RR | 0.66 | 0.27, 1.64 | 0.283 | 2 | 464 | 454 | |
| RD | −4.2% | −9.5%, 1.1% | 0.123 | |||||
|
| ||||||||
| FGR – Varying17,19 | RR | 0.63 | 0.56, 0.70 | <0.001 | 2 | 9656 | 141,651 | |
| RD | −3.5% | −4.0%, −3.0% | <0.001 | |||||
|
| ||||||||
| All studies13,16,19,21,24,26,43,44,47 | SCBC | Rate | 4.8% | 3.4%, 6.2% | - | 9 | 3438 | - |
| FGR – Elevated21,24 | 5.7% | 1.8%, 9.6% | - | 2 | 233 | - | ||
| FGR – Varying13,16,19,43–45,47 | 4.8% | 3.2%, 6.3% | - | 7 | 3205 | - | ||
|
| ||||||||
| All studies16,18,19,21,23,24,26,42 | MCBC | RR | 0.04 | 0.02, 0.08 | <0.001 | 8 | 2325 | 4840 |
| RD | −18.0% | −42.0%, 5.9% | 0.118 | |||||
|
| ||||||||
| FGR – Elevated18,21,23,24 | RR | 0.04 | 0.02, 0.09 | <0.001 | 4 | 764 | 1654 | |
| RD | −24.0% | −35.6%, −12.4% | 0.013 | |||||
|
| ||||||||
| FGR – Varying16,19,26,42 | RR | 0.08 | 0.01, 0.46 | 0.005 | 4 | 1561 | 3186 | |
| RD | −11.1% | −5.9%, 37% | 0.240 | |||||
|
| ||||||||
| All studies16,18,24,26,42 | DMR | RR | 0.64 | 0.51, 0.81 | <0.001 | 5 | 953 | 3323 |
| RD | −4.9% | −7.2%, −2.6% | <0.001 | |||||
|
| ||||||||
| FGR – Elevated18,24 | RR | 0.71 | 0.53, 0.94 | 0.018 | 2 | 464 | 454 | |
| RD | −5.9% | −10.7%, −1.0% | 0.017 | |||||
|
| ||||||||
| FGR – Varying16,26,42 | RR | 0.58 | 0.40, 0.83 | 0.003 | 3 | 489 | 2869 | |
| RD | −4.4% | −6.7%, −2.0% | <0.001 | |||||
BCM, breast-cancer-specific mortality; CI, confidence interval; CPM, contralateral prophylactic mastectomy; DMR, distant/metastatic recurrence; FGR, familial/genetic risk; OS, overall survival; MCBC, metachronous contralateral breast cancer; SCBC, synchronous contralateral breast cancer; RD, risk difference; RR, relative risk
Overall survival (OS)
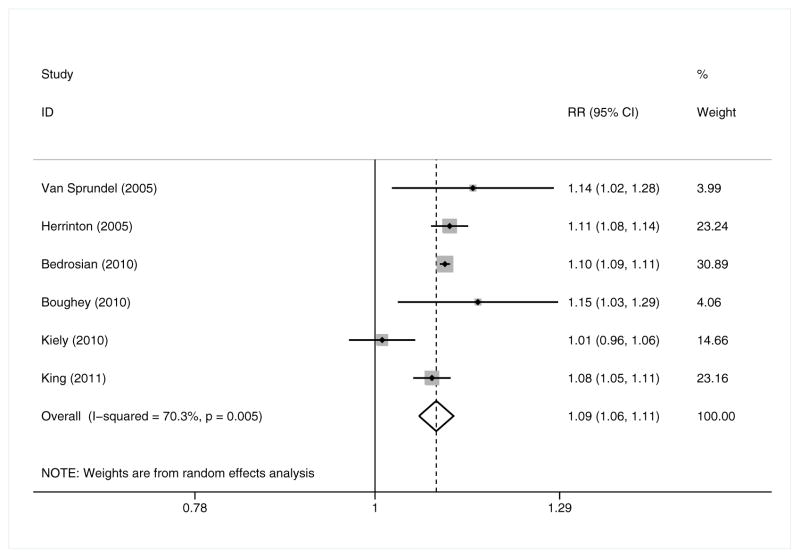
In random-effects meta-analysis (6 studies; CPM n = 10,666, no CPM n = 145,490; Figure 3), OS was 9% more likely for CPM recipients than for those who did not undergo CPM (RR = 1.09 [95% CI 1.06, 1.11, p<0.001]), and seven more UBC patients out of every 100 might be expected to survive if they received CPM (RD = 7.4% [95% CI 5.6%, 9.3%, p<0.001].17–19,21,24,26 Of the six studies included, only one – Kiely et al.21 – had an individual effect size that was not statistically significant, and it was one of only two studies in this meta-analysis – the other being Van Sprundel et al.24 – conducted outside the US.
Figure 3.
Overall survival – Relative Risk
Breast-cancer specific mortality (BCM)
In random-effects meta-analysis (4 studies; CPM n = 10,120, no CPM n = 142,105), CPM recipients had a BCM rate that was 31% lower than that of patients who did not undergo CPM (RR = 0.69 [95% CI 0.56, 0.85, p=0.001]).17–19,24 Out of 100 women with UBC, an additional 3 to 4 might not die of breast cancer if they received CPM (RD = −3.5% [95% CI −4.0, −3.0%, p<0.001]). Two (Bedrosian et al. and Herrinton et al.) of the four included studies had individual BCM rates that were significantly lower among CPM recipients, and both of these studies attempted to control for confounders – including receptor status, non-surgical treatments received, and family history – that might affect interpretation of post-CPM outcomes.17,19
Synchronous contralateral breast cancer (SCBC)
The pooled incidence of SCBC among CPM recipients was 4.8% (9 studies; CPM = 3438; 95% CI 3.4, 6.2%)13,16,19,21,24,43–45,47 and is comparable to recent estimates of SCBC as reported by King et al. (5.9%)45 and Chung et al. (6.6%).6
Metachronous contralateral breast cancer (MCBC)
In random-effects meta-analysis (8 studies; CPM n = 2325, no CPM n = 4840), CPM was associated with a 96% reduction in MCBC (RR = 0.04 [95% CI 0.02, 0.08, p<0.001]).16,18,19,21,23,24,26,42 Notably, CPM was not associated with an absolute reduction in the risk of MCBC incidence (RD = −18.0% [95% CI −42.0%, 5.9%, p=0.118]).
