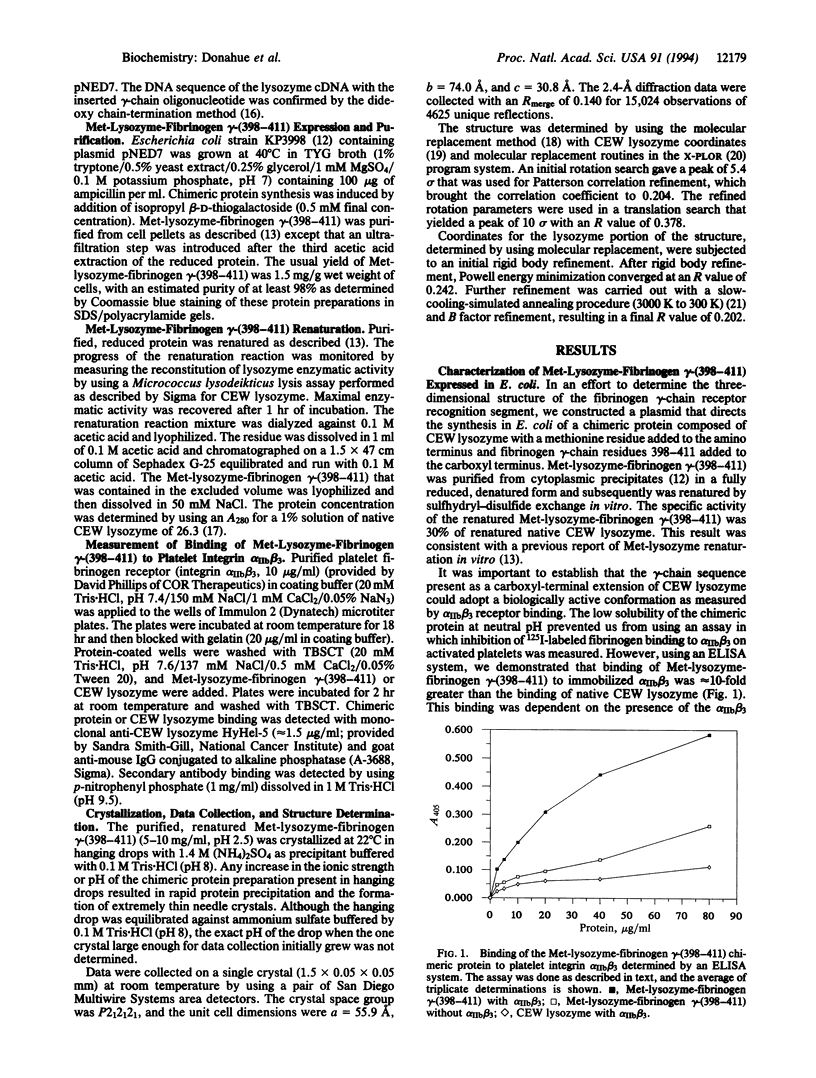
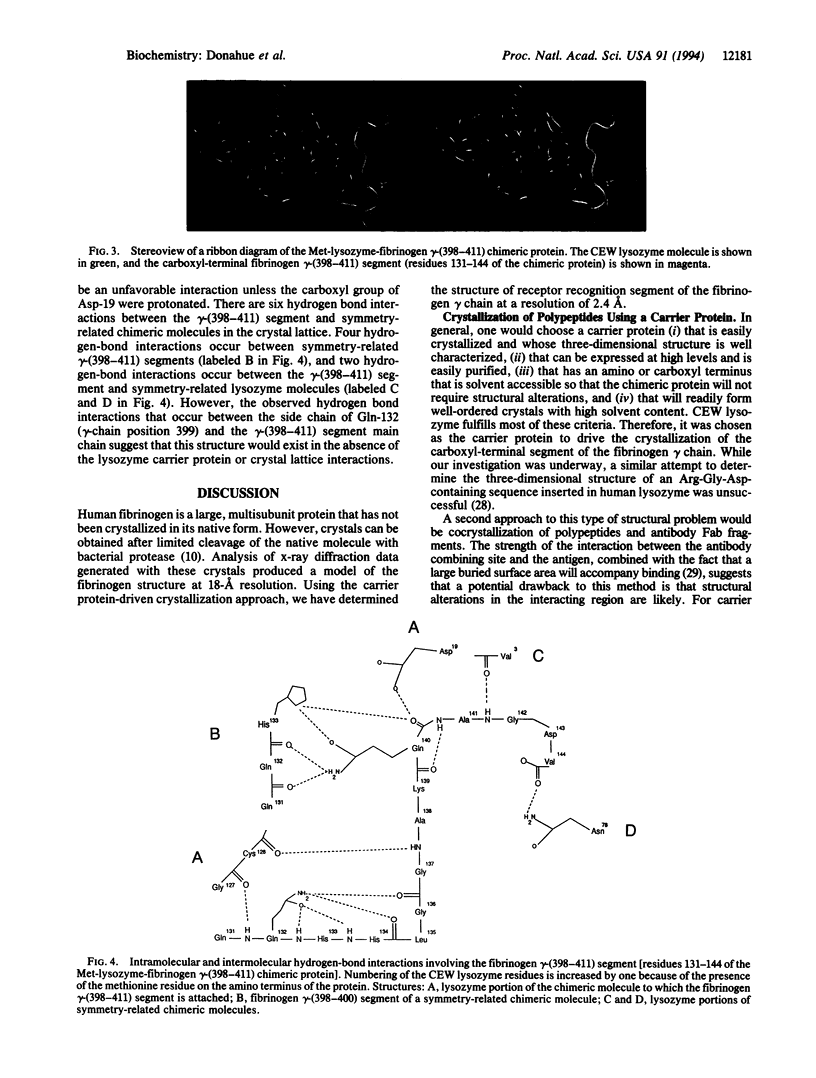
Abstract
We have developed a method for crystallizing small functional protein segments so that their three-dimensional structure can be determined by x-ray diffraction analysis. This method consists of linking a small protein segment of unknown tertiary structure to either the amino or carboxyl terminus of a larger carrier protein of known tertiary structure. Crystallization of the small segment is then driven by crystallization of the carrier protein. Using this approach, we have obtained crystals of the human fibrinogen gamma-chain carboxyl-terminal segment linked to the carboxyl terminus of chicken egg white lysozyme. The three-dimensional structure of the carboxyl-terminal segment of the fibrinogen gamma chain was determined by x-ray diffraction analysis at a resolution of 2.4 A. This segment encompasses the recognition site for the integrin alpha IIb beta 3 receptor on activated platelets and for the clumping receptor on pathogenic staphylococci and also bears donor and acceptor sites for factor XIIIa-catalyzed crosslinking of fibrin. Therefore, the structural information derived from our analysis will provide a rational basis for the design of inhibitors of these important functions of fibrinogen. Moreover, carrier protein-driven crystallization will facilitate the determination of the three-dimensional structure of functional segments of other proteins that are, like fibrinogen, difficult to crystallize in toto.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenstein M., Matsueda G. R., Timmons S., Hawiger J. A beta-turn is present in the 392-411 segment of the human fibrinogen gamma-chain. Effects of structural changes in this segment on affinity to antibody 4A5. Biochemistry. 1992 Nov 10;31(44):10692–10698. doi: 10.1021/bi00159a008. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Krukowski A., Erickson J. W. Slow-cooling protocols for crystallographic refinement by simulated annealing. Acta Crystallogr A. 1990 Jul 1;46(Pt 7):585–593. doi: 10.1107/s0108767390002355. [DOI] [PubMed] [Google Scholar]
- Chen R., Doolittle R. F. - cross-linking sites in human and bovine fibrin. Biochemistry. 1971 Nov 23;10(24):4487–4491. doi: 10.1021/bi00800a021. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Chan W. Y., Davie E. W. Characterization of a complementary deoxyribonucleic acid coding for the gamma chain of human fibrinogen. Biochemistry. 1983 Jun 21;22(13):3250–3256. doi: 10.1021/bi00282a033. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Farrell D. H., Thiagarajan P., Chung D. W., Davie E. W. Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10729–10732. