Abstract
Background
Protease inhibitor (PI)-based therapy is recommended as first-line treatment for HIV-infected infants exposed to nevirapine prophylaxis. However, long-term use poses adherence challenges, is associated with metabolic toxicities, limits second-line options, and is costly. We report long-term outcomes of switching nevirapine-exposed children to nevirapine-based therapy after effective suppression of virus replication on a PI-based regimen.
Methods
At one site in Johannesburg, South Africa, 195 nevirapine-exposed children under 24 months of age who achieved virologic suppression <400 copies/ml while treated with a lopinavir/ritonavir (LPV/r)-based regimen were randomized to switch to nevirapine-based therapy (n=96) or remain on the LPV/r-based regimen (n=99). Children were followed a median of 156 weeks and 3 deaths occurred in each group. HIV RNA was quantified every 3 months.
Findings
By 156 weeks post-randomization, more children in the switch group (23.9%) than in the controls (11.1%) had viral failure (p=0.01) defined as confirmed viremia >1000 copies/ml. However, all (22/22) failures in the switch group were detected by 52 weeks compared with 50% in the controls. Children in the switch group (40.5%) were more likely to suppress to <50 copies/ml (primary endpoint of study) than controls (31.3%, p=0.01) and had better CD4 and growth responses initially after switching. Viral failure was related to non-adherence and pre-treatment drug resistance. In those without pre-treatment drug resistance, no significant difference in viral failure between the switch (14.0%) and control (9.5%) groups (p=0.34) was observed.
Interpretation
Viral load testing through 52 weeks can identify all children likely to fail this PI-switch strategy. Switching children once suppressed to a nevirapine-based regimen may be a valuable treatment option provided that adequate viral load monitoring can be done.
Introduction
Public health approaches to antiretroviral therapy in resource-limited settings have relied on standardizing first- and second-line options to simplify clinical practice and maximize feasibility of programs. For children, protease inhibitor (PI)-based therapy is recommended as first-line treatment for HIV-infected infants and young children who have been exposed to nevirapine used as prophylaxis to prevent mother-to-child HIV transmission.1 This recommendation followed concern about selection of viral resistance to nevirapine after failed prohylaxis2,3 and is supported by the results of a multi-site clinical trial confirming better outcomes with ritonvir-boosted lopinavir (LPV/r)- vs. nevirapine-based therapy in prophylaxis-exposed children.4 Recent data demonstrate that LPV/r-based regimens are virologically-superior to nevirapine-based regimens as primary therapy even in unexposed infants and young children.5 Pediatric treatment recommendations also advise that all HIV-infected infants regardless of their immunologic or clinical status begin therapy due to the poor predictive value of standard markers.6 Thus potentially all newly-diagnosed infants and young children should initiate therapy with LPV/r even though this drug is considered part of second-line therapy in most national programs.
Pediatric treatment requires careful consideration of regimen sequencing as many antiretroviral drugs are not available in formulations suitable for children.7 LPV/r poses adherence challenges for young children as the only suitable formulation is a syrup with poor palatability.8 Concerns have been raised about metabolic toxicities related to long-term PI use over critical periods of child development.9–12 The drug is commonly recommended as a part of second-line regimens and indefinite continuation of LPV/r initiated routinely in infancy as part of first-line regimens, limits later use of this drug.
We conducted a randomized clinical trial of pre-emptive switching to a nevirapine-based regimen of nevirapine-exposed children initially suppressed on a LPV/r-based regimen. This strategy has several advantages, including preserving LPV/r for second-line therapy and/or for use among older children once able to consume tablets. This strategy reduces the cost of pediatric treatment given the price differential between LPV/r and other antiretroviral drugs.13 We have previously reported the results of the trial through 52 weeks post-switch.14 Here we report outcomes over three or more years through the end of the study to evaluate whether targeted virologic and/or drug resistance testing could allow for more effective implementation of the switch strategy.
Methods
Study design
Among 323 nevirapine-exposed children who met clinical and immunologic criteria for treatment when <24 months of age and who initiated a PI-based regimen, 195 were randomized to an open-label trial at one site in Johannesburg, South Africa.14 Excluded from randomization were 38 (11.8%) children who died, 40 (12.4%) who did not remain in follow-up, and 50 (15.5%) who failed to achieve and sustain viral load <400 copies/ml for at least 3 months in the first year of follow-up.15 Children were randomized: (1) to remain on the PI-based regimen (control group); or (2) to substitute nevirapine for PI (switch group). Follow-up continued to a minimum of 90 weeks and maximum of 232 weeks post-randomization. The study was approved by the Institutional Review Boards of Columbia University and the University of the Witwatersrand. The child’s guardian provided signed informed consent.
Study population
HIV-infected children <24 months of age exposed to nevirapine used for prevention of mother-to-child transmission were referred from inpatient wards and pediatric HIV clinics around Johannesburg, South Africa to a research site at Rahima Moosa Mother and Child Hospital, between 8 April 2005 and 10 July 2007. Children requiring antiretroviral therapy based on South African guidelines in place at the time16 or those who had already initiated PI-based therapy were eligible to enroll. Treatment eligibility criteria included WHO stage III or IV disease, CD4 percentage <25 if younger than 12 months or <20 if older than 12 months, or recurrent (> 2/year) or prolonged (>4 weeks) hospitalization for HIV-related complications.16 Children requiring acute treatment for opportunistic infections (except tuberculosis) or tumors were excluded. For most children (n=254) treatment was initiated under supervision of the study team and consent for the study was obtained prior to initiating therapy. A further 69 children had initiated PI-based therapy prior to enrollment but otherwise met all other study eligibility criteria. For these children pre-treatment blood samples could not be stored for resistance testing and pre-treatment viral load results were not consistently available. Determination of nevirapine exposure was based on an internally-consistent and plausible maternal history verified by medical records if available. All children enrolled were exposed only to single-dose nevirapine (to the mother and/or the infant) as inclusion of maternal zidovudine in national prophylaxis protocols began only in 2008. Children who met criteria for randomization tended to be slightly older at treatment start, had less severe disease and were more likely to have mothers on treatment (data not shown).
Pre-randomization treatment
Children older than 6 months were treated with ritonavir-boosted lopinavir (LPV/r) (230 mg/m2), stavudine (1mg/kg) and lamivudine (4mg/kg) every 12 hours. Children younger than 6 months or on tuberculosis treatment received ritonavir (400–450 mg/m2), stavudine and lamivudine every 12 hours. Once older than 6 months of age and/or following completion of tuberculosis treatment, ritonavir was changed to LPV/r. All medications were administered as liquids and drug doses were adjusted at each visit according to growth. Use of ritonavir adversely affected virologic suppression during the pre-randomization period15 but all children were receiving LPV/r-based therapy by randomization. Education about HIV and its treatment and adherence counseling was provided by the clinical team and peer counselors. Caregivers were encouraged to consult the study team for all clinical problems.