Distant/metastatic recurrence (DMR)
In fixed-effects meta-analysis (5 studies; CPM n = 953, no CPM n = 3323), DMR was found to be 36% less likely in CPM patients (RR = 0.64 [95% CI 0.51, 0.81, p<0.001]), and approximately 5 more women out of 100 might be expected to avoid DMR by undergoing CPM (RD = −4.9% [95% CI −7.2%, −2.6%, p<0.001]).16,18,24,26,42 Only two18,26 of the five studies in this meta-analysis had a statistically significant association between CPM and DMR, though all five studies had the same directionality of effect. King and colleagues found that the decrease in DMR they observed was attenuated and rendered insignificant when they adjusted for age and treatments received.26
Stratified subgroup analysis: familial/genetic risk (FGR) – BRCA carrier status and family history
We conducted subgroup analyses in which we separated both the two studies – Metcalfe et al.23 and Van Sprundel et al.24 – in which all patients were BRCA mutation carriers as well as the two studies – Boughey et al. (2010)18 and Kiely et al.21 – in which all patients had a family history positive for breast cancer and analyzed these four studies together to account for the higher familial/genetic risk (FGR) these patients have for CBC (synchronous and metachronous)13,48,49 and the greater benefit these patients might concomitantly derive from CPM. In the study by Boughey and colleagues, all study participants had a parent, sibling, or second-degree relative with breast cancer18 while all the participants in Kiely et al.’s study were members of families enrolled in the Kathleen Cunningham Foundation Consortium for Research into Familial Breast Cancer (kConFab), a collaborative research registry of Australian and New Zealander families with multiple cases of breast cancer.21
In stratified meta-analysis, CPM was not associated with improved OS when looking at the studies in which all patients had elevated FGR (RR = 1.09 [95% CI 0.97, 1.24, p=0.157]; RD = 6.6% [95% CI −1.2%, 14.3%, p=0.096]; 3 studies; CPM n = 618, no CPM n = 1318)18,21,24 but continued to be associated with significantly improved survival in the studies including patients with varying levels of FGR (RR = 1.10 [95% CI 1.09, 1.11, p<0.001]; RD = 8.4% [95% CI 7.8%, 8.9%, p<0.001]; 3 studies; CPM n = 10,048, no CPM n = 144,172).17,19,26 Likewise, BCM was not significantly decreased among studies in which all patients had elevated FGR (RR = 0.66 [95% CI 0.27, 1.64, p=0.283]; RD = −4.2% [95% CI −9.5%, 1.1%, p=0.123]; 2 studies; CPM n = 464, no CPM n = 454),18,24 but for the studies with patients of all FGR levels, CPM continued to be associated with decreased BCM (RR = 0.63 [95% CI 0.56, 0.70, p<0.001]; RD = −3.5% [95% CI −4.0%, −3.0%, p<0.001]; 2 studies; CPM n = 9656, no CPM n = 141,651).17,19
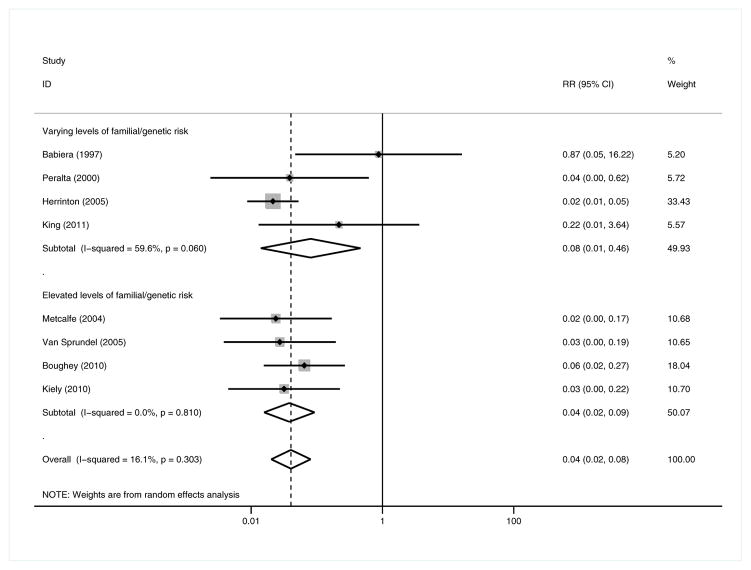
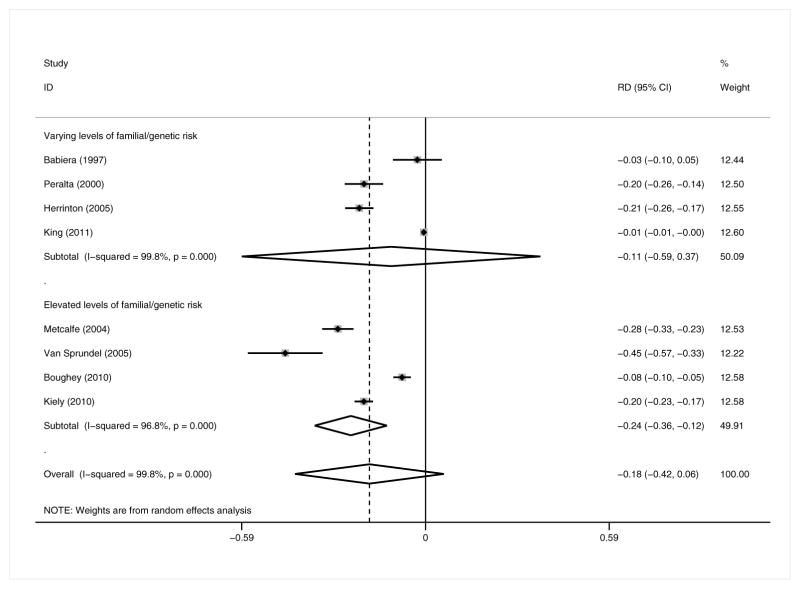
In contrast to the full analysis, both the relative and absolute risks of MCBC incidence (Figures 4 and 5) were significantly decreased among patients with elevated FGR (RR = 0.04 [95% CI 0.02, 0.09, p<0.001]; RD = −24.0% [95% CI −35.6%, −12.4%, p=0.013]; 4 studies; CPM n=764, no CPM n=1654),18,21,23,24 but only the RR of MCBC was significantly decreased among patients of all risk levels (RR = 0.08 [95% CI 0.01, 0.46, p=0.005]; RD = −11.1% [95% CI −5.9%, 37%, p=0.240]; 4 studies; CPM n=1561, no CPM n=3186).16,19,26,42 The effect size and directionality of the pooled measures for SCBC and DMR did not appreciably differ in this subgroup analysis from those of the full meta-analysis.