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto T., Yamada H., Yasukochi T., Yamada E., Ito Y., Ueda T., Nagatani H., Miki T., Horiuchi T. Point mutation of alanine (31) to valine prohibits the folding of reduced lysozyme by sulfhydryl-disulfide interchange. Protein Eng. 1987 Aug-Sep;1(4):333–338. doi: 10.1093/protein/1.4.333. [DOI] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Bednarek M. A., Sakon M., Hawiger J. Platelet receptor recognition domain on the gamma chain of human fibrinogen and its synthetic peptide analogues. Biochemistry. 1989 Apr 4;28(7):2915–2919. doi: 10.1021/bi00433a025. [DOI] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Lukas T. J., Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984 Apr 10;23(8):1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- Kundrot C. E., Richards F. M. Effect of hydrostatic pressure on the solvent in crystals of hen egg-white lysozyme. J Mol Biol. 1988 Mar 20;200(2):401–410. doi: 10.1016/0022-2836(88)90249-5. [DOI] [PubMed] [Google Scholar]
- McDevitt D., Francois P., Vaudaux P., Foster T. J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994 Jan;11(2):237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Miki T., Yasukochi T., Nagatani H., Furuno M., Orita T., Yamada H., Imoto T., Horiuchi T. Construction of a plasmid vector for the regulatable high level expression of eukaryotic genes in Escherichia coli: an application to overproduction of chicken lysozyme. Protein Eng. 1987 Aug-Sep;1(4):327–332. doi: 10.1093/protein/1.4.327. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I., Francis C. W., Marder V. J. Fibrinogen binding to human blood platelets: effect of gamma chain carboxyterminal structure and length. Blood. 1986 Feb;67(2):385–390. [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Rao S. P., Poojary M. D., Elliott B. W., Jr, Melanson L. A., Oriel B., Cohen C. Fibrinogen structure in projection at 18 A resolution. Electron density by co-ordinated cryo-electron microscopy and X-ray crystallography. J Mol Biol. 1991 Nov 5;222(1):89–98. doi: 10.1016/0022-2836(91)90739-s. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry. 1970 Dec 8;9(25):5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- Strong D. D., Laudano A. P., Hawiger J., Doolittle R. F. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982 Mar 16;21(6):1414–1420. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- Yamada T., Matsushima M., Inaka K., Ohkubo T., Uyeda A., Maeda T., Titani K., Sekiguchi K., Kikuchi M. Structural and functional analyses of the Arg-Gly-Asp sequence introduced into human lysozyme. J Biol Chem. 1993 May 15;268(14):10588–10592. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]