Randomization
Children were followed until viral suppression <400 copies/ml was sustained for at least 3 months within the first 12 months of study follow-up. The cut-off of <400 copies/ml was selected for pragmatic reasons because an assay that quantified to this threshold was in routine use at the time the study was designed. Other criteria for randomization included less than grade 2 alanine aminotransferase (ALT) abnormalities17 and not being on tuberculosis treatment. Randomization was done in cohort blocks of variable size between 8 and 12. Allocations were generated by the study statistician and were concealed in opaque envelopes opened on-site at the time of randomization. Once randomization criteria were met, a visit was scheduled to begin the changed regimen for the switch group or to start the post-randomization clock for the controls.
Study intervention
The study intervention required substituting nevirapine for LPV/r. Nevirapine (120mg/m2 ) was introduced once-per-day for the first 2 weeks and thereafter 200mg/m2 every 12 hours. Children randomized to the control group remained on the LPV/r-based regimen. Both groups received additional adherence counseling at the time of randomization which included, for the switch group only, specific instructions about the lead-in schedule and possible new side effects.
Follow-up
Post-randomization, children in both groups were seen at 2, 4, 8, 16, 24, 36, 52, 64 and 76 weeks. Blood samples were collected for viral load tests (HIV-1 RNA quantification) in plasma at 4, 16, 24, 36, 52, 64 and 76 weeks and for CD4 cell determination (counts and percentages) at 16, 24, 36, 52, 64 and 76 weeks. ALT and neutrophil measurements were scheduled at 2, 4, 16, 24, 36, 52, 64 and 76 weeks. Thereafter all children were re-enrolled in an extended follow-up protocol that continued to provide and monitor all clinical care including their randomly-assigned treatment regimen, if appropriate. Blood samples were collected every 3 months and were used to measure viral load, CD4 cell counts and percentages, full blood counts and liver function. Follow-up continued until June 2010 when the study was closed. Child weight and length were measured at every visit. Weight- and height-for-age Z-scores were calculated using WHO software.18 Concomitant medications and all clinical conditions were recorded. Caregivers were asked to return medication containers at all scheduled visits which were weighed and the contents reconciled with expected usage of each drug to determine the extent of adherence. Non-adherence was quantified utilizing the worst medication return of the three drugs excluding those who did not return containers. Poor adherence was defined as medication return for any of the three drugs 20% more than expected.
All children with a viral load test >1000 copies/ml were recalled and re-tested within 4 weeks whenever possible. The study team attempted to establish whether there were any adherence challenges and further counseling was provided. If poor adherence was unlikely, children in the switch group who had two or more viral load tests >1000 copies/ml were returned to the original LPV/r-based regimen. Children in the control group with persistent viremia were managed on a case-by-case basis.
Laboratory methods
Plasma HIV-1 RNA (Roche version 1.5 Amplicor assay, Roche, Branchburg, NJ) measurements, CD4 cell determinations, full blood counts and liver function tests were conducted by Clinical Laboratory Services in Johannesburg and reported directly to the site for use in clinical management. The Roche standard assay (quantification range 400–750,000 copies/ml) was used on pre-treatment samples or if clinicians expected high values and the ultra-sensitive assay (quantification range 50–150,000 copies/ml) for post-treatment samples. Blood counts and liver function tests were categorized into grades based on age and sex-specific norms provided by the local laboratory following guidelines from other clinical trials.17 Pre-treatment plasma samples were tested for viral mutations in the reverse transcriptase gene using bulk population sequencing towards the end of the study.19 Failing samples post-randomization were also tested by population sequencing using in-house methods.19,20 Resistance was defined using the Stanford Algorithm (http://hivdb.stanford.edu/pages/algs/HIVdb.html).
Study endpoints
Any viremia >50 copies/ml post-randomization was defined as the primary endpoint in the study protocol based on the logic that full viral suppression is the goal of antiretroviral treatment.21 This was also the lowest threshold discernable with the ultra-sensitive assay used. However, virologic failure was defined as confirmed viremia (i.e. occurring at least twice) >1000 copies/ml as this was the safety measure used in the protocol to guide regimen change. CD4 cell response, growth, liver function abnormalities, neutropenia and adherence were compared between the groups as secondary endpoints.
Statistical Analysis
The original target sample size was 186 equally divided between two groups to detect a non-suppression rate of 20% in the switch versus 5% in the control group using standard methods for comparing proportions22 and assuming 95% follow-up post-randomization.
All results are presented as modified intent-to-treat analyses that exclude those children (n=3) missing all virologic outcome data and censoring at the last available test all those who died or were lost to follow-up. For time-to-event analyses, results are reported through 156 weeks as this was the point that half of the cohort had surpassed by the time of study closure. Kaplan-Meier methods were used and groups compared using log-rank tests. Virologic outcomes, CD4 measurements, growth and adherence were also compared between groups using a repeat cross-sectional design retaining measurements from scheduled visits and calculating odds ratios (OR) and p-values using Generalized Estimating Equation (GEE) models to account for repeat observations per subjects. Comparisons between groups at specific time-points used Wilcoxon rank sum and t-tests tests for continuous variables and Chi-squared or Fisher’s exact tests for categorical variables. Analyses were done using SAS version 9.1.3 (Cary, NC).
Results
Study population
As previously reported,14 the 195 children randomized were a median of 10 months of age at treatment start, 55% had >750,000 HIV-1 RNA copies/ml, had a median CD4 percentage of 18.5 and a median weight-for-age Z-score of –2.18 pre-treatment. At randomization, a median of 9 months later, when all had <400 HIV-1 RNA copies/ml, the median CD4 percentage was 29.1 and the median weight-for-age Z-score was −0.58. The switch and control groups were similar in characteristics pre-treatment and at the time of randomization.14
Median follow-up post-randomization was 160 weeks among 99 children in the control and 151 weeks among 96 children in the switch group. Overall 3111 and 3519 child-months of follow-up, and 1283 and 1398 viral load assays were accrued in the switch and control groups respectively. Three deaths occurred in each group: in the control group at 4, 8 and 144 weeks and in the switch group at 1, 44 and 96 weeks. Four children were lost to follow-up before 76 weeks in the control and 11 in the switch group. By the end of the study, an additional 6 children in the control and 7 children in the switch group were lost to follow-up (Table 1). Eight children in each group initiated anti-tuberculosis therapy during follow-up and their regimens were modified.
Table 1.