Figure 4.
Metachronous contralateral breast cancer: elevated familial/genetic risk subgroup analysis – Relative Risk
Figure 5.
Metachronous contralateral breast cancer: elevated familial/genetic risk subgroup analysis – Risk Difference
Meta-regression
Bivariate meta-regression analyses demonstrated no significant covariate effect on outcomes (Appendix 2).
DISCUSSION
Summary and contextualization of main results
In our full meta-analysis, CPM was associated with significant relative and absolute increases in the OS of recipients when compared to non-recipients. CPM was also associated with lower relative and absolute risks of BCM and DMR. But while CPM was associated with a decreased relative risk of developing MCBC, it was not associated with an absolute reduction in MCBC risk. In stratified meta-analysis with subgroup divisions based on whether or not participants’ had elevated FGR, results differed from those of the full analysis. Among patients with varying levels of FGR, CPM continued to be associated with improved OS, BCM, and DMR, and was associated with a relative but not an absolute reduction in MCBC risk. Among patients with known elevated FGR, however, there was no relative or absolute improvement in OS or BCM, but there were significant reductions in the relative and absolute risks of both MCBC and DMR among CPM recipients relative to non-recipients.
Although our primary outcome was OS, we focus our discussion on MCBC because we suspect it is less likely to be confounded than any of the other comparative measures in our analysis. There are many more known and unknown covariates – including but not limited to aspects of a patient’s health that are unrelated to her having breast cancer – associated with a breast-cancer patient’s likelihood of survival than there are with a patient’s likelihood of developing another breast cancer. Furthermore, a number of the demographic characteristics known to confer a pro-survival selection bias on patients undergoing CPM (e.g., noninvasive histology)26 may not or only to a lesser extent impact the odds of a patient’s going on to experience MCBC. Finally, through stratification, we controlled for family history and BRCA carrier status, two covariates that are well-documented risk factors for both index and metachronous breast cancers,50 and this subgroup analysis enabled a more insightful understanding of the impact of CPM on MCBC.
In our full meta-analysis, there was no significant decrease in the absolute risk of MCBC incidence when comparing CPM recipients and non-recipients with varying levels of FGR for MCBC. This finding is critically important: it confirms that the risk of MCBC in UBC patients, regardless of whether they undergo CPM or not, is very low at baseline, and it strongly suggests that the decreased rates of mortality observed when comparing CPM recipients to non-recipients in the general population are not attributable to a treatment-derived decrease in MCBC incidence, but rather to other covariates unrelated to the decrease in MCBC risk that CPM would directly provide. This finding is further supported by the results of the subgroup analysis in which we stratified studies according to whether or not they exclusively included patients with known elevated FGR. It is well-established that UBC patients with carcinogenic genetic mutations and/or family histories of breast cancer are significantly more likely to develop MCBC,13,14 and it was only among these high-risk patients that we saw a significant decrease in both the relative and absolute risks of MCBC following CPM, thus implying that, based on our analysis, CPM should be limited to this subset of UBC patients, if performed at all.
DMR was still found to be lower among CPM recipients in the subgroup with elevated FGR, implying that the CPM-associated decrease in MCBC may significantly prevent metastasis from these subsequent cancers but does not improve OS. This apparent discrepancy may reflect selection bias, with CPM recipients’ having less aggressive tumor biology at baseline. But the evidence regarding CPM receipt and index tumor biology in this cohort of women is contradictory. There is evidence that larger tumor size is associated with choosing to undergo CPM,6,9,51 perhaps because larger tumors predispose patients to choose mastectomy over breast conservation therapy (BCT) and women are more likely to undergo CPM if they are already getting a therapeutic mastectomy.6 Furthermore, there is some evidence that women with elevated FGR are actually more likely to have biologically aggressive primary breast cancers that are more likely to recur, metastasize, and result in death and that they are also at increased risk for other extramammary malignancies.48 But other studies have reported an association between small tumor size and receipt of CPM.26 Notably, women with elevated FGR are more likely to undergo radiographic surveillance – including magnetic resonance imaging (MRI) of the breast – that leads to diagnosis of index tumors at earlier stages.52 Accordingly, these women may actually have less advanced disease at diagnosis than CPM recipients without known elevated FGR,52 their index tumors may be less likely to spread, and these women would appear to have lower DMR without a significant difference in OS relative to non-recipients. However, we suspect that the observed discrepancy between CPM’s association with DMR and OS among patients with elevated FGR is due to the increasingly non-lethal nature of breast cancer, even after regional or (to a lesser extent) distant disease extension. Over time, stage for stage, fewer women are dying of breast cancer as a result of improved treatments.53 Hence, diagnosis with advanced disease is less likely than it once was to be associated with death such that we might see CPM have an advantageous effect on MCBC and concomitantly on DFS but not on OS.