Mortality, loss to follow-up and maintenance of virologic suppression among 195 HIV-infected children through 156 weeks post-randomization maintained on their initial ritonavir-boosted lopinavir-based regimen (control group) or switched to nevirapine-based therapy
| N (Kaplan-Meier probability ± standard error) | |||
|---|---|---|---|
|
Event by cumulative time after randomization |
Control Group N=99 |
Switch Group N=96 |
p-value* |
| Death | |||
| By 76 weeks | 2 (0.020 ± 0.014) | 2 (0.021 ± 0.015) | |
| By 156 weeks | 3 (0.036 ± 0.021) | 3 (0.034 ± 0.019) | 0.93 |
| Loss to follow-up | |||
| By 76 weeks | 4 (0.041 ± 0.020) | 11 (0.112 ± 0.033) | |
| By 156 weeks | 8 (0.088 ± 0.030) | 16 (0.185 ± 0.043) | 0.07 |
|
Primary endpoint HIV-1 RNA >50 copies/ml |
|||
| By 76 weeks | 62 (0.645 ± 0.049) | 43 (0.465 ± 0.052) | |
| By 104 weeks | 65 (0.676 ± 0.048) | 47 (0.514 ± 0.053) | |
| By 156 weeks | 66 (0.687 ± 0.047) | 52 (0.595 ± 0.056) | 0.01 |
|
Safety endpoint HIV-1 RNA >1000 copies/ml confirmed |
|||
| By 76 weeks | 9 (0.096 ± 0.030) | 22 (0.239 ± 0.045) | |
| By 104 weeks | 9 (0.096 ± 0.030) | 22 (0.239 ± 0.045) | |
| By 156 weeks | 10 (0.111 ± 0.033) | 22 (0.239 ± 0.045) | 0.009 |
From log-rank test
Timing of virologic endpoints
As observed previously by 52 weeks,14 virologic failure (i.e. confirmed viremia >1000 copies/ml) by 156 weeks occurred more often in the switch (23.9%) than in the control group (11.1%) (p=0.009). However, more children in the switch group (40.5%) maintained suppression to <50 copies/ml than in the controls (31.3%) (p=0.01) (Table 1). The risk of any viremia >1000 copies/ml (i.e. virologic failure or episodes of transient viremia) by 156 weeks was similar in the control (28.1%) and switch (33.2%) groups by 156 weeks (p=0.28). Sensitivity analyses treating loss to follow-up and/or deaths as endpoints yielded essentially the same the results (data not shown).
All 22 failures in the switch whereas only 50% of failures in the control group (5/10) occurred by 52 weeks; with 59.0% of failures (13/22) in the switch versus 10% (1/10) in the control group occurring by 24 weeks. Among those failure-free at 24 weeks post-randomization, the risk of failure by 156 weeks was similar in the switch (11.6%) and control (10.1%) groups (p=0.61).
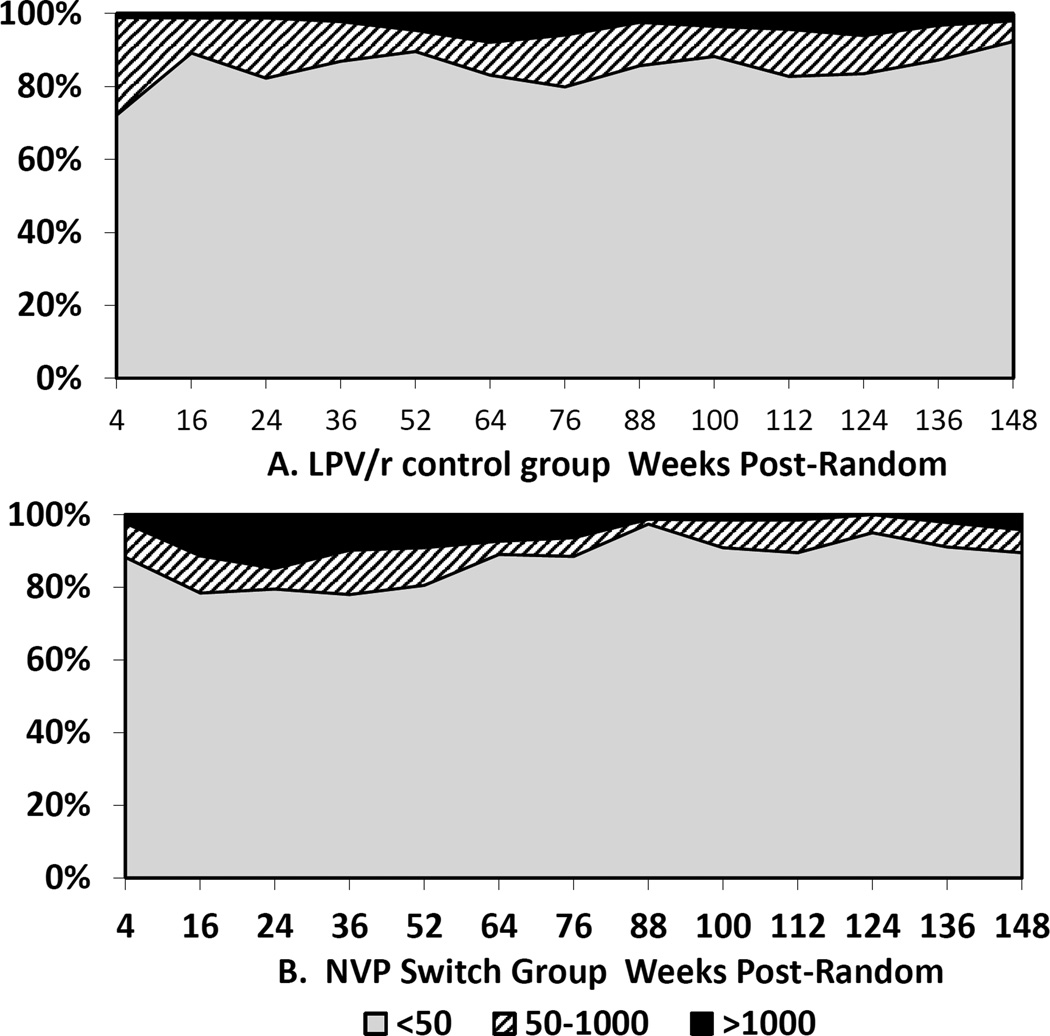
Viremia in categories (<50, 50–1000 and >1000 copies/ml) at each scheduled visit by group is shown in Figure 1. Through 52 weeks post-randomization, 84.1% of 441 scheduled visits in the control group were <50 copies/ml compared to 80.9% of 425 scheduled visits in the switch group (p=0.55). Between 64 and 148 weeks post-random, 85.0% of 594 visits in the control group were <50 copies/ml compared to 91.8% of 516 visits in the switch group (p=0.02). In contrast, through 52 weeks post-randomization, 9 (2.0%) of visits in the control compared to 40 (9.4%) in the switch group were >1000 copies/ml (p=0.0018); after 52 weeks, 27 (4.5%) in the control and 14 (2.7%) in the switch group were >1000 copies/ml (p=0.44).
Figure 1.
Proportions of children with HIV RNA < 50 (Grey), 51–1000 (striped) or >1000 copies/ml (black) at each scheduled visit 4–148 weeks post-randomization: Panel A - Ritonavir-boosted lopinavir-based regimen; Panel B - Nevirapine-based regimen
Risk factors for viral failure
In the control group, poor adherence was significantly associated with viral failure (odds ratio [OR]=2.95 95% CI: 1.28–6.82). Among those who failed, poor adherence was detected at 6/45 (13.3%) prior visits compared to 50/1019 (4.9%) visits among those who did not fail (p=0.01). Age younger than 18 months at the time of randomization was associated with a higher risk of failure in the control group (Table 2). However, only one child failed when younger than 24 months of age, 7/10 failures in the control group occurred when the child was between 24 and 36 months of age (mean age of failure 32.4 months). Of 323 visits between 24 and 35 months of age, 3.4% failed compared to 0.7% in 155 visits in younger and 2.0% in 557 visits in older children (p=0.26). Other characteristics, including all markers of the severity of disease pre-treatment or at the time of randomization, were not associated with failure (data not shown).
Table 2.
Risk factors for viral failure (confirmed viremia > 1000 copies/ml) in those randomized to stay on the initial ritonavir-boosted lopinavir-based regimen (n=99) or to switch to nevirapine-based therapy (n=96)
| Control Group | Switch Group | ||||||
|---|---|---|---|---|---|---|---|
| N | N (Kaplan-Meier probability ± standard error) |
p-value within group |
N | N (Kaplan-Meier probability ± standard error) |
p-value within group |
P-value across groups within strata |
|
| Age at start of therapy | |||||||
| < 1 year | 55 | 8 (0.148 ± 0.048) | 65 | 16 (0.259 ± 0.056) | >0.10 | ||
| ≥ 1 year | 42 | 2 (0.060 ± 0.042) | >0.10 | 30 | 6 (0.200 ± 0.073) | >0.10 | 0.04 |
| Drug resistance pretreatment | |||||||
| None | 59 | 5 (0.095 ± 0.041) | 51 | 7 (0.140 ± 0.049) | >0.10 | ||
| NNRTI* mutations | 11 | 1 (0.091 ± 0.087) | >0.10 | 19 | 10 (0.526 ± 0.115) | 0.0007 | 0.02 |
| Age at randomization | |||||||
| < 18 months | 38 | 7 (0.184 ± 0.063) | 44 | 10 (0.245 ± 0.068) | >0.10 | ||
| ≥ 18 months | 59 | 3 (0.063 ± 0.036) | 0.046 | 51 | 12 (0.236 ± 0.060) | >0.10 | 0.005 |
| HIV-1 RNA at randomization | |||||||
| < 50 copies/ml | 72 | 7 (0.105 ± 0.038) | 71 | 13 (0.189 ± 0.047) | 0.10 | ||
| 51–399 copies/ml | 25 | 3 (0.124 ± 0.067) | >0.10 | 24 | 9 (0.391 ± 0.103) | 0.047 | 0.03 |
| Odds ratio (95% CI)† | Odds ratio (95% CI) † | ||||||
| Non-adherent (>20% of medication returned at prior visits) vs. Adherent |
2.95 (1.28–6.82) | 0.01 | 4.92 (1.84–13.15) | 0.002 | N/A§ | ||
Non-nucleoside reverse transcriptase inhibitors (NNRTI)
Odds ratio calculated using Generalized Estimating Equations (GEE)
N/A Not applicable as adherence is time-depenent.
In the switch group, poor adherence also was significantly associated with viral failure (OR=4.92 95% CI: 1.84–13.15). Among those who failed, poor adherence was recorded at 7/46 (15.2%) prior visits compared to 29/843 (3.4%) visits of those who did not fail (p=0.002). Age either at the start of therapy or at the time of randomization was not associated with failure. The mean age of failure was significantly younger (26.7 months) than in the control group but failure was similarly concentrated 15/22 (68.2%) among children 24–36 months of age. Of 326 visits between 24 and 35 months of age, 10.1% failed compared to 5.4% in 186 visits in younger and 1.4% in 429 visits in older children (p=0.008) There was a trend towards a greater risk of failure if viremia was detectable in the 51–399 copies/ml range at the time of randomization (Table 2).
Mutations associated with nevirapine resistance, predominantly Y181C, detected by bulk sequencing in samples collected pre-treatment were significantly associated with failure in the switch group.14,19,20 In those with no resistance pre-treatment, the probability of failure by 156 weeks was 0.140 in the switch and 0.095 in the control group (p>0.10, Table 2). In those with nevirapine-associated mutation pre-treatment (n=19), 52.6% failed the switch. Those who remained suppressed when nevirapine was re-used despite pre-existing mutations were younger at treatment initiation (mean 5.2 months) than children who failed the switch (mean 10.1 months, p=0.008).
At time of failure, most (18/21 [85.7%]) children in the switch group had NNRTI-associated mutations (Table 3). Among 13/18 with pre-treatment resistance data 8 had NNRTI-associated mutations pre-treatment. Most (17/21 [81.0%]) children who failed the switch also had NRTI-associated mutations at time of failure, mostly M184V/I, but four children had other mutations (K65R, T69N, D67N, L74V). In the control group at the time of failure, only 1/9 had NNRTI-associated resistance mutations and 3 had NRTI-associated mutations, including M184V/I, two with A64V, and one with K219R (Table 3).
Table 3.
Descriptive characteristics, including drug resistance prior to starting therapy and at the time of failure, of the children in each group with confirmed viral failure
| Group | Sex | Age treatme nt started |
Age at rando m |
Viral load at random |
Age at first >1000cp m |
Viral load at failure |
Pre- treatment drug resistance* |
NNRTI resistance at failure |
NRTI resistance at failure |
|---|---|---|---|---|---|---|---|---|---|
| LPV/r control | F | 11 | 25 | <400 | 37 | 6920 | - | WT | WT |
| LPV/r control | F | 13 | 17 | <400 | 35 | 2500 | - | WT | WT |
| LPV/r control | M | 9 | 18 | <50 | 35 | 59600 | - | WT | WT |
| LPV/r control | M | 6 | 16 | <400 | 31 | 3940 | - | K103N | WT |
| LPV/r control | M | 13 | 19 | <50 | 50 | 2850 | WT | - | - |