Quality of evidence
A meta-analysis is only as good as its constitutive studies, which ideally would be RCTs. None of the studies in this meta-analysis were RCTs because none have been conducted on CPM. In our assessment of overall study quality, the 14 studies included in our meta-analysis were of moderate methodological rigor for the purposes of quantitative synthesis. A number of the studies in our meta-analysis made significant efforts to reduce selection bias due to confounders, but we recognize that selection bias is the greatest hindrance to interpreting our results, and we anticipated this issue by using meta-regression and stratification. Bivariate meta-regression failed to demonstrate any significant confounding, though we recognize that these analyses may have been underpowered due to each one’s having a small number of observations. However, our stratified subgroup analysis was very illuminating, as it revealed the extent to which CPM-associated differences in outcome could or could not be ascribed to changes in MCBC incidence.
We would have wished to be able to analyze other patient-specific and disease-specific characteristics that might place patients at significantly lower or increased risk for survival and/or CBC incidence, but by virtue of the data we were able to extract, our stratified analysis was limited to patients with either known BRCA carrier status or a documented family history of breast cancer, who we grouped together as having elevated FGR for both primary and secondary breast cancer incidence. Clinical prediction models such as the Gail54 and Tyrer-Cuzick (i.e., International Breast Cancer Intervention Study [IBIS])55 models provide fuller assessments of breast-cancer risk than simply the binary characteristic of having a family history of breast cancer or not, but these models were not consistently utilized in the studies we ultimately included in this review.
Some might argue that individual patient data (IPD) would be required to enable confounder adjustment through multivariate regression, propensity scores, or instrumental variables. However, we suspect that the benefits of a pooled analysis using IPD from currently available CPM studies would be limited for a number of reasons. First of all, it is not always the case that IPD analysis leads to different conclusions from study-level meta-analysis, especially for models looking at overall estimates of exposure/treatment (in this case, CPM) effects.56 Thus, it is entirely possible that the benefits of IPD – adjustment for confounders, better time-to-event analysis – might not outweigh the significant costs in personnel and time. Furthermore, our meta-regression analyses suggest that using IPD from existing studies might not solve the problem of confounding because many of the covariates (e.g., socioeconomic status39 and surgeon gender9) impacting who among breast-cancer patients receives and benefits from CPM will only rarely and inconsistently have been recorded. Finally, several of the included studies used data from premiere medical centers dedicated to the treatment of cancer, though in reality most patients in the US and throughout the world receive their oncologic surgery through generalists. Thus, the benefits observed in our meta-analysis must be considered with the understanding that realization of said benefits depends not only on who receives CPM but also by whom and in what medical context it is performed.
Implications for practice
In short, CPM decreases MCBC incidence in breast-cancer patients with BRCA mutations and/or family histories of breast cancer, both of which place women at increased risk for MCBC, but CPM does not appear to confer a survival benefit even within this subset of patients. Among breast-cancer patients not otherwise at high risk for MCBC, the improvement observed in OS and BCM is likely secondary to selection bias, as CPM recipients may be more likely to have other characteristics – adequate health insurance,39,57 early-stage tumors26 – that strongly correlate with improved survival; furthermore, the risk of MCBC in most UBC patients is already low and likely does not warrant the morbidity of CPM. We recommend that UBC patients without known elevated FGR be advised against CPM, while patients with elevated FGR should be advised that while CPM would significantly decrease their risk of MCBC, it is unlikely to prolong their lives.
We do not claim that UBC patients with elevated FGR constitute the only type of high-risk group that might benefit from CPM, but it is possible that the conclusions reached regarding this group of women could also be extended after further study to women who, for other reasons, are at high risk for MCBC. Accordingly, a critical next step in evidence-based clinical practice is refinement of our criteria as to who is at high risk for MCBC. Some of the studies in our meta-analysis were limited to early-stage patients18,23,44 and amongst all of the studies, the racial and/or ethnic diversity of included studies’ participants – if reported at all – was very low. Given that CBC is more common in women with late-stage tumors58,59 and in women of African ancestry,60 the benefits of CPM in these populations could be significant but are, as of yet, underexplored. CPM is also more commonly chosen by women with health insurance,39,57 so it will be important to assess the extent to which implementation of the Affordable Care Act in the US impacts the demographics of who gets CPM and the extent to which they realize its potential benefits, particularly if access to CPM alternatives (e.g., MRI surveillance) is significantly affected by insurance type.
Improvements in breast-cancer treatment and diagnosis should also mitigate use of CPM. For example, UBC patients treated with tamoxifen, trastuzumab, or other receptor-targeted therapy for their index breast cancers not only decrease their chances of BCM but also simultaneously treat any occult malignancy or atypia that might exist in contralateral breast tissue.61–65 Furthermore, index tumors, SCBCs, and MCBCs are all increasingly likely to be diagnosed at earlier stages as a result of higher rates of screening participation,66 improvements in mammography, and increased utilization of breast MRI,67–73 and these early-stage tumors are less likely to be associated with recurrence and death.74–77 Currently, the Society of Surgical Oncology (US) only recommends CPM be considered for breast-cancer patients in whom contralateral surveillance would be difficult, post-reconstructive breast symmetry would be improved, and risk reduction would be significant secondary to strong family history, known predisposing genetic mutations, and/or biopsy-proven high-risk pathology (atypical ductal hyperplasia, atypical lobular hyperplasia, and lobular carcinoma in situ).4 We concur that CPM should be limited to patients at high risk for MCBC, but we suspect our current assessment of risk will need to be broadened to incorporate other clinical characteristics – including tumor grade and molecular subtype – at the same time as we collectively refine physicians’ counseling of patients.
Physician recommendations have been repeatedly demonstrated to have a significant impact on patients’ decision to undergo CPM.33,47 As a profession, we are guilty of providing breast-cancer patients with ever increasing amounts of information but with insufficient contextualization regarding their options for treatment and postsurgical cosmesis. CPM is not costless. While many women report satisfaction with both CPM and their post-mastectomy reconstructions, others have reported post-CPM issues with self-esteem, body image, and mental and sexual health.41,47,78–82 Physicians in general, and surgeons in particular, must work to educate patients as to their individual risk of CBC and to better inform them of the costs and benefits of CPM as well as alternatives to CPM such as MRI surveillance and pharmaceutical prophylaxis.