| LPV/r control | M | 8 | 21 | 114 | 33 | 5260 | WT | WT | M184I, A62V |
| LPV/r control | M | 9 | 16 | <50 | 28 | 100000 | WT | WT | WT |
| LPV/r control | F | 6 | 15 | 70 | 19 | 69800 | WT | WT | WT |
| LPV/r control | F | 8 | 17 | 152 | 25 | 35700 | WT | WT | M184V |
| LPV/r control | F | 2 | 16 | <50 | 30 | 8160 | Y181C | WT | A62V, M184V, K219R |
| NVP Switch | F | 3 | 22 | 91 | 34 | 90200 | - | K103N | M184I |
| NVP Switch | M | 14 | 30 | 170 | 33 | 100000 | - | K103N, Y181C | M184V |
| NVP Switch | M | 10 | 20 | <50 | 28 | 4210 | - | V106M, Y181C, Y188C | K65R, M184V |
| NVP Switch | M | 8 | 20 | 192 | 23 | 94300 | - | Y181C | M184V |
| NVP Switch | M | 4 | 16 | <50 | 20 | 15660 | - | Y181C | M184I |
| NVP Switch | M | 10 | 16 | 134 | 22 | 1442 | K103N | - | - |
| NVP Switch | F | 14 | 27 | <50 | 31 | 70800 | WT | K101E, G190A | M184V |
| NVP Switch | F | 9 | 16 | <50 | 28 | 7640 | WT | WT | WT |
| NVP Switch | M | 9 | 16 | <50 | 19 | 36800 | WT | WT | WT |
| NVP Switch | F | 17 | 25 | 252 | 29 | 6900 | WT | V106A | M184V |
| NVP Switch | F | 16 | 26 | <50 | 31 | 2310 | WT | V106A | M184V |
| NVP Switch | M | 8 | 22 | <50 | 25 | 1090 | WT | Y181C | M184V |
| NVP Switch | M | 11 | 19 | 218 | 22 | 4260 | WT | Y181C | M184V |
| NVP Switch | F | 3 | 13 | <50 | 20 | 361000 | Y181C | WT | WT |
| NVP Switch | M | 10 | 18 | 132 | 28 | 4700 | Y181C | V106M, Y181C | M184V |
| NVP Switch | F | 17 | 30 | <50 | 31 | 3000 | Y181C | Y181C | T69N |
| NVP Switch | M | 7 | 16 | <50 | 25 | 100000 | Y181C | Y181C | WT |
| NVP Switch | F | 7 | 14 | <50 | 24 | 1350 | Y181C | Y181C | M184I |
| NVP Switch | M | 17 | 31 | 221 | 32 | 100000 | Y181C | Y181C | D67N |
| NVP Switch | M | 10 | 17 | <50 | 29 | 4360 | Y181C | Y181C | M184I |
| NVP Switch | F | 11 | 25 | 61 | 33 | 18880 | Y181C | Y181C, K103N | L74V, M184V |
| NVP Switch | F | 7 | 14 | <50 | 17 | 7560 | Y188C | K101E, Y181C | M184V |
WT indicates wild type; - indicates no result
Outcomes of children with viral failure
Of 22 children who failed the switch, 21 failed while still treated with nevirapine (the one who was not still being treated with nevirapine had been returned to a LPV/r-based regimen when co-treatment for tuberculosis was required; this child failed thereafter). Of the 22, two children spontaneously re-suppressed without a change in regimen and one child was switched to efavirenz and re-suppressed; three discontinued and thirteen were switched back to their original regimen (LPV/r, lamivudine, stavudine), another one to LPV/r+abacavir+didanosine and one to LPV/r+zidovudine+didanosine. Twelve of 15 returned to a LPV/r-based regimen re-suppressed to <50 copies/ml but two had further elevations towards the end of the study. Follow-up continued for a median of 17 months (minimum and maximum 4 and 37 months) after re-suppression. Eight of ten children who failed in the LPV/r group were able to re-suppress after counseling.
Laboratory abnormalities
Children in the switch group were significantly more likely to develop an ALT abnormality of grade 1 (38.9 vs. 27.6%, p=0.01), grade 2 (15.8 vs. 6.1%, p=0.02) or grade 3 (6.3 vs 1.0%, p=0.06) but not grade 4 (7.4 vs 9.2%, p>0.10) over the duration of follow-up. At individual follow-up visits, there were significantly more ALT abnormalities in the switch group but differences were confined to lower grades. There were no differences between groups in cumulative or cross-sectional risk of neutropenia (5.3% in the switch vs. 4.1% in the control group experienced grade 4 neutropenia during follow-up).
CD4 cell response
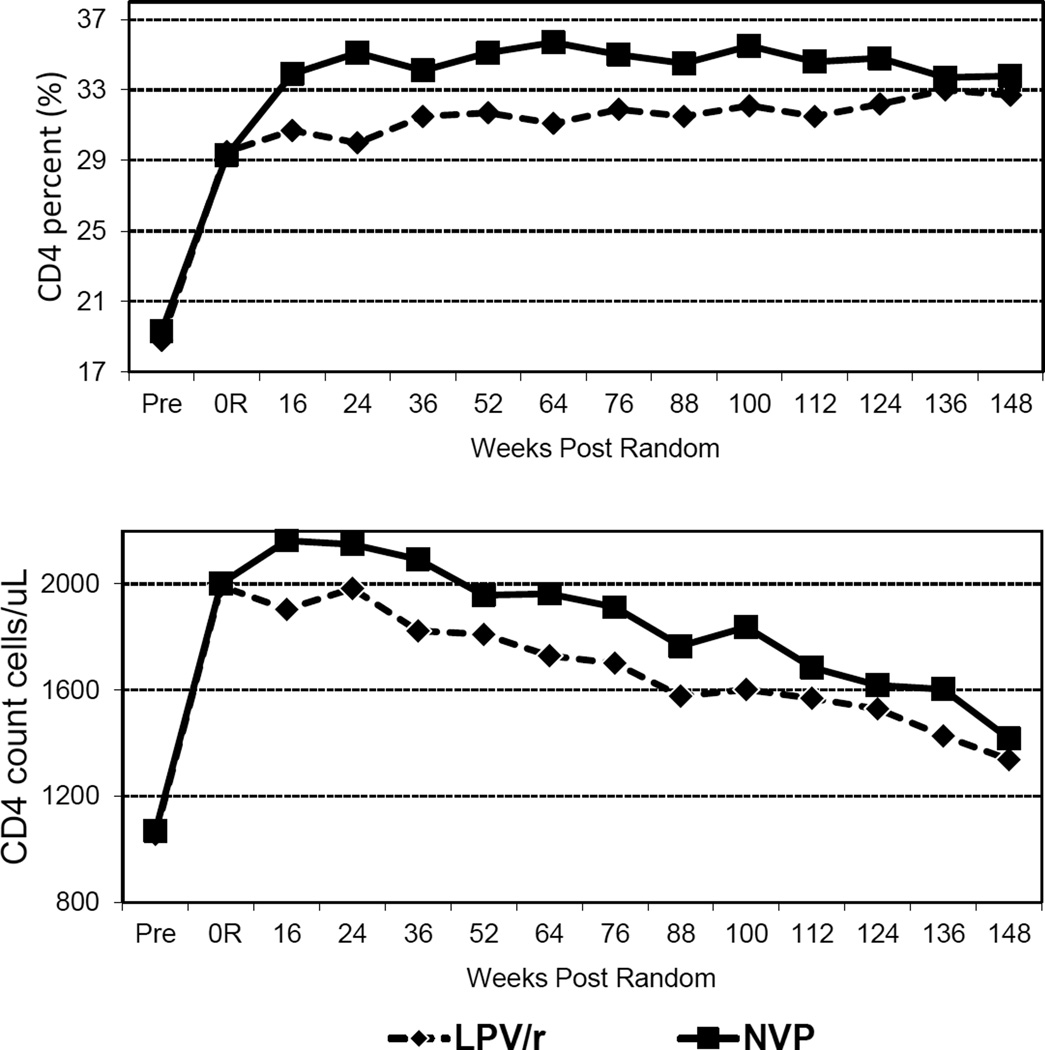
There was a substantial rise in mean CD4 cell count and percent from pre-treatment to randomization, but there was a more robust CD4 response in the switch group after randomization (Figure 2). After randomization, CD4 cell counts declined in both groups consistent with aging of the cohort. Through the first 2 years after randomization, the mean CD4 count was 234 cells/µL (95% CI: 100–369) higher in the switch versus control group (p<0.001). After two years, the differences narrowed and became non-significant (mean difference 128 cells/µL 95% CI: −32–289, p=0.12). Similarly, CD4 percent was an average of 3.15 (95% CI: 1.98–4.31, p<0.001) percentage points higher in the switch than control group through the first 100 weeks post-randomization, with a reduced although still significant difference thereafter (mean difference 1.97 percentage points 95% CI: 0.42–3.53, p=0.01).