Implications for research
We have hypothesized that the improvement in OS and other outcomes seen in our full meta-analysis is secondary to confounding, but we recognize that there may be other unexplored reasons. For example, while young women with breast cancer might have better overall health at baseline and are more likely to choose CPM than not, young age at diagnosis is actually a negative prognosticator with regards to breast cancer, with younger women having worse survival and clinicopathologic features compared with older women.83 However, young CPM recipients are also more likely to have family histories of breast cancer9 and to have had more screening and diagnostic imaging prior to diagnosis,6 so they might actually have lower stage disease than other young breast-cancer patients. Accordingly, one cannot be sure to what extent all of these epidemiologic factors might interact in predicting the benefit or harm of CPM in this population. Given the low probability of a future RCT on CPM, prospective, population-based studies akin to that conducted by Kiely and colleagues21 would be helpful to interrogate post-CPM outcomes and the interaction between baseline FGR, age at diagnosis, the levels of radiographic surveillance and prophylactic therapy (e.g., tamoxifen, oophorectomy) patients with elevated FGR receive, and the extent to which these levels vary regionally and internationally. We encourage the collection of CPM data in prospectively maintained databases such as kConFab’s21 and hope that such databases will be increasingly inclusive of women from all racial, ethnic, and socioeconomic backgrounds.
In conclusion, CPM may hold benefits for UBC patients with elevated FGR for MCBC, but based on the findings of our meta-analysis, its use is not justified in breast-cancer patients not otherwise at elevated risk for developing MCBC. And even among the subset of high-risk patients with elevated FGR, CPM is associated with decreased MCBC incidence but not with improved survival. The temporal and financial challenges of conducting an RCT on CPM will likely preclude one from ever being conducted; post-enrollment periods of at least 10 or 20 years would be required to allow for substantive accumulation of breast-cancer events, and it would be difficult, if not impossible, to achieve that degree of longitudinal observation in retrospective analyses from single or even multiple institutions.58 Accordingly, the contribution of our study, the only quantitative summation of the literature on CPM, is significant: we have demonstrated that, given the minimal decrease in MCBC risk conferred by CPM in the general population of UBC patients, CPM should not be offered to those whose FGR does not otherwise place them at high risk for MCBC.
Acknowledgments
Funding
OMF is supported by the National Institutes of Health Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant (5T32CA009621-22). GAC and CRTS are supported by the Foundation for Barnes-Jewish Hospital.
The authors would like to thank Isabelle Bedrosian and Chung-Yuan Hu of MD Anderson Cancer Center as well as Tari King and Sujata Patil of Memorial Sloan-Kettering Cancer Center for sharing their primary data with us.
Appendix 1 (Online only). PubMed database search strategy for systematic review and meta-analysis of contralateral prophylactic mastectomy
(“Women”[Mesh] OR “Female”[Mesh] OR “women” OR “woman” OR “female” OR “females”) AND (“Breast Neoplasms”[Mesh] OR “Hereditary Breast and Ovarian Cancer Syndrome”[Mesh] OR “breast carcinomas” OR “breast carcinoma” OR “Breast Neoplasms” OR “Breast Neoplasm” OR “Breast Tumors” OR “Breast Tumor” OR “Mammary Carcinomas” OR “Mammary Carcinoma” OR “Mammary Neoplasm” OR “Mammary Neoplasms” OR “Breast Cancer” OR “breast cancers” OR “Cancer of the Breast” OR “Cancer of Breast” OR “Mammary Ductal Carcinomas” OR “Mammary Ductal Carcinoma” OR “Breast Invasive Ductal Carcinoma” OR “Hereditary Breast and Ovarian Cancer Syndrome” OR “HBOC Syndrome” OR “HBOC Syndromes” OR “BRCA1” OR “BRCA2” OR “breast gland cancer” OR “breast gland neoplasm” OR “mamma cancer” OR “mammary cancer” OR “mammary gland cancer” OR “breast adenocarcinoma” OR “mammary adenocarcinoma” OR “breast carcinogenesis” OR “breast cancerogenesis” OR “mammary gland carcinogenesis” OR “breast carcinoma” OR “mamma carcinoma” OR “breast metastasis” OR “mammary gland metastasis” OR “breast tissue metastasis” OR “breast sarcoma” OR “mammary gland sarcoma” OR “mammary sarcoma” OR “cystosarcoma phylloides” OR “breast phylloid tumor” OR “cysto sarcoma phylloides” OR “cystosarcoma” OR “giant fibroadenoma” OR “phyllodes tumor” OR “phylloides tumor”) AND (“prevention and control” [Subheading] OR “prevention and control” OR “preventive therapy” OR “prophylaxis” OR “preventive measures” OR “prevention” OR “control” OR “asepsis” OR “disease eradication” OR “protection” OR “prophylactic” OR “Prophylactically”) AND (“Mastectomy”[Mesh] OR “Mastectomy” OR “Mastectomies” OR “Mammectomy” OR “Mammectomies” OR “breast amputation” OR “breast resection” OR “Halsted operation”) AND (“unilateral” AND “contralateral”) AND (“Mortality”[Mesh] OR “mortality” [Subheading] OR “Mortality” OR “Mortalities” OR “Case Fatality Rate” OR “Case Fatality Rates” OR “Death Rate” OR “Death Rates” OR “survival” OR “disease free” OR “cancer free” OR “asepsis” OR “Recurrence”[Mesh] OR “Recurrence” OR “Recurrences” OR “Relapse” OR “Relapses” OR “Recrudescence” OR “Recrudescences” OR “cancer recidive” OR “cancer regeneration”) NOT ((“Animals”[Mesh] NOT (“Animals”[Mesh] AND “Humans”[Mesh]))
Limits: English
Appendix 2 (Online only). Results of bivariate meta-regression analyses
Meta-regression analyses for the outcome OS were conducted using covariates representing age (5 studies, p=0.491), length of follow-up (5 studies, p=0.107), receipt of chemotherapy (3 studies, p=0.776), and lymph node involvement (4 studies, p=0.410). For the outcome BCM, there was only sufficient data to conduct meta-regression analyses using age (3 studies, p=0.639) and length of follow-up (3 studies, p=0.380). Meta-regression analyses were conducted for the outcome MCBC using the following covariates: age (6 studies, p=0.651); length of follow-up (7 studies; p=0.404); stage (4 studies; p=0.238); receipt of systemic (5 studies, p=0.856), endocrine (4 studies, p=0.732), and radiation therapy (3 studies, p=0.684); family history (5 studies, p=0.504); and lymph-node status (3 studies, p=0.597). For the outcome DMR, meta-regression was conducted for six covariates: age (4 studies, p=0.683); length of follow-up (4 studies, p=0.544); stage (3 studies, p=0.683); receipt of endocrine (3 studies, p=0.664) and systemic therapy (3 studies, p=0.752); and family history (3 studies, p=0.576). There were insufficient observations to enable meta-regression analysis of any outcomes examining race, ER status, index tumor histology, multifocality/multicentricity, BRCA carrier status, or receipt of prophylactic oophorectomy as covariates.
References
- 1.Tuttle T, Habermann E, Abraham A, et al. Contralateral prophylactic mastectomy for patients with unilateral breast cancer. Expert Rev Anticancer Ther. 2007;7:1117–1122. doi: 10.1586/14737140.7.8.1117. [DOI] [PubMed] [Google Scholar]
- 2.Abbott AM, Rueth NM, Kuntz KM, et al. Perceptions of contralateral breast cancer: An overestimation of risk. Ann Surg Oncol. 2011;18:S154. doi: 10.1245/s10434-011-1914-x. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry A, Sahu A. Patient request for contralateral prophylactic mastectomy is due to a false perception of increased risk at time of intial diagnosis. European Journal of Cancer, Supplement. 2010;8:126. [Google Scholar]
- 4.Giuliano AE, Boolbol S, Degnim AC, et al. Society of Surgical Oncology: Position statement on prophylactic mastectomy. Ann Surg Oncol. 2007;14:2425–2427. doi: 10.1245/s10434-007-9447-z. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MD, Lerman C, Brogan B, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22:1823–1829. doi: 10.1200/JCO.2004.04.086. [DOI] [PubMed] [Google Scholar]
- 6.Chung A, Huynh K, Lawrence C, et al. Comparison of Patient Characteristics and Outcomes of Contralateral Prophylactic Mastectomy and Unilateral Total Mastectomy in Breast Cancer Patients. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2299-1. [DOI] [PubMed] [Google Scholar]
- 7.Manson J, Brooks SE, Hembree T, et al. Exploratory study of contralateral prophylactic mastectomy in women with breast cancer: Potential role for preoperative genetic counseling. Cancer Prevention Research. 2010;3:B6. [Google Scholar]
- 8.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prevention Research. 2010;3:1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrington A, Jarosek S, Virnig B, et al. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 10.Nichols HB, Berrington de Gonzalez A, Lacey JV, et al. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29:1564–1569. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heron DE, Komarnicky LT, Hyslop T, et al. Bilateral breast carcinoma: Risk factors and outcomes for patients with synchronous and metachronous disease. Cancer. 2000;88:2739–2750. [PubMed] [Google Scholar]
- 12.Obedian E, Fischer D, Haffty B. Second malignancies after treatment of early-stage breast cancer: lumpectomy and radiation therapy versus mastectomy. J Clin Oncol. 2000;18:246–2412. doi: 10.1200/JCO.2000.18.12.2406. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell S, Schaid D, Myers J, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol. 2001;19:3938–3943. doi: 10.1200/JCO.2001.19.19.3938. [DOI] [PubMed] [Google Scholar]
- 14.Graeser MK, Engel C, Rhiem K, et al. Contralateral Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers. J Clin Oncol. 2009;27:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 15.Reiner AS, John EM, Brooks JD, et al. Risk of Asynchronous Contralateral Breast Cancer in Noncarriers of BRCA1 and BRCA2 Mutations With a Family History of Breast Cancer: A Report From the Women’s Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2013;31:433–439. doi: 10.1200/JCO.2012.43.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peralta EA, Ellenhorn JDI, Wagman LD, et al. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg. 2000;180:439–445. doi: 10.1016/s0002-9610(00)00505-5. [DOI] [PubMed] [Google Scholar]
- 17.Bedrosian I, Hu C, Chang G. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst. 2010;102:401–409. doi: 10.1093/jnci/djq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boughey J, Hoskin T, Degnim A, et al. Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol. 2010;17:2702–2709. doi: 10.1245/s10434-010-1136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23:4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 20.Jones NB, Wilson J, Kotur L, et al. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol. 2009;16:2691–2696. doi: 10.1245/s10434-009-0547-9. [DOI] [PubMed] [Google Scholar]
- 21.Kiely BE, Jenkins MA, McKinley JM, et al. Contralateral risk-reducing mastectomy in BRCA1 and BRCA2 mutation carriers and other high-risk women in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) Breast Cancer Res Treat. 2010;120:715–723. doi: 10.1007/s10549-009-0497-8. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Grant CS, Donohue JH, et al. Arguments against routine contralateral mastectomy or undirected biopsy for invasive lobular cancer. Surgery. 1995;118:640–647. doi: 10.1016/s0039-6060(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 23.Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer. 2005;93:287–292. doi: 10.1038/sj.bjc.6602703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database of Systematic Reviews. 2010;(11) doi: 10.1002/14651858.CD002748.pub3. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD002748/frame.html http://onlinelibrary.wiley.com/store/10.1002/14651858.CD002748.pub3/asset/CD002748.pdf?v=1&t=h406p1vl&s=0849c49574aa5139e9d87d6939618fc773adc340. [DOI] [PubMed]
- 26.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29:2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heemskerk-Gerritsen BAM, Hooning MJ, Jager A, et al. Is risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer beneficial with respect to distant disease free survival and overall survival? European Journal of Cancer, Supplement. 2010;8:206. [Google Scholar]