Figure 2.
Mean CD4 percent and CD4 count pre-treatment, at randomization and at scheduled visits 16–148 weeks post-randomization in the ritonavir-boosted lopinavir-based regimen and switch to nevirapine-based regimen groups
Growth
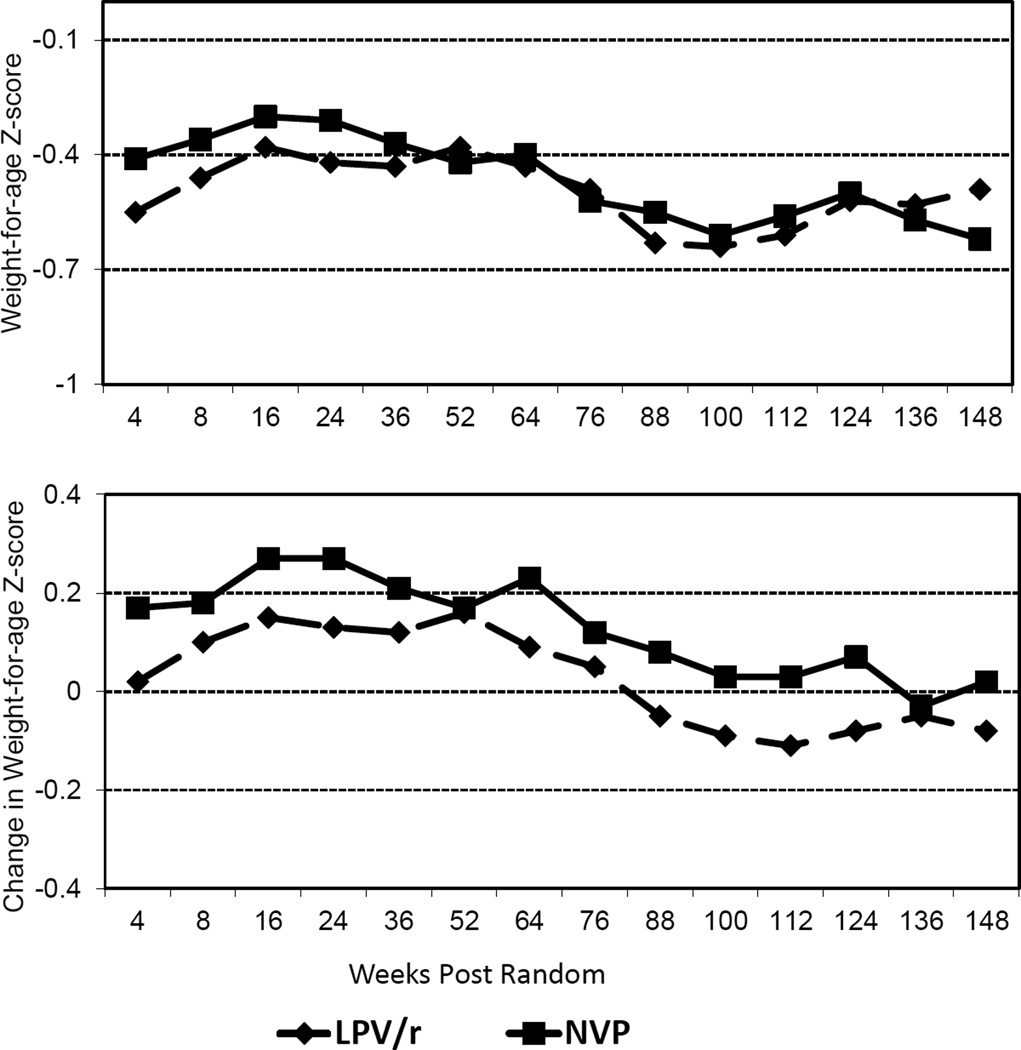
At randomization, there were no significant differences in mean weight-for-age Z-scores (−0.59 and −0.56) between the switch vs. control groups (p=0.84). Post-randomization, weight change relative to the time of randomization was 0.16 Z-score points higher (p=0.01) in the switch than control group before 52 weeks. Differences in the mean weight-for-age Z-score did not reach significance (p=0.36). Children in the control group were more likely to experience a >1 Z-score drop in their weight-for-age before 52 weeks (13.1%) than the switch group (4.2%) (P=0.03). After 52 weeks post-randomization, differences were attenuated and less consistent (Figure 3). Mean height-for-age Z-score showed a non-significant advantage favoring the switch group before 52 weeks (0.10 units, p=0.54) and thereafter was similar.
Figure 3.
Mean weight-for-age Z-score and mean change from randomization weight-for-age Z-score at scheduled visits 4–148 weeks post-randomization in the ritonavir-boosted lopinavir-based regimen or switch to nevirapine-based regimen groups
Discussion
Switching children who failed nevirapine chemoprophylaxis, but initially controlled on a LPV/r-based regimen, may be a valuable treatment strategy to preserve LPV/r for future treatment while at the same time minimizing adherence challenges for parents, limiting metabolic toxicities and reducing cost. Consistent with our previous report,14 these results confirm that re-use of nevirapine is an effective strategy for maintaining viral suppression below 50 copies/ml, is without major side effects, and results in better immunologic and growth outcomes in the short-term. Enthusiasm for the strategy was tempered by our prior observation that viral failure –defined as confirmed viremia >1000 copies/ml which was the safety endpoint in the study to prompt consideration of regimen change–was more frequent in the switch than in the control group. The new follow-up data presented here demonstrate that those children likely to fail the switch strategy can all be detected by 52 weeks. Thus focused viral load testing during the first year after switch could identify those in need of return to the original regimen allowing the remaining children to reap the advantages of the nevirapine-based regimen.
The utility of viral load monitoring in low resource settings is controversial because of high cost. Many programs in sub-Saharan Africa do not include routine viral load monitoring as part of their clinical services.23,24 Recent data in adults suggest that programs that include viral load monitoring have better clinical outcomes, including mortality,25 than programs that do not but ecological comparisons across countries limits interpretation. For the switch strategy to be safely implemented at least three viral load tests are necessary to identify children and at least one further test to confirm any elevations. One prior to the switch to determine virologic response to the initial regimen, and two after the switch, at e.g. 24 and 52 weeks post-switch, to promptly identify failures. Children with elevated viral load results will need further tests to confirm results. Our data indicate that after excluding failures occurring in the first 24 weeks post-switch, rates of failure are similar between the two groups suggesting that the later failures are unrelated to underlying resistance selected by nevirapine prophylaxis. By a year post-switch, children could be monitored as per routine clinical guidelines. Cost analyses of the additional resources required to monitor the switch strategy versus the savings from utilizing the less expensive regimen are warranted.