- 29.Lee JS, Grant CS, Donohue JH, et al. Arguments against routine contralateral mastectomy or undirected biopsy for invasive lobular breast cancer. Surgery. 1995;118:640–647. doi: 10.1016/s0039-6060(05)80030-3. discussion 647–648. [DOI] [PubMed] [Google Scholar]
- 30.Barry M, Sacchini V. When is contralateral mastectomy warranted in unilateral breast cancer? Expert Rev Anticancer Ther. 2011;11:1209–1214. doi: 10.1586/era.11.100. [DOI] [PubMed] [Google Scholar]
- 31.Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;16:935–941. doi: 10.1634/theoncologist.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Pena-Salcedo JA, Soto-Miranda MA, Lopez-Salguero JF. Prophylactic mastectomy: is it worth it? Aesthetic Plast Surg. 2012;36:140–148. doi: 10.1007/s00266-011-9769-x. [DOI] [PubMed] [Google Scholar]
- 33.Gershenwald JE, Hunt KK, Kroll SS, et al. Synchronous elective contralateral mastectomy and immediate bilateral breast reconstruction in women with early-stage breast cancer. Ann Surg Oncol. 1998;5:529–538. doi: 10.1007/BF02303646. [DOI] [PubMed] [Google Scholar]
- 34.Morrow M. Prophylactic contralateral surgery: Current recommendations and techniques. Breast Cancer Res. 2009;11:S4. [Google Scholar]
- 35.Nekhlyudov L, Bower M, Herrinton LJ, et al. Women’s decision-making roles regarding contralateral prophylactic mastectomy. J Natl Cancer Inst Monogr. 2005:55–60. doi: 10.1093/jncimonographs/lgi038. [DOI] [PubMed] [Google Scholar]
- 36.Newman LA, Kuerer HM, Hung KK, et al. Prophylactic mastectomy. J Am Coll Surg. 2000;191:322–330. doi: 10.1016/s1072-7515(00)00361-6. [DOI] [PubMed] [Google Scholar]
- 37.Schrag D, Kuntz KM, Garber JE, et al. Life expectancy gains from cancer prevention strategies for women with breast cancer and BRCA1 or BRCA2 mutations. JAMA. 2000;283:617–624. doi: 10.1001/jama.283.5.617. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle TM, Abbott A, Arrington A, et al. The increasing use of prophylactic mastectomy in the prevention of breast cancer. Curr Oncol Rep. 2010;12:16–21. doi: 10.1007/s11912-009-0070-y. [DOI] [PubMed] [Google Scholar]
- 39.Yao K, Stewart AK, Winchester DJ, et al. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. 2010;17:2554–2562. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 40.Zendejas B, Moriarty JP, O’Byrne J, et al. Cost-effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. J Clin Oncol. 2011;29:2993–3000. doi: 10.1200/JCO.2011.35.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frost M, Slezak JM, Tran N, et al. Satisfaction after contralateral prophylactic mastectomy: The significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol. 2005;23:7849–7856. doi: 10.1200/JCO.2005.09.233. [DOI] [PubMed] [Google Scholar]
- 42.Babiera GV, Lowy AM, Davidson BS, et al. The role of contralateral prophylactic mastectomy in invasive lobular carcinoma. The Breast Journal. 1997;3:2–6. [Google Scholar]
- 43.Boughey J, Khakpour N, Meric-Bernstam F, et al. Selective use of sentinel lymph node surgery during prophylactic mastectomy. Cancer. 2006;107:1440–1447. doi: 10.1002/cncr.22176. [DOI] [PubMed] [Google Scholar]
- 44.Goldflam K, Hunt KK, Gershenwald JE, et al. Contralateral Prophylactic Mastectomy Predictors of Significant Histologic Findings. Cancer. 2004;101:1977–1986. doi: 10.1002/cncr.20617. [DOI] [PubMed] [Google Scholar]
- 45.King T, Gurevich I, Sakr R, et al. Occult malignancy in patients undergoing contralateral prophylactic mastectomy. Ann Surg. 2011;254:2–7. doi: 10.1097/SLA.0b013e3182125b26. [DOI] [PubMed] [Google Scholar]
- 46.Metcalfe KA, Lubinski J, Ghadirian P, et al. Predictors of Contralateral Prophylactic Mastectomy in Women With a BRCA1 or BRCA2 Mutation: The Hereditary Breast Cancer Clinical Study Group. J Clin Oncol. 2008;26:1093–1097. doi: 10.1200/JCO.2007.12.6078. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery L, Tran K, Heelan M, et al. Issues of regret in women with contralateral prophylactic mastectomies. Ann Surg Oncol. 1999;6:546–552. doi: 10.1007/s10434-999-0542-1. [DOI] [PubMed] [Google Scholar]
- 48.Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998;351:316–321. doi: 10.1016/s0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 49.Verhoog LC, Brekelmans CTM, Seynaeve C, et al. Survival in Hereditary Breast Cancer Associated With Germline Mutations of BRCA2. J Clin Oncol. 1999;17:3396–3402. doi: 10.1200/JCO.1999.17.11.3396. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Thompson W, Semenicw R, et al. Epidemiology of contralateral breast cancer. Cancer Epidemiology, Biomarkers, and Prevention. 1999;8:855–861. [PubMed] [Google Scholar]
- 51.Tuttle T, Habermann E, Grund E, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 52.Warner E, Hill K, Causer P, et al. Prospective Study of Breast Cancer Incidence in Women With a BRCA1 or BRCA2 Mutation Under Surveillance With and Without Magnetic Resonance Imaging. J Clin Oncol. 2011;29:1664–1669. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buzdar A, Hunt K, Buchholz TA, et al. Improving survival of patients with breast cancer over the past 6 decades: The University of Texas M. D. Anderson Cancer Center experience. American Society of Clinical Oncology (ASCO) Breast Cancer Symposium; 2 October 2010; 2010. [Google Scholar]
- 54.Gail MH, Brinton LA, Byar DP, et al. Projecting Individualized Probabilities of Developing Breast Cancer for White Females Who Are Being Examined Annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 55.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 56.Olkin I, Sampson A. Comparison of Meta-Analysis Versus Analysis of Variance of Individual Patient Data. Biometrics. 1998;54:317–322. [PubMed] [Google Scholar]
- 57.McLaughlin C, Lillquist P, Edge S. Surveillance of prophylactic mastectomy: trends in use from 1995 through 2005. Cancer. 2009;115:5404–5412. doi: 10.1002/cncr.24623. [DOI] [PubMed] [Google Scholar]
- 58.Khan SA. Contralateral Prophylactic Mastectomy: What Do We Know and What Do Our Patients Know? J Clin Oncol. 2011;29:2132–2135. doi: 10.1200/JCO.2010.33.4482. [DOI] [PubMed] [Google Scholar]
- 59.Vichapat V, Garmo H, Holmqvist M, et al. Tumor stage affects risk and prognosis of contralateral breast cancer: results from a large Swedish-population-based study. J Clin Oncol. 2012;30:3478–3485. doi: 10.1200/JCO.2011.39.3645. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Fisher S, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 61.Bertelsen L, Bernstein L, Olsen JH, et al. Effect of Systemic Adjuvant Treatment on Risk for Contralateral Breast Cancer in the Women’s Environment, Cancer and Radiation Epidemiology Study. J Natl Cancer Inst. 2008;100:32–40. doi: 10.1093/jnci/djm267. [DOI] [PubMed] [Google Scholar]
- 62.Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer Causes Control. 2012;118:1982–1988. doi: 10.1002/cncr.26484. [DOI] [PubMed] [Google Scholar]
- 63.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. The Lancet. 2012;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 64.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 65.Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136:627–633. doi: 10.1007/s10549-012-2318-8. [DOI] [PubMed] [Google Scholar]
- 66. [Accessed 30 November 2012];Disparities in Breast Cancer Screening. 2012 http://ww5.komen.org/BreastCancer/RacialEthnicIssuesinScreening.html.
- 67.Bedrosian I, Mick R, Orel S, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468–473. doi: 10.1002/cncr.11490. [DOI] [PubMed] [Google Scholar]
- 68.Biglia N, Bounous V, Martincich L, et al. Role of MRI (magnetic resonance imaging) versus conventional imaging for breast cancer presurgical staging in young women or with dense breast. Eur J Surg Oncol. 2011;37:199–204. doi: 10.1016/j.ejso.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Bilimoria K, Cambic A, Hansen N, et al. Evaluating the impact of preoperative breast magnetic resonance imaging on the surgical management of newly diagnosed breast cancers. Arch Surg. 2007;142:441–445. doi: 10.1001/archsurg.142.5.441. [DOI] [PubMed] [Google Scholar]
- 70.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI Evaluation of the Contralateral Breast in Women with Recently Diagnosed Breast Cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 71.Slanetz P, Edmister W, Yeh E, et al. Occult contralateral breast carcinoma incidentally detected by breast magenetic resonance imaging. Breast J. 2002;8:145–148. doi: 10.1046/j.1524-4741.2002.08304.x. [DOI] [PubMed] [Google Scholar]
- 72.Smith R. The evolving role of MRI in the detection and evaluation of breast cancer. N Engl J Med. 2007;356:1364–1364. doi: 10.1056/NEJMe078006. [DOI] [PubMed] [Google Scholar]
- 73.Sorbero M, Dick A, Beckjord E, et al. Diagnostic breast magnetic resonance imaging and contralateral prophylactic mastectomy. Ann Surg Oncol. 2009;16:1597–1605. doi: 10.1245/s10434-009-0362-3. [DOI] [PubMed] [Google Scholar]
- 74.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic Factors in Breast Cancer. Archives of Pathology & Laboratory Medicine. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 75.Carter CL, Allen C, Henson DE. Relation of Tumor Size, Lymph Node Status, and Survival in 24,740 Breast Cancer Cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 76.Richards M, Westcombe A, Love S, et al. Influence of delay on survival in patients with breast cancer: a systematic review. The Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 77.Gentry S, Davis DL. Influencing factors for a contralateral prophylactic mastectomy. American Journal of Clinical Oncology: Cancer Clinical Trials. 2011;34:550. [Google Scholar]
- 78.Bresser P, Seynaeve C, Van Gool A, et al. Satisfaction with prophylactic mastectomy and breast reconstruction in genetically predisposed women. Plast Reconstr Surg. 2006;117:1675–1682. doi: 10.1097/01.prs.0000217383.99038.f5. [DOI] [PubMed] [Google Scholar]
- 79.Contant C, Menke-Pluijmers M, Seynaeve C, et al. Clinical experience of prophylactic mastectomy followed by immediate breast reconstruction in women at hereditary risk of breast cancer (HB(O)C) or a proven BRCA1 and BRCA2 germ-line mutation. Eur J Surg Oncol. 2002;28:627–632. doi: 10.1053/ejso.2002.1279. [DOI] [PubMed] [Google Scholar]
- 80.Geiger A, West C, Nekhlyudov L, et al. Contentment with quality of life among breast cancer survivors with and without contralateral prophylactic mastectomy. J Clin Oncol. 2006;24:1350–1356. doi: 10.1200/JCO.2005.01.9901. [DOI] [PubMed] [Google Scholar]
- 81.Schwartz MD. Contralateral prophylactic mastectomy: Efficacy, satisfaction, and regret. J Clin Oncol. 2005;23:7777–7779. doi: 10.1200/JCO.2005.08.903. [DOI] [PubMed] [Google Scholar]
- 82.Tercyak K, Peshkin B, Brogan B, et al. Quality of life after contralateral prophylactic mastectomy in newly diagnosed high-risk breast cancer patients who underwent BRCA1/2 gene testing. J Clin Oncol. 2007;25:285–291. doi: 10.1200/JCO.2006.07.3890. [DOI] [PubMed] [Google Scholar]
- 83.Anders CK, Hsu DS, Broadwater G, et al. Young Age at Diagnosis Correlates With Worse Prognosis and Defines a Subset of Breast Cancers With Shared Patterns of Gene Expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]