Viral mutations conferring resistance to nevirapine, predominantly Y181C, detected by standard population sequencing in samples collected pre-treatment were strongly associated with switch failure. This indicates that methods for ascertaining drug resistance already in routine clinical use are adequate to identify children most likely to benefit from the switch strategy. Although a larger proportion of children harbor resistance mutations below the detection threshold of these standard assays, we have shown previously that these low frequency mutations are not associated with switch failure.19,20 Theoretically, pre-treatment resistance results could be used to guide the switch strategy as the long time window would allow samples to be batched and tested in central laboratories. However, such testing is logistically-complex and expensive and it is doubtful whether there is adequate laboratory capacity to support routine testing. With adequate viral load monitoring, pre-treatment drug resistance testing would be useful but not essential.
We observed that children between two and three years of age were at higher risk of failure while on therapy than children either older or younger. This may reflect developmental challenges related to adherence in this age group. Contrary to expectations, young age at initiating therapy was not associated with switch failure despite the higher prevalence of measured drug resistance in young children. To investigate this further, analysis was restricted to children with detectable resistance pretreatment. Among this small sub-group, older children were more likely to fail the switch. We can infer that population sequencing in younger children recently exposed to nevirapine detects viral mutations destined to decline as well as those destined to persist, whereas testing in older children with nevirapine exposures in the more distant past, detects only persisting mutations. Thus it appears that only the persisting mutations influence treatment response. These findings raise the caveat that the predictive capability of drug resistance testing in children may be related to age. As the new guidelines recommending early treatment in newly-diagnosed young children become more widely implemented, the predictive utility of drug resistance testing may need to be revisited.
Multi-class drug resistance at time of viral failure was more common in the children who failed the switch than in the controls. This is consistent with observations of slower selection of NRTI-associated mutations in PI-treated children.26 The most common mutation was M184V/I which is rapidly selected with lamivudine exposure, may be related to reduced viral fitness, and confers increased susceptibility to zidovudine.27 Resistance detected at the time of failure did not influence treatment response when children were returned to the original regimen: all of whom re-suppressed if adequate adherence could be attained. Our data reinforce the importance of supporting caregivers to maintain adherence with pediatric treatment.
In our study population, ~85% of surviving children attained <400 copies/ml within the 12 month time window and were eligible for the switch strategy but only ~75% of these were <50 copies/ml at the time of randomization. The subset who were <50 copies/ml at randomization were slightly less likely to fail the switch than those with viremia in the 51–399 copies/ml range. It may be prudent to wait until the more stringent threshold is reached before selecting children to consider for regimen switch which may limit the applicability of the strategy.
The better outcome achieved in the switch versus control group when viral suppression was defined as <50 copies/ml is intriguing. It is concerning that less than a third of children treated with the LPV/r-based regimen maintained suppression at this level throughout follow-up. Whether intermittent, low-level viremia has clinical significance needs further investigation.28 The superior profile of nevirapine in maintaining viremia <50 copies/ml may be due to its greater penetration into extra-vascular compartments. This has been hypothesized as an explanation for the drug’s greater efficacy in suppressing residual viremia below the usual limits of detection i.e. <1–2 copies/ml.29,30
The better outcomes we report in the switch group for CD4 response and growth in the short-term are consistent with those reported from the trial of LPV/r- versus nevirapine-based primary therapy for exposed infants despite the clear-cut virologic superiority of LPV/r in that trial.4 Inadequate growth in children treated with ritonavir has also been previously reported.31 In adults, these paradoxical findings have not been reported.32 The unpleasant taste of LPV/r may contribute to poor appetite or other developmental vulnerabilities to LPV/r may exist among infants and young children. Although intriguing, the differences did not persist and the clinical significance is unclear.
Our new results indicate that with adequate virologic monitoring the switch strategy may be a valuable option for HIV-infected children. The switch strategy allows a LPV/r-based regimen to be used to achieve viral suppression initially and then once suppressed, switching may be considered if regular viral load monitoring for at least a year can be implemented. The strategy results in better immunologic and growth outcomes initially and allows LPV/r to be preserved for second-line treatment. Despite its proven virologic superiority,4 some programs in low resource settings have not been able to implement pediatric treatment using LPV/r as part of primary therapy because it is more expensive than nevirapine. The lower cost of long-term maintenance therapy with nevirapine, often part of pediatric fixed-dose combinations,33 may be able to offset some of the initial drug costs as well as the extra virologic testing making LPV/r-based regimens as first-line regimens for infants and young children economically-viable in a wider range of settings (Panel). Nevertheless, the practical challenges of identifying and monitoring children undergoing changing regimens should not be under-estimated. Although new guidelines for prevention of mother-to-child transmission will result in fewer children acquiring infection, those who do will have a greater likelihood of having resistance to nevirapine.34 Thus it continues to be essential that optimal treatment strategies utilizing the limited number of antiretroviral drugs suitable for young children be evaluated.
Panel
Research in Context
Systematic review
When children born to HIV-infected women acquire infection despite use of prophylactic antiretroviral regimens that include nevirapine, drug resistant variants are commonly selected.2 A randomized clinical trial demonstrated that HIV-infected children need to start antiretroviral therapy as early as possible.6 A search of PubMed for articles published in English between January 2006 and December 2011 could locate only one clinical trial of starting regimens for HIV-infected children who failed nevirapine prophylaxis.4 This trial, which compared nevirapine- to ritonavir-boosted lopinavir (LPV/r)-based therapy among nevirapine-exposed children, found better virologic suppression and improved survival with a LPV/r-based regimen,4 consistent with an adult trial.32 Current guidelines recommend LPV/r-based regimens as first-line therapy for nevirapine-exposed infants and young children.
Interpretation
For HIV-infected children who failed nevirapine prophylaxis and are controlled on an initial LPV/r-based regimen, pre-emptive switching to a nevirapine-based regimen once suppressed may be a valuable treatment strategy to preserve LPV/r for future treatment while at the same time minimizing adherence challenges for parents, limiting metabolic toxicities and reducing cost. The switch strategy is effective for maintaining viral suppression below 50 copies/ml, is without major side effects, and results in better immunologic and growth outcomes in the short-term. Virologic failure associated with the switch only occurred within the first year after switch. Switching children once suppressed to a nevirapine-based regimen may be a valuable treatment option provided that adequate viral load monitoring can be done.
Summary.
Antiretroviral therapy using regimens including ritonavir-boosted lopinavir (LPV/r) are recommended as first-line treatment for HIV-infected infants exposed to nevirapine prophylaxis. However, this regimen poses adherence challenges, is associated with metabolic toxicities, limits second-line options, and is costly. In Johannesburg, South Africa, 195 nevirapine-exposed <24 months of age who achieved virologic suppression <400 copies/ml while treated with a LPV/r-based regimen were randomized to switch to nevirapine-based therapy or remain on the LPV/r-based regimen. By 156 weeks post-randomization, more children in the switch (23.9%) than in the control group (11.1%) had confirmed viral failure >1000 copies/ml (p=0.01) but all of these failures were detected by 52 weeks. Children in the switch group (40.5%) were more likely to maintain full suppression <50 copies/ml than controls (31.3%, p=0.01) and had better CD4 and growth responses initially after switching. Switching children once suppressed to a nevirapine-based regimen may be a valuable treatment option provided that adequate viral load monitoring can be done.
Acknowledgements
We would like to thank the following people for assistance with the study: Edmund Caparelli, M.D., Mark Cotton, M.D., Victor DeGruttola, Ph.D., Brian Eley, M.D., Mary-Glenn Fowler, M.D., Lynne Mofenson, M.D., Paul Palumbo, M.D., Andrea Ruff, M.D. and Kevin Ryan, Ph.D. We also thank the children and their care-givers for their dedication to the study and all the members of the NEVEREST study team.
Funding: The study was supported in part by grants from the National Institutes of Child Health and Human Development (NICHD) HD 47177 and HD057784 (to DP) and Secure the Future Foundation RES 219.
The funding sources had no role in the writing of the manuscript or the decision to submit for publication.
Footnotes
Conflict of interest statement: Dr. Persaud served on an Advisory Board for Glaxo Smith Kline on use of dried blood spots for HIV monitoring. None of the other authors has any conflicts of interest to declare.
Trial Registration: Clinical Trials.gov NCT00117728
Reference List
- 1.World Health Organization. [accessed Aug 2008];WHO Antiretroviral Therapy for Infants and Children: Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting. 2008 Apr 10–11; http://www who int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008 pdf. 2008.
- 2.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palumbo P, Violari A, Lindsey J, et al. NVP- vs LPV/r-based ART among HIV + infants in resource-limited settings: the IMPAACT P1060 trial; Boston, MA. 18th Conference on Retroviruses and Opportunistic Infections; 2011. Feb 27, Abstract 129LB ed. Mar 2. [Google Scholar]
- 6.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn AH, Nuttall JJC, Zhang F. Sequencing of antiretroviral therapy in children in low- and middle-income countries. Current Opinion in HIV & AIDS. 2010;5:54–60. doi: 10.1097/COH.0b013e3283339bd8. [DOI] [PubMed] [Google Scholar]
- 8.Davies MA, Boulle A, Fakir T, Nuttall J, Eley B. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatrics. 2008;8:34. doi: 10.1186/1471-2431-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McComsey GA, Leonard E. Metabolic complications of HIV therapy in children. AIDS. 2004;18:1753–1768. doi: 10.1097/00002030-200409030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitnun A, Sochett E, Babyn P, et al. Serum lipids, glucose homeostasis and abdominal adipose tissue distribution in protease inhibitor-treated and naive HIV-infected children. AIDS. 2003;17:1319–1327. doi: 10.1097/00002030-200306130-00006. [DOI] [PubMed] [Google Scholar]
- 12.Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 13.Ciaranello AL, Lockman S, Freedberg KA, et al. First-line antiretroviral therapy after single-dose nevirapine exposure in South Africa: a cost-effectiveness analysis of the OCTANE trial. AIDS. 2011;25:479–492. doi: 10.1097/QAD.0b013e3283428cbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201:1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health. Guidelines for the management of HIV-infected children. Pretoria: 2005. http://www.doh.gov.za/docs/policy-f.html; [Google Scholar]
- 17.Division of AIDS (DAIDS) Table for grading the severity of adult and pediatric adverse events. 2009 http://rsc tech-res com/document/safetyandpharmacovigilance/Table_for_grading_severity_of_adult_pediatric_adverse_events pdf.
- 18.WHO Child Growth Standards (2005) and the WHO Anthro 2005 software and macros. http://www who int/childgrowth/software/en/.
- 19.Hunt GM, Coovadia A, Abrams EJ, et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS. 2011;25:1461–1469. doi: 10.1097/QAD.0b013e3283492180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorthy A, Kuhn L, Coovadia A, et al. Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children. Clin Infect Dis. 2011;52:514–521. doi: 10.1093/cid/ciq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the international AIDS society - USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 22.Fleiss JL. Statistical Methods for Rates and Proportions. Second Edition. New York: Wiley; 1981. [Google Scholar]
- 23.Schneider K, Puthanakit T, Kerr S, et al. Economic evaluation of monitoring virologic responses to antiretroviral therapy in HIV-infected children in resource-limited settings. AIDS. 2011;25:1143–1151. doi: 10.1097/QAD.0b013e3283466fab. [DOI] [PubMed] [Google Scholar]
- 24.Walker AS, Gibb DM. Monitoring of highly active antiretroviral therapy in HIV infection. Curr Opin Infect Dis. 2011;24:27–33. doi: 10.1097/QCO.0b013e3283423e0e. [DOI] [PubMed] [Google Scholar]
- 25.Keiser O, Chi BH, Gsponer T, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in southern Africa. AIDS. 2011;25:1761–1769. doi: 10.1097/QAD.0b013e328349822f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babiker A, Castro nee GH, Compagnucci A, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11:273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner D, Brenner BG, Routy JP, Petrella M, Wainberg MA. Rationale for maintenance of the M184v resistance mutation in human immunodeficiency virus type 1 reverse transcriptase in treatment experienced patients. New Microbiol. 2004;27:31–39. [PubMed] [Google Scholar]
- 28.Cohen C. Low-level viremia in HIV-1 infection: consequences and implications for switching to a new regimen. HIV Clin Trials. 2009;10:116–124. doi: 10.1310/hct1002-116. [DOI] [PubMed] [Google Scholar]
- 29.Bonora S, Nicastri E, Calcagno A, et al. Ultrasensitive assessment of residual HIV viraemia in HAART-treated patients with persistently undetectable plasma HIV-RNA: a cross-sectional evaluation. J Med Virol. 2009;81:400–405. doi: 10.1002/jmv.21405. [DOI] [PubMed] [Google Scholar]
- 30.Haim-Boukobza S, Morand-Joubert L, Flandre P, et al. Higher efficacy of nevirapine than efavirenz to achieve HIV-1 plasma viral load below 1 copy/ml. AIDS. 2011;25:341–344. doi: 10.1097/QAD.0b013e3283427de3. [DOI] [PubMed] [Google Scholar]
- 31.Nachman SA, Lindsey JC, Pelton S, et al. Growth in human immunodeficiency virus-infected children receiving ritonavir-containing antiretroviral therapy. Arch Pediatr Adolesc Med. 2002;156:497–503. doi: 10.1001/archpedi.156.5.497. [DOI] [PubMed] [Google Scholar]
- 32.Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulenga V, Cook A, Walker AS, et al. Strategies for nevirapine initiation in HIV-infected children taking pediatric fixed-dose combination "baby pills" in Zambia: a randomized controlled trial. Clin Infect Dis. 2010;51:1081–1089. doi: 10.1086/656628. [DOI] [PubMed] [Google Scholar]
- 34.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4(1):e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]